2022 Volume 21 Issue 4 Pages 111-117
2022 Volume 21 Issue 4 Pages 111-117
Thermodynamic properties and atomic configuration of PdRu alloy were investigated using Wang-Landau Monte Carlo method in combination with a newly-developed universal neural network potential. By using this new potential, excess energy of PdRu alloy was calculated. It is found that PdRu alloy in FCC lattice is unstable in the full range of alloy composition. This agrees with previous study based on density functional theory. The combined method was able to determine the configurational density of states, from which thermodynamic properties of the alloy were derived. It is found that when temperature increases, the excess free energy of the alloy is reduced, increasing the possibility of alloy mixing. Depending on the composition, transition peaks appear at finite temperatures where there are changes of preferable atomic arrangement due to the effect of temperature via the configurational entropy. In addition, the analyses on short-range order parameter and bond fraction show that PdRu alloy prefers to be in segregated form, where Pd and Ru are immiscible at low temperature, consistent with the experimental observations. The random mixing of Pd and Ru atoms in the form of solid-solution can occur at high temperature.
The discovery of novel alloy and composite materials with good performance for specific applications has been a hot topic in the field of materials science. For instance, the materials discovery is being applied to the research on the improvement of catalytic performance and long-term stability of electrodes in fuel cells [1, 2] or the research on the improvement of the cathode life cycle under charging and discharging processes in Li-ion batteries by mixing different metal elements with different compositions [3]. Traditionally, materials discovery often consumes a vast amount of time and resources in experiments and computational simulations because of the limitations of experimental conditions or theoretical foundations. Thus, it is crucial to approach this task in a new way. In recent years, due to the availability of large experimental and simulation datasets in combination with advanced algorithms and computer resources, materials discovery using data-driven and machine learning approaches has been receiving increasing attention as well as achievements in both time efficiency and prediction accuracy. One of the approaches in this new field is to use neural network potentials (NNP) [4], which model the interactions of atoms by mimicking the neural networks in the human brain. These kinds of potentials are expected to retain the accuracy up to the quantum mechanical calculation level and speed up to the force field calculation level. In addition, due to the rapid development of advanced computational techniques, such as the multicanonical Monte Carlo method [5], a method for predicting the stability of alloys can be implemented.
Thermodynamic properties of alloys are calculated based on the multicanonical ensemble method [5, 6]. Here, instead of sampling the probability distribution at a fixed temperature in configurational space as usually carried out in conventional Metropolis Monte Carlo (MC) methods, energy space is explored to estimate the configurational density of states (DOS), g(E). This is a central quantity in the Wang-Landau (WL) sampling technique [7, 8]. Once this is known, the partition function can be calculated from [9]:
| (1) |
From the partition function above, other thermodynamic quantities of a given system such as free energy (F), internal energy (U), and specific heat (Cv) can be derived:
| (2) |
| (3) |
| (4) |
In the WL sampling method, the probability of transition from state A to B is evaluated from:
| (5) |
Trial move is performed by swapping two arbitrarily atoms of dissimilar types in the
alloy compound. The sampling starts with an initial guess of
g(E) (=1) and zero-component energy histogram,
H(E). Every time when a trial move is accepted,
g(E) and H(E) of new
state are updated. Otherwise, those of old state will be modified. The updates are done by
multiplying g(E) with a modification factor,
f, and adding 1 to H(E). The
criterion for stopping the updates for H(E) is if any of
its value is larger than 0.8 multiplied by its average. At this point,
H(E) is considered flat with a flatness criterion of
0.8. When this condition is met, f will be reduced to
f1/2 and H(E) is reset to
0. The initial value of f was set as the Napier's constant (
For each trial move in the WL sampling, total energy of the given alloy compound is calculated. This is the most time-consuming part in WL method as well as other conventional MC techniques. To accelerate the energy calculations, a recently developed universal NNP, namely Preferred Potential (PFP version 2.0.0) [12], is used due to its calculation speed, versatility, and wide-range of support for many elements. There are several calculation modes in PFP. In the case of PdRu alloy, the mode for crystal systems with Hubbard and dispersion corrections (CRYSTAL_PLUS_D3) is selected.
