2015 Volume 62 Issue 2 Pages 37-45
2015 Volume 62 Issue 2 Pages 37-45
Glycogen is the predominant polysaccharide in living cells. Many microorganisms accumulate glycogen, which serves as an energy reserve to cope with harsh environmental conditions. Therefore, the functions of enzymes involved in glycogen synthesis and degradation must be deciphered to understand the survival mechanisms of microbes. However, these enzymes in bacteria, most of which are glycosyl transferases or glycosidases, have not been fully characterized. Although there are similarities, the processes of glycogen synthesis and degradation in bacteria are quite distinct from the same processes in eukaryotes. Considerable progress has been made in understanding the mode of glycogen metabolism in Escherichia coli. In addition to the common core pathway, the virulence factors of infecting enteropathogenic bacteria appear to be involved in glycogen degradation. This review will focus on the following: (i) enzymes involved in glycogen degradation in E. coli, (ii) comparisons of the glycogen enzymes within enterobacteria, and (iii) glycogen as a carbon source for infectious microbes.
DP, degree of polymerization; GDE, debranching enzyme from eukaryote; GH, glycoside hydrolase; Glc3, maltotriose; Glc4, maltotetraose; α-glc-1-P, α-glucose 1-phosphate; GlgA, glycogen synthase; GlgB, branching enzyme; GlgC, ADP-glucose pyrophosphorylase; GlgP, glycogen phosphorylase; GlgX, glycogen debranching enzyme; Glk, glucokinase; GT, glycosyltransferase; MAase, maltogenic amylase; MalP, maltodextrin phosphorylase; MalQ, amylomaltase; MalZ, maltodextrin glucosidase; MTase, maltosyltranferase; NPDE, Nostoc punctiforme debranching enzyme; TreX, debranching enzyme from Sulfolobus solfataricus.
Glycogen forms a major energy reserve in eukaryotes and many bacteria.1) 2) 3) It consists of α-1,4-glucosidic linkages with α-1,6 branches (8‒12%) and particles 20‒50 nm in diameter. In many microorganisms, including bacteria and yeast, glycogen reserves enable survival when energy consumption is low. Escherichia coli and Vibrio sp. accumulate glycogen in larger amounts (50% of their cell mass) than other microorganisms under conditions of N shortage.4) 5) In stationary phase, Bacillus cereus accumulates glycogen, which is a source of carbon and energy during sporulation.6) Recently, glycogen accumulation in cyanobacteria has attracted a great deal of attention. These bacteria have the same photosynthetic pigment, chlorophyll a, that is present in plants and enables cyanobacteria to accumulate glycogen in the absence of carbon.7)
Bacterial glycogen biosynthesis was described in detail by Preiss8) 9), and there have been several recent comprehensive reviews on glycogen metabolism and regulation in bacteria and yeast.4) 10) Boos,11) Montero et al..,12) and Preiss13) 14) published reviews on the structure–function relationships of enzymes involved in bacterial glycogen metabolism. In addition, there have been reviews of the maltose system in E. coli.11) Bacterial species share a very similar glycogen synthesis operon structure. Glycogen metabolism in numerous bacteria can be derived from a common pathway. Park et al.. proposed a model for glycogen synthesis in E. coli. In that model, when E. coli MC4100 is grown on maltose, the maltose utilization system is associated with a glycogen synthase-dependent pathway.5)
The glycogen degradation processes in eukaryotes have been studied extensively. During this process, debranching enzymes exhibit both hydrolysis and transferase activity. The glycogen degradation pathway is less well understood in bacteria and archaea. Several recent studies have linked glycogen to pathogen colonization and virulence.15) 16) 17) 18) 19) The breakdown of glycogen may play an important role in the interactions between the host and pathogenic bacteria, such as E. coli, Salmonella, Shigella, and Vibrio, which accumulate glycogen throughout their life cycles.20) However, the mechanisms and roles of glycogen during infection by microbes such as E. coli are still not fully understood. Elucidation of the mechanisms underlying glycogen metabolism and degradation may reduce the impact of pathogens within the human body. This review focuses on the biochemical properties and functions of enzymes important in the glycogen degradation pathway in E. coli.
