2015 年 14 巻 4 号 p. 124-130
2015 年 14 巻 4 号 p. 124-130
The nature of Si-O bonding and Si-O-Si bridging is discussed using molecular orbital calculations. We found the equilibrium geometries for two pyrosilisic acid molecules (C2V and 60° torsion) using Møller-Plesset perturbation theory and 6-311G (d,p) split valence basis set. The bent configuration of the Si-O-Si angle in equilibrium geometries can be explained by the balance of Coulombic repulsion between SiO4 tetrahedra and the energy of lone pair orbitals belonging to bridging oxygen atom without concerning the contribution of d-p π-bonding from the results of natural bonding orbital analysis. The energy surfaces of two pyrosilisic acid molecules with varying Si-O length to the bridging oxygen and Si-O-Si angle were calculated and we found the relationship between Si-O length to the bridging and Si-O-Si bridging angle. The Si-O bonding strengthens with increasing Si-O-Si angle because of stabilization in energy of Si-O bonding orbital with decreasing the hybridization index λ in spλ orbital of bridging oxygen and increase of Coulombic interaction between Si and bridging oxygen atom.
Understanding the nature of Si-O bond and Si-O-Si bridging is important for mineralogy, material science and metallurgy. It is well known that the variation of Si-O-Si angle in silicates is caused by difference of composition, temperature and pressure. The change in angle of Si-O-Si bridging affects the strength of Si-O bond. For instance, decrease of Si-O-Si angle increases the Si-O bond length in coesite crystal (Gibbs et al. [1]). The decrease of Si-O-Si angle of liquid silicates as a result of compression was reported by various researchers (e.g., Navrotsky et al. [2], Ohtani et al. [3], Sakamaki et al. [4]). The decrease of Si-O-Si angle is thought to be the trigger of decrease of viscosity of liquid silicates (Navrotsky et al. [2], Noritake et al. [5]). Quantum chemical properties of Si-O-Si bridging was investigated to understand the relationship between Si-O-Si angle, Si-O bond length and its strength (e.g., Newton and Gibbs [6], Tsuneyuki [7], Kubicki and Sykes [8]). Newton and Gibbs [6] reported that a pyrosilisic acid molecule has energy minimum at Si-O-Si angle of 145° using Hartree-Fock method and STO-3G basis set (Hehre et al. [9]). Tsuneyuki [7] reported that the bent configuration of Si-O-Si angle in pyrosilisic acid molecule is not reproduced using double-zeta function basis set nevertheless increase of the number of basis function generally increases reproducibility. The relationship between Si-O bond length and Si-O-Si angle, that is, the nature of Si-O-Si bridgings seems not to be reproduced by increase of basis function using Hartree-Fock method. In this paper, we show the molecular orbital calculations about the pyrosilisic acid molecule using post-Hartree-Fock method and more precise basis set to understand the nature of Si-O-Si bridging.
Molecular orbital calculations were performed using the Gaussian 09 code [10]. We first calculate the optimized structure of disiloxane molecule by Hartree-Fock (HF), second-order Møller-Plesset perturbation theory (MP2) [11], and two density functional theory (three-parameter hybrid functional (B3LYP) [12] which is a mixture of Hartree-Fock exchange with DFT exchange functional by Becke and correlation functional by Lee, Yang and Parr [13], and generalized gradient approximation by Perdew, Burke and Ernzerhof (PBE) [14]) with 6-311G (d,p) split valence basis set [15,16]. The values of structural parameters of disiloxane molecule optimized by various models and experimental data by Almenningen et al. [17] are shown in Table 1. The Si-O-Si angle is not reproduced by HF method as shown in Tsuneyuki [7]. The bent configuration of Si-O-Si angle is reproduced by use of MP2 and density functional theory with PBE. The optimized angle of Si-O-Si in disiloxane molecule by MP2 is closer to the experimental value than that by PBE. Then we apply the MP2 method with 6-311G (d,p) basis set to the calculation of the pyrosilisic acid molecule, H6Si2O7. Kubicki and Sykes [8] reported that hydrogen bonding is made in the molecule in optimizing the molecular structure of pyrosilisic acid. We optimized pyrosilisic acid molecule with z-matrix to define the point symmetry of pyrosilisic acid molecule to avoid the effect of hydrogen bonding. Two types of structure were optimized, one is the C2v point symmetry as shown in Figure 1a, the other is structure in which each tetrahedra is in conformational relation of 60° torsion as shown in Figure 1b. The optimized structural parameters are shown in Table 2.