To examine the accuracy of this potential, excess energy of PdRu alloy is calculated and compared with the results from density functional theory (DFT). Here, the excess energy is estimated by:
| (6) |
In this work, a 4 × 4 × 4 supercell of FCC PdxRu1-x (x = 0.25, 0.50, 0.75) alloy containing 256 atoms is used for WL sampling. To speed up the evaluation of energy, all atomic positions and cell volume/shape were kept fixed with pre-optimized lattice constants, which are 3.797 Å, 3.824 Å, and 3.858 Å for Pd64Ru192, Pd128Ru128, and Pd192Ru64, respectively. While, for the comparison of excess energies by PFP and DFT calculations, a 2 × 2 × 2 supercell was considered. DFT calculations were conducted using VASP [13, 14], where the exchange-correlation functionals based on the Perdew-Burke-Ernzerhof generalized gradient approximation [15] was used, the electron-core interactions are described by the projector-augmented wave method [16, 17]. Optimization criterion for atomic forces is below 0.02 Å/eV when applicable. For the consistency with PFP calculations, dispersion correction with Becke-Johnson damping (DFT-D3 (BJ)) [18] was introduced. Furthermore, to examine the performance of PFP, calculation time was monitored. Using the 2 × 2 × 2 supercell as a reference, we found that the optimization time for the Pd128Ru128 is about 2.1 seconds, while for DFT calculation running on a 32-core Intel Xeon CPU, it took about 9.2 hours. For fixed total energy calculations, calculation time are 0.1 seconds and 1.1 hours for PFP and DFT, respectively.
2.3 Short-range order parametersTo evaluate the atomic ordering in PdRu alloy from WL simulation, the Warren-Cowley short-range order (SRO) parameter [19, 20] was used. In binary alloy, the SRO parameter of dissimilar types is expressed as:
| (7) |
Here, PAB and cB represent the probability of finding the B atom surrounding the A atom and the overall composition of B atom in the compound, respectively. When αAB is close to zero, it means that two elements are randomly mixed.
In Figures 1 (a) and (b), the excess energies calculated from DFT and PFP are compared. Here, 30 random configurations with different alloy compositions and 3 ordered structures with respect to L12-type and L10-type orderings are chosen. We can see that the consistency of excess energies is better for the ordered structures than the random ones. The coefficients of determinant (R2) from fixed and relaxed calculations are 0.660 and 0.674, respectively. These values are moderate in the PFP version 2.0.0 but further checks for latest version (3.0.0) showed that PFP excess energies are comparable to DFT values, 0.926 and 0.956 for fixed and relaxed calculations, respectively. The difference of R2 between the fixed and relaxed calculations are very small, for both PFP versions. This means that energy calculations using PFP are well consistent with DFT. In addition, the compositional dependence of excess energy was also examined for FCC PdRu alloy and is shown in Figure 1 (c). Here, calculations using PFP were carried out by alloying Ru in FCC Pd lattice at different concentrations in both the randomly mixed and segregated atomic arrangements. For each value of Ru concentration in the randomly-mixed case, 2000 atomic configurations were considered. We can see that PdRu alloy is unstable in the whole range of Ru composition. For the randomly-mixed arrangement, the maximum mean value of excess energy is about 0.135 eV per atom with the maximum error bar (standard deviation) of about 0.008 eV at Ru concentration of 0.4-0.5. The segregated arrangement of atoms seems to be more preferable than the randomly-mixed cases. In the Pd- or Ru-rich conditions, the alloy tends to be more stable. The results calculated by PFP in this work is in good agreement with previous work using DFT [21].

Comparison between excess energy of FCC PdRu alloy in bulk state by (a) fixed and (b) relaxed DFT and PFP calculations; and (c) compositional dependence of excess energy for the cases of segregated and randomly-mixed atomic arrangements.
The configurational DOS for 3 PdRu alloy compositions (Pd64Ru192, Pd128Ru128, and Pd192Ru64) were estimated from WL sampling and depicted in Figure 2 (a). The range of excess energy is smaller than that calculated by regression equation [21]. This is because the energy range of each alloy was pre-estimated from two randomly-mixed atomic configurations. For each alloy composition, the two configurations above are the most stable and unstable ones among more than 10 million scanned structures. As shown in previous sub-section, the excess energy of randomly-mixed arrangements is larger than that of segregated one. Hence, the excess energy range is smaller in this work. From the configurational DOS, thermodynamic quantities were calculated using thermodynamic equations together with the partition function in equation 1. In Figures 2 (b) and (c), the excess free energy and specific heat are presented. It is noted that in the WL simulation, only contribution from atomic arrangement (or configurational contribution) was included in calculation of thermodynamic properties. Vibrational contribution was not considered in this current work. In principle, the two phenomena above are system-dependent. However, in the case of PdRu alloy, one expects that the former is more pronounced than the latter (i.e. the latter offsets between different configurations) [22]. We can see that when temperature increases, the excess free energies in the three compounds are decreased due to the contribution of configurational entropy. The change is more dramatical for Pd128Ru128. This might be the fact that Pd128Ru128 with Ru concentration of 0.5 is very much unstable as mentioned in previous sub-section. When temperature increases, the configurational entropy works to stabilize the system more significantly. Although the attenuation of the excess free energy with increase in temperature is obvious, they are still positive. This means that the PdRu compound is unstable than the monometallic systems.