Starch is a polysaccharide carbohydrate found in plants. The two types of polyglucans present in starch are amylopectin, a highly branched component, and amylose, which is moderately branched. The branched chains are intertwined and associated as double-helical structures. Glucose units are linked by α-1,4-glycosidic linkages and branching takes place with α-1,6-glycosidic linkages occurring every 24‒30 glucose units.
Glycogen is a highly (8‒12%) branched polymer, in which approximately 1 in 12 glucose residues (α-1,4-linked) form an α-1,6-glycosidic bond. In bacteria, the particle diameter is in the range of 20‒50 nm, the average length of the branch chains is 8‒12 glucose units, and the molecular mass is about 107‒108 Da. A mathematical model can provide a better understanding of the physiological significance and the relationships between structure and enzymatic degradation of glycogen. The structure of glycogen was fit to a mathematical model21) that optimizes the degree of branching and length of the chains. Each A- or B-chain has 12‒14 glucose residues (mean, 13) and there are 12 tiers in the molecule, which consists of about 53,000 glucose residues (molecular mass 106 Da) and about 2,100 non-reducing ends (Fig. 1). The optimal values of these two parameters are identical to those found in cellular glycogen.

The model structure of the glycogen molecule. Modified from reference21) with permission of the publisher.
Glycogen efficiently stores large amounts of cytoplasmic glucose without causing a significant increase in osmolarity. The total amount of glucose stored in liver cells as glycogen is equivalent to 400 mM, whereas the concentration of glycogen is only 0.01 μM. The number of non-reducing ends in a glycogen particle is much greater than the number of active phosphorylase sites. There are actually about 20‒25 phosphorylase tetramers in one β-particle of glycogen which has a diameter of 40 nm, about 55,000 glucose residues (106 Da), and about 2,100 nonreducing ends.22) The molecular weight and chain length of glycogen appear to vary, depending on growing conditions and other factors. For example, the molecular weight and branch chain length of glycogen in wild-type and various E. coli MC4100 knockout mutants vary depending on carbon source and specificity of the enzyme that is knocked out. The molecular weight varies from 106‒107 Daltons and chain lengths are in the range of degree of polymerization (DP) = 9‒16.5) The structure of yeast glycogen is similar to that of bacteria, with a chain length of 11‒12 glucose units.4)
Several reviews have compared bacterial glycogen and starch metabolism.23) 24) 25) Cenci et al.24) proposed the switch of glycogen to starch metabolism in Archaeplastida. Both bacteria and plants synthesize storage polysaccharides by an ADP-glucose-based pathway. The bacterial debranching enzyme, GlgX, which appears to be involved in glycogen degradation, shares substrate specificity for Glc4-branch chains with Isa3, a GlgX-derived debranching enzyme in plants. Therefore, comparison of bacterial glycogen with starch in plants may yield a better understanding of glycogen metabolism.
On the other hand, the complexity in structure of glycogen makes it difficult to analyze the changes in structure. This appears to be one of the major reasons that the reaction mechanism of enzymes in glycogen metabolism has been poorly understood. Thus, a glycogen mimic substrate, β-cyclodextrin, which is branched with maltodextrin, was proposed to be suitable for examining the branch chain specificity and transglycosylation mechanisms of glycogen enzymes.26) 27) Maltodextrin-branched cyclodextrin was prepared by incubation of maltosyl transferase (MTase) from Thermotoga maritima with maltosyl-β-cyclodextrin26) (Fig. 2).

Enzymatic preparation of the glycogen-mimic substrate.
