2016 年 85 巻 3 号 p. 209-216
2016 年 85 巻 3 号 p. 209-216
‘Hasshu’, a dwarf budsport that originated from the leading persimmon cultivar ‘Hiratanenashi’ (Diospyros kaki Thunb.), was discovered in Japan in 2005. Although ‘Hiratanenashi’ is seedless because of anisoploidy (2n = 135 = 9x), ‘Hasshu’ produces some small normal seeds. In this study, we investigated differences in the morphological characteristics of the vegetative organs and fruits and in the ploidy level between ‘Hasshu’ and ‘Hiratanenashi’. The shoot length, internode length, and leaf size of ‘Hasshu’ were smaller than those of ‘Hiratanenashi’. ‘Hasshu’ bore smaller flowers than those of ‘Hiratanenashi’ in late May, and had consistently smaller fruit than ‘Hiratanenashi’ at all fruit development stages. ‘Hasshu’ ripened in late October, similar to ‘Hiratanenashi’. Both ‘Hiratanenashi’ and ‘Hasshu’ are pollination-variant astringent (PVA) cultivars. Except for the deletion of one allele at ssrdk10, no differences were detected between the two cultivars at four simple sequence repeat (SSR) marker loci. By flow cytometric analysis and chromosome observation, we confirmed that ‘Hasshu’ was octoploid (2n = 120 = 8x), indicating that it was both a dwarf and a ploidy-reduction mutation. These results suggest that recovery of the ability to produce some normal seeds by ‘Hasshu’ may have been caused by the change in ploidy from anisoploid to isoploid.
Persimmons belong to the genus Diospyros in the family Ebenaceae. The genus contains nearly 400 species, most of which are distributed in tropical and subtropical regions (Sugiura, 2005). Japanese or Oriental persimmon (Diospyros kaki Thunb.) is believed to have originated somewhere along the Yangtze River in China (Sugiura, 2005). It spread to Korea and Japan many years ago, where additional cultivars were developed. Persimmons have been cultured for centuries in Japan, China, and Korea, and are now gaining in popularity throughout the world (Yamada, 2006).
Although most Diospyros species such as date plum (D. lotus L.) and velvet apple (D. discolor Willd.) are diploid (2n = 2x = 30, x = 15), most Japanese persimmon cultivars are hexaploid with a somatic chromosome number of 90 (2n = 6x) (Namikawa, 1930; Namikawa and Higashi, 1928). Zhuang et al. (1990) found that a few seedless Japanese persimmon cultivars are nonaploid with a somatic chromosome number of 135 (2n = 9x), in contrast to most of the seeded cultivars (6x). Tamura et al. (1995) established somatic hybrids of Japanese persimmon by electrofusion of protoplasts, and produced intraspecific dodecaploid hybrids (2n = 12x = 180) between two hexaploid cultivars, D. kaki ‘Jiro’ and ‘Suruga’. Dodecaploid persimmons have also been produced by colchicine treatment (Chijiwa et al., 2011; Tamura et al., 1996) or by crossing between the hexaploid cultivar ‘Fujiwaragosho’ that can produce an unreduced megaspore and hexaploid unreduced pollen (Yamada and Tao, 2006, 2007). Additionally, Tamura et al. (1998) succeeded in the production of artificial interspecific octoploid hybrids (2n = 8x = 120) between hexaploid D. kaki and the wild diploid species D. glandulosa. However, neither octoploid nor dodecaploid Japanese persimmon has been found in nature.
‘Hiratanenashi’, which is a pollination-variant astringent (PVA) and seedless cultivar, is the leading cultivar in Japan. The ploidy level of ‘Hiratanenashi’ is nonaploid (Zhuang et al., 1990). ‘Hiratanenashi’ is known as the source of some important bud sport mutants (Yonemori et al., 2000) such as an early-maturing mutant (‘Tonewase’). Another bud sport, ‘Totsutanenashi’, is a small-fruited dwarf mutant that originated from ‘Hiratanenashi’ in Japan (Tao et al., 2013; Yamane et al., 2008). Like ‘Hiratanenashi’, these mutants are seedless cultivars.
In 2005, we discovered a dwarf bud sport bearing a small fruit that originated from ‘Hiratanenashi’ planted in our orchard in Higashihiroshima, Hiroshima Prefecture, Japan. Interestingly, this dwarf mutant produces some normal seeds, unlike ‘Hiratanenashi’ and mutants previously derived from it such as ‘Totsutanenashi’ (Yamane et al., 2008). We named this dwarf bud sport ‘Hasshu’, which was registered as a new cultivar under the Seeds and Seedlings Law in 2015. We propagated ‘Hasshu’ by top-grafting for several years to compare the morphological characteristics of vegetative organs and fruit between ‘Hasshu’ and ‘Hiratanenashi’. In this report, we examined their genetic relationship using simple sequence repeat (SSR) markers, and determined the ploidy level of ‘Hasshu’.