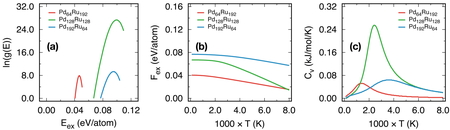
(a) Configurational density of states, and temperature dependence of (b) excess free energy and (c) specific heat in FCC Pd64Ru192, Pd128Ru128, and Pd192Ru64 alloys.
From the configurational specific heat, we can see that peaks appear in all

Specific heat calculated from different segments of the configurational DOS for FCC Pd128Ru128. (a) Full DOS and selected DOS segments. Here, all segments are shifted up for easy viewing. In (b), (c), and (d), the specific heat calculated from each selected DOS segment is compared to that calculated from full DOS. Full DOS data was taken from ref [21]. with some extrapolation to the high temperature range.
The SRO parameter as well as the bond fraction in alloy compounds are thermodynamic observables and can be estimated from WL simulation via the 2-dimensional configurational DOS, g(E,σ). Here, the second variable in g(E,σ) is the thermodynamic observable. The results of Warren-Cowley SRO parameter and Pd-Ru bond fraction as functions of temperature in Pd128Ru128 alloy is shown in Figure 4. These two quantities are in principle interchangeable as shown in equation 7 and lead to similar discussion on atomic ordering in the alloy. We can see that at low temperature, the Pd-Ru bond fraction and SRO parameter are about 0.4 and 0.2, respectively. These correspond to the segregation of Pd and Ru atoms in the alloy. The result is consistent with the experimental works which stated that at room temperature, there is no combination of Pd and Ru in the form of solid solution in bulk alloy [23]. This happens only when they are in the form of nanoparticles [24]. When temperature overcomes the transition point (around 1200 K, corresponding to the highest peak in the specific heat curve in Figure 3 (b)), the bond fraction and the SRO parameter are drastically changing. At very high temperature, the values are 0.5 and 0.0 for the bond fraction and the SRO parameter, respectively. These indicate a random mixture state of Pd and Ru atoms. In this case, the probability of finding A surrounding B is large and vice versa.
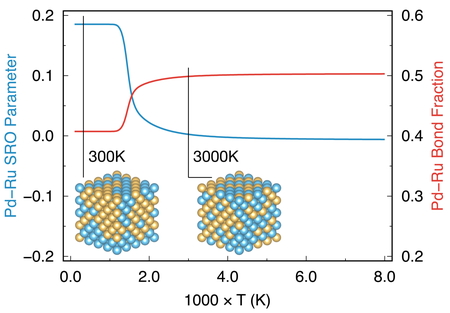
Temperature dependence of bond fraction (red) and Warren-Cowley short-range order parameter (blue) in Pd128Ru128 alloy.
In summary, we have successfully combined current advanced techniques, namely the Wang-Landau sampling and universal neural network potential, to study the thermodynamic properties and atomic arrangement in metallic alloy compounds. The method has been applied to study the stability of PdRu alloy. The novel neural network potential was proven to improve significantly the calculation speed as compared to the state-of-art DFT method. At the same time, it reproduced well the composition-dependent excess energy of PdRu alloy when compared to other study using DFT and supervised learning model. Furthermore, the combined method was shown to be a good way to investigate the contribution of configurational entropy to the thermodynamic properties and atomic ordering of alloys. It can reproduce well the atomic mixing phenomena in PdRu as compared to previous works. Obviously, this method can be applied not only to the PdRu alloy but also to the study any kind of alloy systems, due to the "universal" feature of the neural network potential. However, it is very important to choose the energy range in WL sampling. This can strongly govern the accuracy description of the thermodynamic properties of alloy systems.
This work was supported by the JST CREST (Grant No. JPMJCR21B3) and JSPS KAKENHI (Grant No. 20H05623). The calculations were partly conducted using the Supercomputing system resources (MASAMUNE-IMR) from the Center for Computational Materials Science of the Institute for Materials Research, Tohoku University.