In E. coli and some mildly pathogenic bacteria, all genes involved in glycogen metabolism steps are clustered in two operons. The operons are formed by the sequentially transcribed glgBX and glgCAP genes encoding a branching enzyme (GlgB), a debranching enzyme (GlgX), an ADP-glucose pyrophosphorylase (GlgC), a glycogen synthase (GlgA), and a glycogen phosphorylase (GlgP) involved in synthesis and degradation of glycogen11) 28) 29) (Fig. 3). In addition, E. coli possesses are three cytoplasmic enzymes, amylomaltase (MalQ), maltodextrin phosphorylase (MalP), and maltodextrin glucosidase (MalZ), which are known to participate in maltose/maltodextrin metabolism11) and are also actively involved in glycogen metabolism.5) 30) Enzymes involved in maltose utilization were discussed in a review by Boos.11) GlgB can catalyze transglycosylation of an α-1,6-linkage from an α-1,4-linkage. α-Glucan, with branches longer than DP = 1, is the substrate for GlgB in E. coli.31) 32) 33)

Glycogen metabolism in various bacteria, such as Bacillus species, has deviated from the pathway in E. coli. Shim et al.. investigated the physiological functions of maltogenic amylase (MAase) and pullulanase, focusing on the degradation of maltodextrin and glycogen in Bacillus subtilis.34) It was suggested that glycogen breakdown may be a sequential process that involves pullulanase and MAase. Pullulanase hydrolyzes the α-1,6-glycosidic linkage at the branch point to release a linear maltooligosaccharide that is then hydrolyzed into maltose and maltotriose by MAase.34) Several fully sequenced genomes of bacteria known to accumulate glycogen were shown not to contain prokaryote glycogenin homologs.28) The roles of the enzymes important in glycogen degradation in E. coli are discussed below.
Amylomaltase (4-α-glucanotransferase), encoded by malQ in E. coli, is an enzyme that catalyzes transfer of an α-glucan moiety to the C-4 position of the acceptor.
(α-1,4-glucan)n + (α-1,4-glucan)m
(α-1,4-glucan)n+x + (α-1,4-glucan)m-x
MalQ and MalP are considered to be key enzymes in maltose utilization in E. coli.5) 30) 37) Maltose utilization involves recognition of maltose and the longer maltodextrin, and preferential removal of glucose from their reducing ends. The glucosyl or maltodextrinyl residues are transferred to other maltodextrins, thus forming longer maltodextrin chains. The elongated maltodextrins can be further processed by MalP to α-glucose 1-phosphate (α-glc-1-P), which enters the glycolysis pathway.
A study on mutants with deletions in the glycogen and mal gene clusters indicated that the action of MalQ on maltose or maltodextrin can lead to glycogen formation. Park et al.5) proposed a model of maltodextrin utilization for the formation of glycogen in the absence of GlgA. As shown in Fig. 4, MalQ plays an important role in the interconnection between GlgA-dependent glycogen synthesis and the maltose utilization system, as glucose formed from maltose by MalQ may also enter the glycogen synthesis pathway in E. coli via GlgA. Moreover, E. coli can form the primer required for the elongation process by the disproportionation reaction of MalQ. This was consistent with the lack of glycogenin analogs in bacteria and the genomes of bacteria known to accumulate glycogen.5) 28)

A proposed model of glycogen metabolism in Escherichia coli. Reprinted from reference5).
The roles of MalQ enzymes from various microbes have been investigated. The amino acid sequence deduced from E. coli MalQ was 85% identical to the sequence of Salmonella typhimurium (Enterobacteriaceae) and 49% identical to Vibrio vulnificus.38) The disproportionation enzyme, D-enzyme (corresponding to MalQ in E. coli), is considered to play an important role in starch metabolism in plants. The plant pathway of maltose metabolism is similar to that of bacteria, including E. coli.5) 11) 25) 39) Ruzanski et al.40) successfully replaced the plant 4-α-glucanotransferase (DPE2; corresponding to MalQ) with the bacterial amylomaltase MalQ. Maltose metabolism was compared between E. coli and Arabidopsis thaliana. Interestingly, the D-enzyme in Arabidopsis may be equally involved in starch anabolism and catabolism.23) Similarly, given the main physiological role of MalQ in Saccharophagus degradans 2‒40, it has been suggested that MalQ may have roles not only in maltose utilization but may be involved in metabolism of maltodextrins formed during glycogen degradation in this bacterium.36) Therefore, enzymes involved in both the maltose system and biosynthesis of glycogen may participate in the degradation process in E. coli.