‘Hasshu’ was discovered in 2005 at the Grape and Persimmon Research Station, NARO Institute of Fruit Tree Science in Japan (34°N, 132°E). We used seven trees each of ‘Hasshu’ and ‘Hiratanenashi’ top-grafted onto ‘Shinshu’ growing in the orchard. All trees were hardwood-grafted at the same time in May 2010. Flower buds were thinned before blossoming, with a target of approximately 13 leaves to one flower. All flowers were artificially pollinated with pollen from ‘Zenjimaru’. Fruit was finally thinned to a ratio of 20 leaves to one fruit in early July.
Morphological characteristics of the shoots, flowers, and leavesIn December 2013, the shoot and internode lengths of 15 one-year-old shoots were measured for both cultivars. The diameter and weight of 15 flowers in full bloom (late May) were measured in 2014. Twenty mature leaves per cultivar were collected from the mid-position of the shoots in August 2013, and their leaf length, leaf width, and petiole length were measured using digital calipers (CD-S15C; Mitutoyo Co., Kanagawa, Japan). The leaf area was calculated with a leaf area meter (LI-3050C; LI-COR, Lincoln, NE, USA).
Comparison of fruit qualityTo examine the fruit development of ‘Hasshu’, the transverse diameters of ‘Hasshu’ and ‘Hiratanenashi’ fruit were measured periodically during fruit development in 2014. Mature fruits of each cultivar were harvested in late October, when they measured 5.0 to 5.5 on the ‘Hiratanenashi’ peel-color chart index (Yamazaki and Suzuki, 1980). The peel color (L*, a*, and b* values) was determined in the equatorial zone of mature fruit using a color-difference meter (CR-400; Konicaminolta Co., Tokyo, Japan). Astringency was removed by the constant-temperature short-duration (CTSD) method. At harvest, 20 to 25 fruits of each cultivar were placed in an incubator at 26°C for 16 h. These fruits were then placed in a thick, gas-tight polypropylene container. The internal gas in the container was replaced by an atmosphere of > 95% CO2, and the container was sealed, and maintained at 26°C for 24 h. The container was opened and kept in air at 26°C and RH 80% for 48 h. Flesh firmness and soluble solids concentration (SSC) were measured for each of 20 fruits. Flesh firmness was determined at two points on the cut surface horizontally using a firmness meter with a 5-mm-diameter cylindrical plunger (KM-5; Fujiwara Scientific Co. Ltd., Tokyo, Japan). The SSC of the juice was measured with a digital refractometer (PR-101α; Atago, Tokyo, Japan). Soluble tannin contents were measured as described by Oshida et al. (1996). Five fruits were used for this analysis before and after astringency-removal treatment by CTSD. Mesocarp tissues were homogenized in 80% methanol, and centrifuged. Folin-Ciocalteu reagent (Wako, Tokyo, Japan) was added to the supernatant. The soluble tannin concentration was determined by an ultraviolet/visible spectrophotometer (725 nm) (UV-1240; Shimadzu, Kyoto, Japan), and expressed as (+)-catechin hydrate equivalents (%).
SSR analysisFor SSR analysis, we examined ‘Hasshu’, ‘Hiratanenashi’, and three references: ‘Fuyu’, ‘Zenjimaru’, and ‘Saijo’. Total DNA was extracted from fresh young leaves in accordance with the method of Kobayashi et al. (2002). SSR analysis was conducted by using four SSR markers (ssrdk01, ssrdk02, ssrdk09, and ssrdk10) in accordance with the method of Soriano et al. (2006) and Naval et al. (2010). Polymerase chain reaction (PCR) was performed in 10 μL of reaction mixture containing 50 mM KCl, 2 mM MgC12, 200 μM of each dNTP, 0.5 mM of each forward primer labeled with a fluorescent chemical (FAM or HEX), 0.5 mM of each unlabeled reverse primer, 30 ng of genomic DNA, and 0.5 units of Ex Taq polymerase (Takara, Tokyo, Japan). The PCR cycling conditions were an initial 94°C for 3 min; 38 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 1 min for denaturation, annealing, and primer extension, respectively, and a final extension at 72°C for 5 min. The PCR products were separated and detected using a 3100-Avant Genetic Analyzer (Applied Biosystems, Carlsbad, CA, USA). The size of each amplified band was calculated on the basis of the sizes of internal standard DNA markers by using version 4.0 of the GeneMapper software program (Applied Biosystems).