| Exp. | HF | Δ | MP2 | Δ | B3LYP | Δ | PBE | Δ | |
| Si-O | 1.634 | 1.621 | −0.013 | 1.642 | 0.008 | 1.640 | 0.006 | 1.635 | 0.001 |
| Si-H | 1.486 | 1.476 | −0.010 | 1.476 | −0.010 | 1.485 | −0.001 | 1.488 | 0.002 |
| Si-O-Si | 144.1 | 180.0 | 35.9 | 156.5 | 12.0 | 179.2 | 35.1 | 169.7 | 25.6 |
| O-Si-H | 109.9 | 109.9 | 0.0 | 110.4 | 0.5 | 109.9 | 0.0 | 110.1 | 0.2 |
| H-Si-H | 109.1 | 109.0 | −0.1 | 109.0 | −0.1 | 109.0 | −0.1 | 109.0 | −0.1 |

Calculated equilibrium geometries of pyrosilisic acid molecule. (a) C2V model. (b) Each tetrahedra mutually twisted 60° model.
| C2V | 60° torsion | |
| Si-Obr | 1.601171 | 1.604154 |
| Si-Onbr | 1.648782 | 1.648563 |
| O-H | 0.954669 | 0.954561 |
| Si-O-Si | 172.006 | 159.647 |
| Si-O-H | 117.705 | 117.857 |
| Total Energy (Hartree) | −1107.5476706 | −1107.5482842 |
Then we calculate the potential energy surfaces of two pyrosilisic acid molecules varying the Si-Obr bond length and Si-O-Si angle with fixing other structural parameters obtained by optimization. To analyze the nature of bonding, we use the Natural Bonding Orbital (NBO) analysis method (Foster and Weinhold [18], Reed et al. [19, 20]). NBO analysis originated as a technique for studying hybridization and covalency effects in molecular wave functions. In NBO analysis, the calculated molecular orbitals are decomposed and recomposed visualized bond orbital that corresponds to the picture of interatomic bonds and lone pairs. It gives us energy of bonding, degree of hybridization of bonding, overwrap integration weighted bond order and charge of atoms. In NBO analysis, we firstry define natural atomic orbital and attribute the electrons. Natural atomic orbital can be obtained by block diagonalization of density matrix by each angular momentum of atomic orbital of each atom. Then we define the spλ natural hybrid orbitals, hλ(θ) as follows,
| (1) |
| (2) |
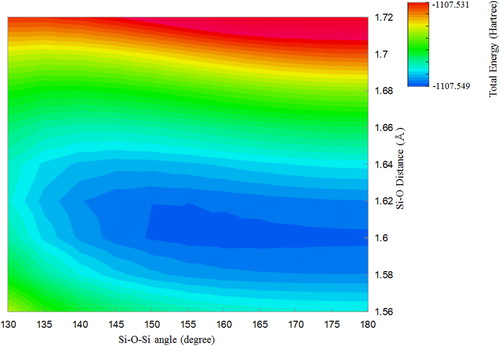
The optimized structural parameter of the pyrosilisic acid molecules is shown in Table 2. Stable Si-O-Si angle and total energy of C2V model are larger than those of 60° torsion model. However, the bond length between Si and bridging oxygen (Obr) is not different significantly by the torsion angle. Figures 2 and 3 are contour maps of the potential energy surface of the pyrosilisic acid molecules (C2V and 60° torsion models). The stable Si-O bond lengths decrease with increasing Si-O-Si angle in both molecules. Figure 4 shows the sum of orbital energy of valence electrons belonging to bridging oxygen, energy of each valence orbital, and overlap weighted bond order of Si-Obr. The sum of orbital energy of valence electrons belonging to the bridging oxygen decreases with decreasing Si-O-Si angle. Decomposing into each orbital, orbital energy of sum of two lone pairs decreases with decreasing Si-O-Si angle, however the energy of Si-O bonding orbital increases with decreasing Si-O-Si angle. Note that the calculated orbitals are decomposed based on natural Lewis structure and lone pairs are orthogonal with each other. The overlap weighted bond order has local minimum at 140 to 145 degrees whereas the changes in orbital energies change monotonously. Calculated charges of Si and Obr and electrostatic potential between Si and bridging oxygen calculated using charges are shown in Figure 5. In NBO analysis, the charge at each atom is calculated from occupancy of natural atomic orbital. The absolute value of atomic charge increases with increasing Si-O-Si angle. The order of changes in electrostatic potential between Si and bridging oxygen with varying Si-O-Si angle is comparable to that in the energy of Si-O bonding orbital. Figure 6 shows the coefficient cSi and cO in equation (2) with varying Si-O-Si angle. The coefficient of cSi decreases and cO increases with increasing Si-O-Si angle.