The E. coli maltodextrin/glycogen degradation process requires four enzymes, i.e., GlgP, GlgX, MalQ, and MalP. Of these, GlgX is the key enzyme involved in initiating the debranching process. The debranching enzymes are either direct or indirect. The direct debranching enzymes found in bacteria and plants show α-1,6-glucosidase activity, in which the branched chains are directly released from glycogen or starch. GlgX is a direct debranching enzyme and belongs to the glycoside hydrolase GH13 in the CAZy database.41) 42) 43) The role of GlgX in glycogen degradation involves selectively catalyzing the debranching of glycogen outer chains previously processed by glycogen phosphorylase.44) 45) 46) In contrast, direct debranching enzymes have not been found in eukaryotes. The glycogen debranching enzymes present in mammals and yeast, known as indirect debranching enzymes, are bifunctional and possess both α-1,4-transferase and α-1,6-glucosidase activities with two distinct catalytic sites on a single polypeptide chain.27) 47) 48) The enzyme transfers Glc3 residues from a Glc4 branch chain of limit-glycogen to the neighboring branch chain that can be further phosphorolyzed to α-glc-1-P by GlgP. The remaining glucose is debranched by the action of α-1,6-glucosidase activity of the same enzyme (Fig. 5), in which 90% of the glycogen is converted to α-glc-1-P through transfer reaction by the debranching enzyme.
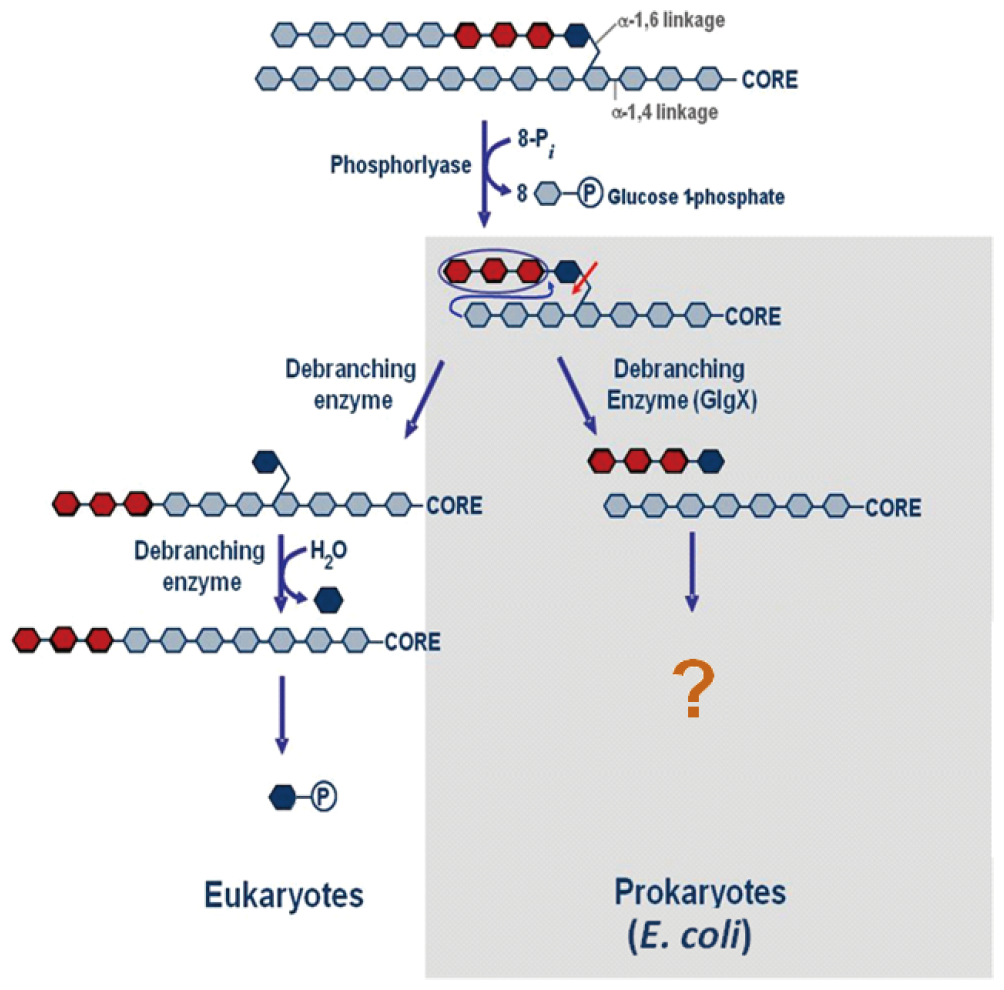
Schematic diagram for glycogen degradation in eukaryote and prokaryote.
The three-dimensional structure of GlgX from E. coli K12 was determined at 2.25 Å resolution.49) The structural features observed at the substrate binding groove provided a molecular explanation for the unique substrate specificity of the Glc4 branch chain (Fig. 6) suitable only for Glc4 of GlgP-limit dextrin. This strict specificity ensures that the debranching enzyme will not