Determination of ploidy levelsYoung leaves were sampled from ‘Hasshu’ and the reference cultivars ‘Hiratanenashi’ (9x) and ‘Fuyu’ (6x) in early May of 2012. Young leaf tissues (approximately 0.5 cm2), including the main vein for flow cytometry were immersed in nuclei extraction buffer (10 mM Tris-HCl (pH 7.5), 50 mM sodium citrate dihydrate, 2 mM MgCl2, 1.0% (w/v) polyvinylpyrrolidone K-30, 0.1% (v/v) Triton X-100), and quickly chopped with a razor blade by hand in a Petri dish. The suspension was filtered using a 30-μm nylon mesh to remove debris. The filtrate, which contained the nuclei, was stained with buffer containing 2 mg·L−1 of 4',6-diamidino-2-phenylindole (DAPI), and then injected into the flow cytometric analysis equipment. The fluorescence of the nuclei was determined by a Partec PA flow cytometer (Partec, Münster, Germany), using ‘Fuyu’ leaf nuclei as a reference standard.
Chromosome countingSomatic chromosome numbers were counted in the metaphase cells of the shoot apex including the shoot primordia and regenerated shoots obtained from axially buds by Feulgen’s method. The shoot tips of axial buds were immersed in 2 mM 8-hydroxyquinoline solution for 3 h at 20°C. Then, they were fixed in a fixative solution (ethanol:chloroform:acetic acid = 2:3:1, v/v), and stored at 4°C until use. The fixed samples were rehydrated by successive incubations from 70% to 15% ethanol for 5 min and finally in water. The rehydrated shoot tips were hydrolysed with 1 N HCl for 8 min at 60°C. After hydrolysis, the samples were washed in distilled water, and then stained with Schiff’s reagent for 1 h at 25°C. In order to observe somatic chromosome numbers, stained tissue of the primary shoot primordia was dissected out with tweezers under a light microscope. The tissue segment was squashed on a slide glass after an additional staining with 1% acetic orcein for 10 min. Well-spread chromosomes were photographed with a digital microscope system (DP72; Olympus Co., Tokyo, Japan).