The contour map of energy surface of pyrosilisic acid molecule (C2V) with varying Si-Obr and Si-O-Si angle.
The contour map of energy surface of pyrosilisic acid molecule (60° torsion) with varying Si-Obr and Si-O-Si angle.

The plots of (a) sum of energy of the valence orbitals belongs to the bridging oxygen atom, (b) energy of each valence orbitals (two lone pairs (LP1, LP2) and Si-O bonding orbital (Si-Obr)) belongs to the bridging oxygen atom and (c) overlap weighted bond order of Si-Obr bonding with varying Si-O-Si angle in the case of 60° torsion model.

The plots of (a) electrostatic potential energy (ESP) between Si and bridging oxygen atoms calculated from charge and (b, c) charge of Si and bridging oxygen atoms calculated by NBO analysis with varying Si-O-Si angle in the case of 60° torsion. Si-Obr is the orbital energy of Si-Obr σ bond and plotted for comparison.

The plots of bonding coefficients Si and O atoms for Si-Obr σ-bonding with varying Si-O-Si angle in the case of 60° torsion.
The bent configuration of Si-O-Si bridging is reproduced in this study using Møller-Plesset perturbation theory and 6-311G (d,p) split valence basis set. The bent configuration of Si-O-Si bridging is often explained by three contributions, Coulombic repulsion between two SiO4 tetrahedra, lone pair (or valence shell electron pair repulsion rule) of bridging oxygen, and the d-p π-bonding model. The contribution of Coulombic repulsion to energy surface seems to be important. Because the bent configuration cannot be reproduced in some combinations of model chemistry and basis set (Tsuneyuki [7]). The contribution of lone pair electrons of bridging oxygen decreases the bridging angle. The oxygen atomic orbitals make sp3 hybrid orbitals that form tetrahedral configuration constructed by two bonding orbitals and two orbitals with lone pairs because of Coulombic repulsion between these orbitals. This stabilization with decreasing Si-O-Si angle is represented in Figure 4. The sum of the energy of valence orbitals decreases with decreasing Si-O-Si angle. The energy of lone pair orbitals split with decreasing Si-O-Si angle, but one of them does not change because of the orthogonal decomposing of lone-pair electrons by NBO method, unlike with bonding orbitals. However the changes in orbital energy of valence orbital of oxygen atom shows the tendency of equalization of each orbital. Consequently the effect of lone pairs potentially narrows the Si-O-Si bridging angle. The decrease in the energy of lone pair orbital is comparable to the electrostatic potential of the molecule calculated from charge of atom by NBO analysis (Figure 7). The sum of the electrostatic potential calculated from charge and the energy of lone pair electron has local minimum around 165 degrees. The d-p π-bonding between Si and O atoms is considered to play an important role in Si-O-Si bridging [21]. Newton and Gibbs [6] calculated the contributes of 3d-orbital to Si-O bond by Mulliken population analysis [22] from the results of MO calculation with Hartree-Fock method and STO-3G basis. In Newton and Gibbs [6], the total overlap populations in Si-O bond is 0.832 to 0.862. The population of σ-bonding between sp hybrid orbital of Si and O is 0.556. The populations of σ-bonding and π-bonding which 3d-orbital involves are 0.113 and 0.163, respectively. The occupancy of valence orbitals analyzed by natural atomic orbital analysis is shown in Table 3. The ratio of occupancy of d-orbital to s- and p-orbital is about 1% whereas the population analysis by Newton and Gibbs [6] shows several tens of percent. The effect of d-p π-bonding seems to be negligible in Si-O-Si bridging. Consequently, the bending of Si-O-Si bridging can be explained by the balance of Coulombic repulsion between SiO4 tetrahedra and lone pair electrons of bridging oxygen atom.