interfere with the normal process of branching during glycogen biosynthesis in E. coli.23) Consequently, GlgX does not hydrolyze native glycogens, starches, or linear maltooligosaccharides. As Glc4 released from GlgP-limit glycogen by GlgX must be further metabolized, it has been postulated that the product, Glc4, may be elongated through the action of MalQ and further processed by GlgP.11) However, a better understanding of the mechanisms and kinetics is needed due to the complex enzyme system that includes MalQ, GlgX, GlgP, and MalP.

(a) Schematic overview of GlgX superimposed on the TreX structure, (b) Schematic view of GlgX superimposed on the isoamylase from Pseudomonas, (c) Substrate binding groove of GlgX modeled with α-1,6-linked maltotetraose.
Reprinted from reference 49).
Sequence alignment between GlgX, TreX from Sulfolobus solfataricus, and isoamylase from Pseudomonas showed common features, including four conserved regions among amylases in the GH13.42) 43) The three-dimensional structure of an archaeal glycogen debranching enzyme, TreX, shows similarity to the structure of GlgX.50) 51) However, TreX has both glucosidase and transferase activities.51) The preference for branch chain length varies among bacteria. TreX is unique in exhibiting higher activity on branched substrates with longer maltooligosaccharides,50) while the debranching enzyme from Nostoc punctiforme (NPDE) shows high specificity for long branch chains (DP > 8).51) 52) These differences in specificity for branch chain length between GlgX, TreX, and NPDE suggest that glycogen degradation in other bacteria may differ from that in E. coli.
Phosphorylases catalyze the phosphorolytic cleavage of glycogen or dextrin to release α-glc1-P via the sequential removal of glycosyl residues from the nonreducing ends of the glycogen molecule. α-glc-1-P released from glycogen can be readily converted into glucose 6-phosphate.