In a comparison of one-year-old shoots, the shoot and internode lengths of ‘Hasshu’ were significantly shorter than those of ‘Hiratanenashi’ (Table 1; Fig. 1A). Both cultivars flowered in late May, but ‘Hasshu’ produced smaller flowers than those of ‘Hiratanenashi’ (Table 1; Fig. 1B). The leaf length and leaf width of ‘Hasshu’ were less than those of ‘Hiratanenashi’, and the leaf area of ‘Hasshu’ was only about 70% that of ‘Hiratanenashi’. Additionally, the petiole of ‘Hasshu’ was significantly shorter than that of ‘Hiratanenashi’.

Comparisons of vegetative growth characteristics between ‘Hasshu’ and ‘Hiratanenashi’ top-grafted trees.
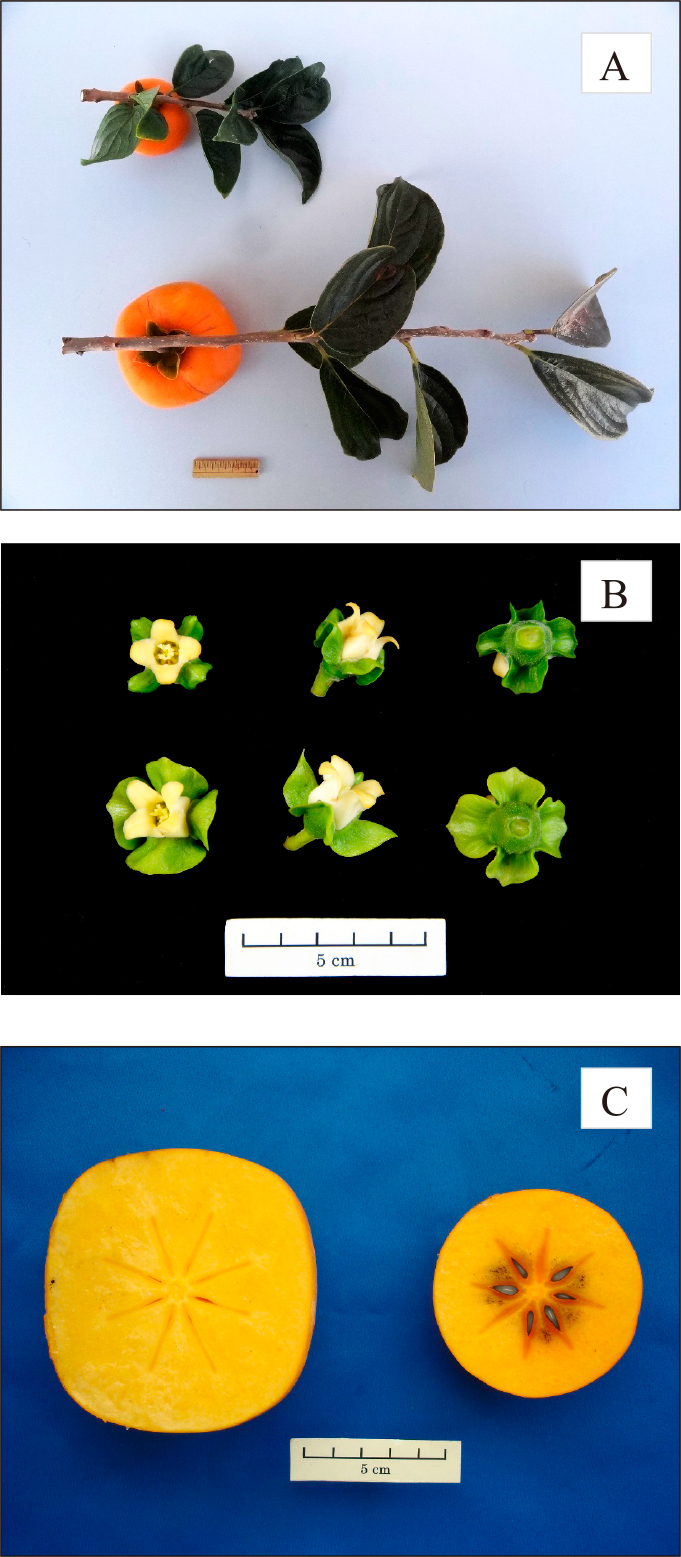
Photographs of shoot with leaves and fruit (A), flowers (B), and cross sections of mature fruit (C) of ‘Hasshu’ and ‘Hiratanenashi’. In A and B, ‘Hasshu’ is at the top and ‘Hiratanenashi’ is at the bottom. In A, the scale bar represents 5 cm. In C, ‘Hasshu’ is on the right and ‘Hiratanenashi’ is on the left.
The fruit growth and development of ‘Hasshu’ showed a more pronounced double-sigmoidal curve than that of ‘Hiratanenashi’ (Fig. 2); however, the diameter of ‘Hasshu’ fruit changed less than that of ‘Hiratanenashi’ from young fruit to harvest. The harvest time of both cultivars was in late October. The fruit weight of ‘Hasshu’ was approximately a quarter (72 g) that of ‘Hiratanenashi’ (267 g), and the calyx width of ‘Hasshu’ was significantly narrower than that of ‘Hiratanenashi’ (Table 2). The peel color of ‘Hasshu’ was superior to that of ‘Hiratanenashi’ owing to higher a* value (indicating a red color). ‘Hasshu’ fruit had softer flesh and higher SSC than ‘Hiratanenashi’ fruit after removal of astringency by CTSD. Although the soluble tannin content (1.21%) of ‘Hasshu’ fruit before CTSD treatment was higher than that of ‘Hiratanenashi’ (0.71%), it was reduced to a non-astringent level (0.10%) by the CTSD treatment. While ‘Hiratanenashi’ had almost no seeds or only imperfect seeds (empty seed), ‘Hasshu’ produced both imperfect seeds and an average of 3.7 normal seeds (seed weight: 0.2 g) per fruit (Table 2; Fig. 1C). These data show that ‘Hasshu’ is a pollination variant astringent-type persimmon like the parent cultivar, ‘Hiratanenashi’.

Seasonal changes in fruit transverse diameter of ‘Hasshu’ and ‘Hiratanenashi’ during fruit growth and development. SE bars (n = 10–15) smaller than the symbol width are not visible.

Comparisons of fruit characteristics ‘Hasshu’ and ‘Hiratanenashi’ top-grafted trees.