The plots of (a) electrostatic potential energy of pyrosilisic acid molecule calculated from charge calculated by NBO analysis and (b) sum of the electrostatic potential energy and the energy of lone pair orbital with varying Si-O-Si angle.
| 130° | 155° | 180° | |
| 3s | 0.44388 | 0.43775 | 0.43546 |
| 3px + 3py + 3pz | 0.92376 | 0.91504 | 0.91303 |
| 3dxy + 3dyz + 3dzx + 3dx2y2 + 3d2 | 0.05959 | 0.05834 | 0.05808 |
The general result of works about the nature of Si-O-Si bridging is Si-O bond lengthens with decreasing Si-O-Si angle [2,6,23]. This phenomenon is also reported by experiments [1]. Our study shows the same tendency in energy surface map (Figure 2 and 3). The mechanism of Si-O bond lengthening with decreasing of Si-O-Si angle can be explained by the energy of Si-O bonding orbital, overlap weighted bond order and Coulombic interaction.
The energy of Si-O bonding orbital increases with decreasing Si-O-Si angle (Figure 4). This means that the Si-O bonding stabilizes when the Si-O-Si angle is 180°. The ideal energy of sp hybrid orbital should be lower than that of sp3 hybrid orbital because the energy of s-orbital is lower than that of p-orbital with the same principal quantum number, qualitatively. The changes in orbital energy during decrease of Si-O-Si angle might be the result of increase of ratio of p character in spλ hybrid orbital in bridging oxygen. The hybridization may be considered to make Si-O bond weakened with decreasing Si-O-Si angle, from the viewpoint of energy of bonding orbital.
Another view point is overlap-integration weighted bond order. An increase of overlap weighted bond order increases bond strength especially in the case of homonuclear diatomic molecule. The calculated overlap weighted bond order of Si-Obr bonding has local minimum at Si-O-Si angle of 140 to 145 degrees whereas the energy of Si-O bonding orbital monotonously increases with decreasing Si-O-Si angle (Figure 4). However the decrease of overlap weighted bond order can weaken the Si-O bonding at least at high-angle region.
The changes in the charge of atom (Figure. 5) seem to support the weakening of Si-O bonding with decreasing Si-O-Si angle because the absolute value of charge of Si and bridging oxygen decreases with decreasing Si-O-Si angle.
Consequently the Si-O bond weakens and lengthens with decreasing Si-O-Si angle because of stabilization of Si-O bonding orbital with decreasing the hybridization index λ in spλ orbital of bridging oxygen and increase of Coulombic interaction between Si and bridging oxygen atom.
We hypothesize that the variation of Si-O-Si angle strongly affects the transportation coefficient of silicates. The decrease of Si-O-Si angle and viscosity of acidic silicate liquid with increasing pressure is well known experimental fact [2,3,4,5,24,25].These phenomena of silicate liquids can be explained by weakening of Si-O bonding by bending of Si-O-Si angle, from the view point of quantum chemical study. The Eyring's viscosity equation is written as follows,
| (3) |
We here showed the results and discussion of molecular orbital calculations of pyrosilisic acid molecule. We found the equilibrium geometries for two pyrosilisic acid molecules (C2V and 60° torsion) using Møller-Plesset perturbation theory and 6-311G (d,p) split valence basis set. The bent configuration of Si-O-Si angle in equilibrium geometries can be explained by the balance of Coulombic repulsion between SiO4 tetrahedra and the energy of lone pair orbitals belonging to bridging oxygen atom without concerning the contribution of d-p π-bonding. We calculated the energy surface with varying Si-Obr length and Si-O-Si angle and found the relationship between Si-Obr length and Si-O-Si bridging angle. The Si-O bond weakens with decreasing Si-O-Si angle because of instabilization in energy of Si-O bonding orbital with increasing the hybridization index λ in spλ orbital of bridging oxygen and decrease of Coulombic interaction between Si and bridging oxygen atom.