Glycogen (or Maltodextrin) + Pi
(n-residues)

Glycogen (or Maltodextrin) + α-glc-1-P
(n-1 residues)
The three-dimensional structure shows a narrow 30-Å crevice that binds glycogen and accommodates 4‒5 residues. The pyridoxal-5-phosphate cofactor is located near the active site. The structure of a bacterial phosphorylase, MalP, of E. coli was described by Watson et al.54) 55) As expected from sequence comparisons, the structures of GlgP and MalP are similar. However, the active site of E. coli MalP is open and has a structure to promote degradation of maltodextrin but lacks regulatory sites. In contrast, mammalian GlgP consists of subunits a and b that allow allosteric transition.56) 58)
According to the CAZy classification, both GlgP and MalP can be grouped into the GT35 family based on an α-glucosidic linkage. In addition, β-1,4- and β-1,3- glucoside-specific phosphorylases have been reported.59) 60) 61) The biochemical properties and physiological functions of bacterial α-glucan phosphorylases, including GlgP and MalP, have been reviewed.62) 63) In E. coli, the amino acid sequence of MalP shows 45% similarity to that of GlgP. The catalytic domains are highly conserved, while regulatory sites are poorly conserved, and the overall structure of MalP appears to be similar to that of GlgP. Thus, bacterial phosphorylases GlgP and MalP follow the same catalytic mechanisms, but differ in substrate specificity and regulation. E. coli MalP has a preference for linear maltodextrins with DP = 7, whereas activity for glycogen is approximately 1/10 lower.62) Klebsiella pneumonia (Enterobacteriaceae) GlgP shows similar substrate preferences to those of MalP.
The amino acid sequence of E. coli MalP is 87% identical to that of S. typhimurium (Enterobacteriaceae) and 55% identical to that of V. vulnificus.38) Similarly, comparison of the amino acid sequence of MalP from Corynebacterium glutamicum with that of E. coli revealed 41% identity. This protein also showed 40% identity to GlgP. MalP of C. glutamicum shows a preference for maltodextrin with at least five glucose residues. In contrast, the specificity of E. coli GlgP for glycogen is four times greater than that for maltodextrin. Thus, GlgP involved in the degradation of glycogen cannot replace MalP in utilization of maltose and maltodextrins.11) MalP recognizes maltodextrins with DP > 4‒5 and catalyzes the sequential removal of glucosyl residues from the nonreducing ends of the dextrins by phosphorolysis, forming α-glc1-P.11) 39) 54) 55) 64) 65) 66)
The reaction catalyzed by phosphorylase is readily reversible in vitro. At pH 6.8, the equilibrium ratio of orthophosphate to α-glc1-P is 3.6. The value of ΔG for this reaction is small (+3.6 kJ/mol), as a glycosidic bond is replaced by a phosphoryl ester bond that has nearly equal transfer potential. However, phosphorolysis proceeds far in the direction of glycogen breakdown in vivo because the [Pi]/[glc-1-P] ratio is usually about 30‒100 under physiological conditions, favoring phosphorolysis with ΔG = -5 to -8 kJ/mol. In general, the phosphorolytic cleavage of glycogen is energetically advantageous, because the released sugar is already phosphorylated. In contrast, hydrolytic cleavage would yield glucose, which would then have to be phosphorylated at the expense of hydrolysis of a molecule of ATP to enter the glycolytic pathway. An additional advantage of phosphorolytic cleavage for muscle cells is that α-glc-1-P, which is negatively charged under physiological conditions, cannot diffuse from the cell.67)
The physiological role of MalP has been investigated in several bacteria. Mutants lacking MalP accumulate large amounts of long-chain maltodextrins when grown on maltose or maltodextrin, suggesting that MalP is involved in both maltose utilization and glycogen synthesis pathways.5) The deletion mutant of C. glutamicum, ΔmalP, showed reduced intracellular glycogen degradation, confirming the pathway for glycogen degradation via GlgP, GlgX, and MalP. C. glutamicum also forms maltodextrin in the course of glycogen degradation,68) 69) indicating that glycogen is degraded in this bacterium by a pathway similar to that in E. coli. GlgP catalyzes the phosphorolysis of glucose residues at least five units from the branch point of glycogen, accumulating the glycogen phosphorylase-limit glycogen.