We examined the alleles of four cultivars, including ‘Hasshu’, at four SSR marker loci (Table 3). A total of 30 putative alleles were obtained from the four loci. The number of alleles per locus ranged from 5 to 11, with an average value of 7.5. All four SSR loci showed polymorphisms among ‘Fuyu’, ‘Zenjimaru’, ‘Saijo’, and ‘Hiratanenashi’. The genotype of ‘Hasshu’ was completely identical to that of ‘Hiratanenashi’ at the four SSR loci except for ssrdk10. ‘Hiratanenashi’ had alleles of 189, 191, 192, 198, 200, and 206 bp at ssrdk10, whereas ‘Hasshu’ did not have the 192 bp allele. Although the deletion of an allele was detected, this was consistent with the observation that ‘Hasshu’ originated from ‘Hiratanenashi’ by bud sport mutation.
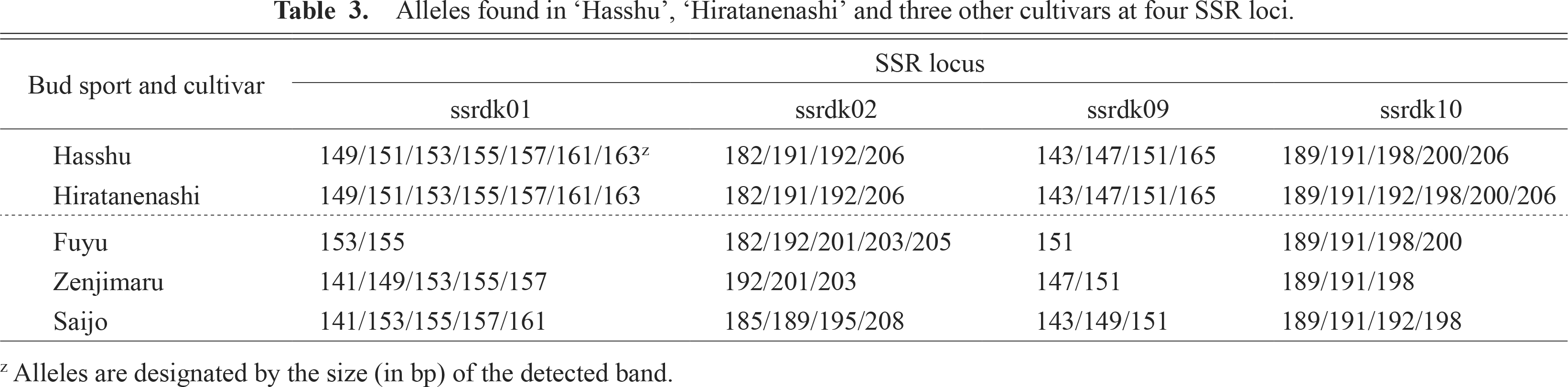
Alleles found in ‘Hasshu’, ‘Hiratanenashi’ and three other cultivars at four SSR loci.
Ploidy levels of young leaves were determined by flow cytometry. The nuclei from young leaves of hexaploid (2n = 6x) ‘Fuyu’ (Fig. 3A) and nonaploid (2n = 9x) ‘Hiratanenashi’ (Fig. 3C) were used as reference standards. The fluorescence peak of nuclei from young leaves of ‘Hasshu’ (132) was located between those of ‘Fuyu’ (101) and ‘Hiratanenashi’ (158) (Fig. 3B); thus, it had 1.31 times the fluorescence (DNA content) of ‘Fuyu’ and 0.84 times that of ‘Hiratanenashi’. By microscopic observation of shoot apex cells, 120 chromosomes were observed in the cells of ‘Hasshu’ (Fig. 4), indicating that it is octoploid (2n = 120 = 8x).

Flow cytometric histograms of relative fluorescence intensity in the nuclei of ‘Fuyu’, ‘Hasshu’, and ‘Hiratanenashi’ obtained from young leaves. A: ‘Fuyu’, hexaploid reference standard (2n = 6x = 90). B: ‘Hasshu’ (determined here to be 2n = 8x = 120). C: ‘Hiratanenashi’, nonaploid reference standard (2n = 9x = 135).
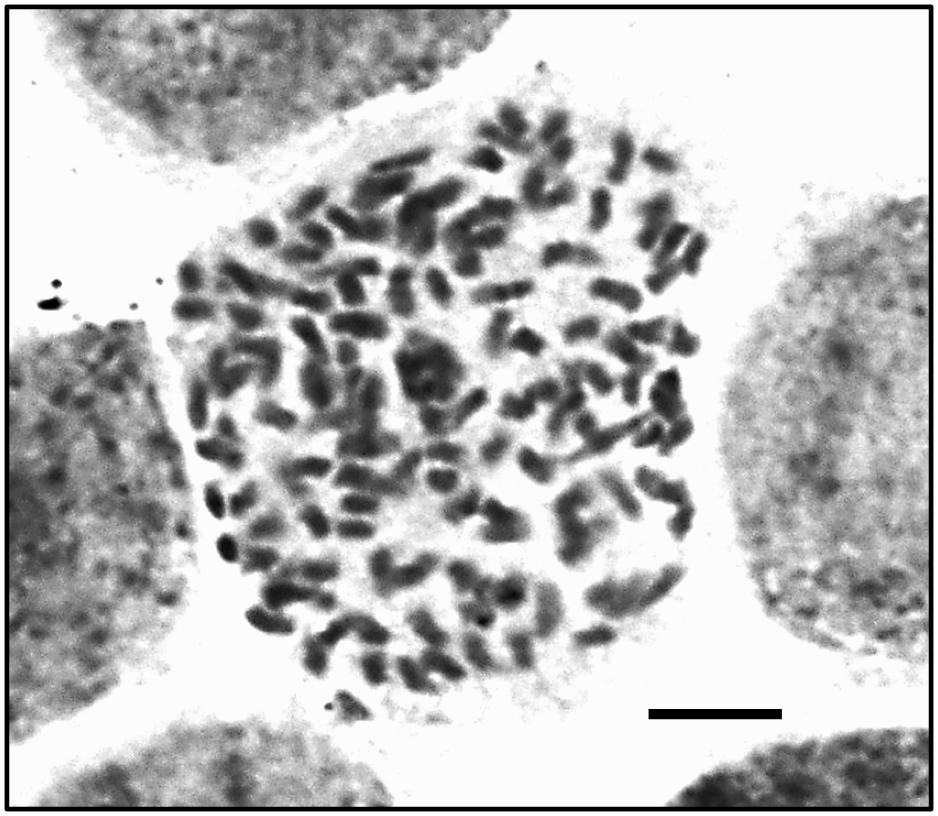
Chromosomes of ‘Hasshu’ (120 chromosomes; 2n = 8x). Scale bar = 5 μm.
‘Hasshu’ originated from a dwarf bud sport discovered on a mature ‘Hiratanenashi’ persimmon tree growing in an orchard at our research site. On comparing the vegetative growth structures of ‘Hasshu’ with those of the parent cultivar ‘Hiratanenashi’, we found that the structures of ‘Hasshu’ were clearly dwarf, with internode and shoot lengths being markedly shorter, and its leaf area only 70% that of ‘Hiratanenashi’, with petiole length being significantly shorter (Table 1). Dwarf characteristics were also confirmed in top-grafted trees. A comparison of the reproductive organs showed ‘Hasshu’ flowers to be significantly smaller than those of ‘Hiratanenashi’ (Fig. 1B). ‘Hasshu’ flowered in late May, about the same time as ‘Hiratanenashi’. ‘Hasshu’ fruit showed the same double-sigmoid growth curve as ‘Hiratanenashi’ (Fig. 2), although the transverse diameter of ‘Hasshu’ fruit was smaller than that of ‘Hiratanenashi’ fruit from flowering until harvest. These observations revealed ‘Hasshu’ to be a bud sport in which all tissues including the fruit exhibit dwarfing.
Among the dwarf bud sports derived from ‘Hiratanenashi’, the morphological characteristics of ‘Hasshu’ most resemble those of the small-fruit variety ‘Totsutanenashi’, a nonaploid, seedless cultivar originating from a bud sport discovered in Niigata Prefecture (Yamane et al., 2008). The enlargement of persimmon fruit indicates that in addition to cell size, cell number is also closely involved in determining fruit size. Yamane et al. (2008) compared the fruit flesh cells of ‘Totsutanenashi’ with those of ‘Hiratanenashi’, and reported that small cell size was the cause of small fruit in ‘Totsutanenashi’. On the other hand, Hamada et al. (2008) reported that the cell number and cell size of ‘Otanenashi’, a large-fruit bud sport mutant of ‘Hiratanenashi’, were greater than those of ‘Hiratanenashi’. Nonaka et al. (2010) have reported that spraying ‘Totsutanenashi’ fruit with 1-(2-chloro-4-pyridinyl)-3-phenylurea (CPPU), a synthetic cytokinin, restored fruit size to that of ‘Hiratanenashi’. This suggests that a mutation that affects the biosynthesis pathway or receptor for cytokinin is involved in the small-fruit mutation or the dwarfing of vegetative growth structures in persimmon fruit. We anticipate that both the size and number of ‘Hasshu’ fruit cells will be small, as it is in ‘Totsutanenashi’. To elucidate the mechanism of dwarfing in Japanese persimmon fruit going forward, we will need to investigate the role of plant hormones in the cause of dwarfing in ‘Hasshu’.