Glycogen is one of the key carbon sources for infectious microbes, such as E. coli, Salmonella, and Vibrio, that are classified as clinically important human pathogens.70) Thus, microbes must obtain nutrients from their host environment during infection.71) 72) 73) 74) 75) 76) 77) The interaction of bacteria with plants is solely extracellular,78) 79) while interaction with animal hosts can be intracellular or extracellular. They contain a minimal set of enzymes, with activities sufficient to use glycogen as a source of carbon energy. Several bacterial genomes, such as those of E. coli, Vibrio cholera, and Clostridium perfringens, appear to have the minimal set of glycogen-active enzymes GT5 (GlgA), GT35 (GlgP and MalP), and GH13 (GlgX and GlgB), but no GH15 (glucoamylase).78) Therefore, investigation of the microbial complex carbohydrate utilization pathways may generate novel insights into host-pathogen interactions.
This concept has been proposed as the key factor for virulence of pathogens. Consequently, recent investigations have focused on bacterial metabolism during infection, whereas traditional studies of bacterial pathogenesis have focused on the pathogen-specific virulence properties that include toxins, adhesion, and secretion mechanisms.15) 80) 81) E. coli metabolism has been explored during infection, focusing on colonization of the intestine. E. coli was shown to grow in the mouse intestine using simple sugars released by the breakdown of complex polysaccharides by anaerobes.82) Metabolism by enteric pathogens during colonization of the gastrointestinal tract is not understood. Alteri et al.80) investigated the expression of uropathogenic E. coli (UPEC) proteins and bacterial metabolic pathways during infection in the urinary tract. The results suggested that central metabolic pathways of bacteria are critical components for virulence of pathogenic microbes. The glycogen metabolism-related enzymes, GlgX, MalQ, GlgP, GlgC, and GlgA, were investigated during infection by chlamydial organisms. GlgA of Chlamydia trachomatis was detected in the cytosol of host cells, suggesting that GlgA is expressed and secreted into the host cell cytosol during C. trachomatis infection in humans.83)
In the cytosol of eukaryotes, the indirect debranching enzyme cannot use maltooligosaccharides during glycogen metabolism, and enzymes such as MalP are present to catabolize the maltooligosaccharides. Chlamydia pathogens may produce maltotetraose through secretion of their own GlgX in the host cytosol, triggering the formation of a substrate that can be further metabolized.24) Streptococcus pneumonia has a multicomponent system to metabolize exogenous glycogen in the host cell.84) SpuA (pullulanase type debranching enzyme), MalX (solute binding protein), MalP, and MalM (α-glucanotransferase) are sequentially involved in debranching of glycogen, transport of the products, and processing of the internalized glycogen products.
MalQ and MalP have been shown to be involved in glycogen synthesis and degradation, as there is genetic evidence suggesting their importance in bacterial glycogen metabolism. Glycogen degradation in E. coli requires four enzymes: GlgP, GlgX, MalQ, and MalP. The direct debranching enzyme, GlgX, is present in E. coli and possesses α-1,6-glucosidase activity. GlgX directly cleaves the Glc4-branch chain of glycogen. The indirect debranching enzyme (GDE) in eukaryotes possesses α-1,4-transferase and α-1,6-glucosidase activity. GDE initially transfers a Glc3-residue to an adjacent branch chain and then removes the remaining glucose from glycogen. This suggests that both enzymes provide an efficient degradation pathway separate from the synthesis pathway. The process following the direct GlgX-mediated cleavage of a branch chain in E. coli and other bacteria is not obvious. Further studies are required to determine the precise molecular functions of the enzymes responsible for glycogen degradation in E. coli and other bacteria.
This work was supported in part by the Basic Research Program through the National Research Foundation (2012R1A1A2005012) and in part by the Next Generation BioGreen21 Program (SSAC, No. PJ009086), Rural Development Administration, Republic of Korea.