The polyploidy of ‘Hasshu’ is an octoploid derived from a nonaploid (Figs. 3 and 4) while ‘Totsutanenashi’ is a nonaploid (Yamane et al., 2008). This suggests that the dwarfing mechanism of ‘Hasshu’ is different from that of ‘Totsutanenashi’. Since hexaploid cultivars such as ‘Fuyu’ and nonaploid cultivars, such as ‘Hiratanenashi’, are not dwarf, it seems unlikely that the dwarfing in persimmon is directly caused by octoploidy. Actually, Tamura et al. (1998) reported that artificial interspecific octoploid hybrids between D. kaki (hexaploid) and D. glandulosa (diploid) morphologically resembled ‘Jiro’ (hexaploid). It is possible that dwarfing in ‘Hasshu’ is due to losing chromosomes located on genes associated with growth regulation factors such as cytokinin biosynthesis with decreasing ploidy. For an alternative explanation of dwarfing in ‘Hasshu’, similar dwarfing in ‘Totsutanenashi’ and polyploidy reduction may simultaneously occur.
In this study, reduced fruit size, higher SSC and better peel coloration were observed in ‘Hasshu’ (Table 2). Similarly, ‘Totsutanenashi’ produced smaller fruit with higher soluble sugar content compared to ‘Hiratanenashi’ (Yamane et al., 2008). This suggests that a higher SSC or the promotion of peel coloration is caused by its reduced fruit size. On the other hand, ‘Hasshu’ showed a higher soluble tannin content than ‘Hiratanenashi’ at harvest, whereas the soluble tannin content in ‘Totsutanenashi’ fruit was constituently lower than that of ‘Hiratanenashi’ throughout fruit development (Yamane et al., 2008). This implies that tannin biosynthesis in ‘Hasshu’ fruit may be higher than in both ‘Hiratanenashi’ and ‘Totsutanenashi’. Further studies of fruit qualities including tannin biosynthesis and tannin cells are required for ‘Hasshu’ and other mutations originating from ‘Hiratanenashi’.
‘Hiratanenashi’ is a PVA cultivar and its fruit rarely contain normal seeds; even when they do, those seeds tend to be empty. ‘Hiratanenashi’ is one of the few varieties of astringent persimmons for which astringency can be removed relatively quickly through the use of alcohol or CO2 (i.e., the CTSD method) (Yamada et al., 2002). ‘Hasshu’ is also a PVA variety like ‘Hiratanenashi’ (Fig. 1C), with significantly higher available tannin content than ‘Hiratanenashi’ before astringency removal. However, this content decreased to 0.10% after CTSD treatment (Table 2), showing that astringency removal can be carried out in ‘Hasshu’ as easily as in ‘Hiratanenashi’.
SSR markers have been developed for identifying persimmon cultivars (Naval et al., 2010; Soriano et al., 2006). We used this method to compare ‘Hasshu’ and ‘Hiratanenashi’ with each other and with ‘Fuyu’, a pollination-constant non-astringent (PCNA) persimmon, ‘Saijo’, a pollination-constant astringent (PCA) persimmon, and ‘Zenjimaru’, a pollination-variant non-astringent (PVNA) persimmon (Table 3). We found polymorphism for the four SSR markers used, with ‘Hiratanenashi’ showing clear differences from ‘Fuyu’, ‘Saijo’, and ‘Zenjimaru’ but no distinguishable difference from its bud sport ‘Hasshu’, except for the deletion of one allele at ssrdk10. This allelic loss could be due to chromosomal loss in ‘Hasshu’. Bud sport cultivars of Japanese pear and peach have also been reported to show SSR patterns identical to those of the cultivars from which they were derived (Sawamura et al., 2008; Yamamoto et al., 2003). Additionally, Bowers et al. (1996) reported that 77 cultivars of Vitis vinifera could be distinguished by using four SSR markers, with the exception of those cultivars considered to be somatic variants of another cultivar in the set. Thus, our SSR analyses demonstrated that ‘Hasshu’ is also a bud sport of ‘Hiratanenashi’. However, more SSR markers for ‘Hasshu’ are needed to clarify allelic losses associated with the chromosome loss as with ssrdk10.
Interestingly, although the fruit of ‘Hasshu’ is quite small, it contains an average of 3.7 normal seeds per fruit, unlike ‘Hiratanenashi’ and its bud sports ‘Totsutanenashi’ and ‘Tonewase’, all of which are seedless (Table 2; Fig. 1C). Flow cytometry analysis of the ploidy of ‘Hasshu’ showed the fluorescence (DNA content) of its nuclei peak to be approximately 1.31 times that of ‘Fuyu’, a known hexaploid cultivar, and 0.84 times that of ‘Hiratanenashi’, a known nonaploid, suggesting that ‘Hasshu’ is a putative octoploid (Fig. 3). Furthermore, microscope observation of shoot tip cells showed a somatic chromosome count of 120, confirming that ‘Hasshu’ is indeed an octoploid bud sport (Fig. 4). Until now, only hexaploid and nonaploid persimmons have been reported to exist in nature (Namikawa, 1930; Namikawa and Higashi, 1928; Zhuang et al., 1990). Although Tamura et al. (1998) succeeded in breeding octoploid plants through protoplast fusion of D. kaki and D. glandulosa, ‘Hasshu’ represents a spontaneous somatic cell mutation to octoploidy from its nonaploid parent cultivar, ‘Hiratanenashi’.
There are a number of reports of grape bud sports formed under natural conditions that show doubling of the ploidy of their parent cultivars, such as the tetraploid ‘Muscat Cannon Hall’ derived from a diploid cultivar ‘Muscat of Alexandria’ (Olmo, 1942). Conversely, ploidy reduction has been reported for the Japanese cypress that have reverted from octoploidy to tetraploidy (Sasaki et al., 1998). Spontaneous mutations that cause ploidy reduction, as in the case of ‘Hasshu’ from nonaploidy to octoploidy, are thought to be extremely rare. Because bud sport mutations are somatic cell mutations, it is likely that as many chromosomes as in the basic chromosome number of Diospyros plants (n = 15) were lost during cell division, suggesting a concerted chromosome loss. In mitotically reproducing yeast, large-scale transitions in genome size from both tetraploid and triploid to diploid were observed (Gerstein et al., 2008). Gerstein et al. (2008) suggested that nearly full sets of chromosomes were lost together, with some additional chromosome mis-segregation events. Alternatively, unequal mitosis may occur in a manner that ensures a balanced set of chromosomes in each daughter cell; one possibility is that a multipolar mitotic division occurs with three spindle pole bodies on one side of the dividing cell and one on the other (Storchová and Pellman, 2004). Although these considerations suggest that unequal mitosis or mitotic spindle abnormalities could underlie the spontaneous appearance of octoploid bud sports from the nonaploid ‘Hiratanenashi’, further research is required on such genome reduction.
Nonaploid ‘Hiratanenashi’ and some of its bud sports, including ‘Totsutanenashi’, are known to be seedless (Yamane et al., 2008; Zhuang et al., 1990). It is thought that these cultivars hardly produce any normal seeds because their nonaploidy prevents normal ovule development due to unbalanced chromosome numbers in gametes (Zhuang et al., 1990). ‘Hasshu’, on the other hand, is thought to owe its restored fertility to its octoploidy, which in meiotic cell division enables the chromosomes in each genome to be divided equally. The only persimmon cultivars (D. kaki) and lines known to produce male flowers including ‘Zenjimaru’ are all hexaploid. Since we confirmed that ‘Hasshu’ normal seeds had a relatively high germinability (over 50%) (data not shown), it is likely that even-numbered polyploids such as hexaploid and octoploid regularly form bivalents, and then the ovules and endosperms in these hybrids seedlings develop normally. Similarly, Takayama et al. (1996) reported that the heptaploid of Isoetes Japonica was obtained from the F1 hybrid between the hexaploid and octoploid. Thus, hybrid seedlings obtained from ‘Hasshu’ are predicted to be heptaploid the same as Isoetes Japonica. For this reason, the ability of ‘Hasshu’ seeds to germinate, and the ploidy of the F1 hybrids produced as a result, should be investigated further. It will also be critical to determine whether dwarfing is limited to ‘Hasshu’ or expressed in its F1 progeny.
The octoploid dwarf ‘Hasshu’ that we have discovered promises to be an extremely useful bud sport mutation because it produces normal seeds, opening the way for genetic analysis of its dwarfism, rearing of heptaploid plants and elucidation of their ecology, and many other interesting areas of investigation.
We are grateful to Dr. K. Taniguchi, Faculty of Science, Hiroshima University, for his helpful suggestions and comments of chromosome counting. We thank T. Morishige for her support and technical assistance.