Abstract
Because bud mutation occurs in a specific part of a plant, the genomic backgrounds of mutant and wild-type branches are identical except for mutations. Therefore, bud mutants are ideal for identifying key genes governing important crop traits. We studied Giant La France (GLaF), a bud mutant setting large fruit, which appeared spontaneously in the European pear (Pyrus communis L.) ‘La France’. In GLaF, increased cell size and DNA reduplication occurred specifically in fruit flesh. With the goal of identifying genes expressed differentially between GLaF and ‘La France’, microarray analysis was performed with RNA extracted from the receptacle (fruit flesh) 1 week before the full bloom stage. The receptacle was isolated by laser microdissection. Genes encoding proteins localized in the nucleus and cytoskeleton were up-regulated in GLaF. Among these genes, several were homologous to genes previously described to be associated with DNA reduplication. These might be related to the molecular mechanism of GLaF fruit size mutation.
Introduction
Fruit size is one of the most important commercial traits of a fruit. Many attempts have been made to identify the key that determines fruit size (Martre et al., 2011). Final fruit size is determined by both cell number and cell size. Among the genes involved in the regulation of cell number in fruits, FW2.2 and FASCIATED were identified by quantitative trait locus (QTL) analyses of tomato (Tanksley et al., 2004). FW2.2 encodes ORFX, whose molecular function is unclear. FW2.2 negatively regulates fruit size by controlling cell division during early fruit development (Cong et al., 2002; Frary et al., 2000). FASCIATED encodes a YABBY-like transcription factor and regulates fruit carpel number (Cong et al., 2008). Cell size is positively correlated with polyploidization, which increases the amount of DNA in cells (Cheniclet et al., 2005; Chevalier et al., 2011). Two types of polyploidization in which the cell cycle is modified are endoreduplication and endomitosis. Endoreduplication is chromosome duplication without sister chromatid segregation, leading to an increase in chromosome size. Endomitosis is a mitotic cycle within the nuclear envelope that leads to an increase in the number of chromosomes (D’Amato, 1984). Endoreduplication is observed in the fruit flesh of several species, such as tomato, peach, cherry, and strawberry (Chevalier et al., 2011). Representative examples of genes regulating fruit endoreduplication and cell size are WEE1 and CCS52A. Suppression of the gene encoding WEE1, a cell cycle-associated protein kinase, in tomato reduced endoreduplication level, fruit cell size, and fruit size (Gonzalez et al., 2007). Similarly, suppression of the gene encoding CCS52A, an anaphase-promoting complex activator, in tomato reduced endoreduplication, cell size, and fruit size (Mathieu-Rivet et al., 2010).
As mentioned above, in an annual crop, such as tomato, key genes for fruit size, such as FW2.2, FACIATED, WEE1, have been identified by QTL analysis or by analyses of transgenic plants. On the other hand, in fruit tree research, heterozygosity, long juvenility, large plant size, and difficulty in gene transformation hinder the search for such genes. However, fruit trees show an interesting phenomenon called bud mutation, also called bud sport. A bud mutation occurs in a single branch and confers a different phenotype from the other branches. Bud mutations occur naturally, but can be induced artificially with gamma-ray radiation. New cultivars have often been bred by isolation of mutant branches from mother trees and further propagation. Among the cultivars derived from natural bud mutants are the self-compatible Japanese pear cultivar ‘Osanijisseiki’ (Furuta et al., 1980) and the high lycopene-accumulating sweet orange cultivars ‘Cara Cara’ and ‘Hong Anliu’ (Lee, 2001; Liu et al., 2007). Cultivars derived from artificial bud mutants induced by gamma-ray radiation include the Japanese pear cultivars ‘Osa Gold’, ‘Gold Nijisseiki’, and ‘Kotobuki Shinsui’, which are resistant to black spot disease (Yoshioka et al., 1999). Because bud mutation occurs in a specific part of the plant, the genomic background of the mutant and wild-type branches are identical except for mutations. For this reason, bud mutants are ideal for identifying key genes governing important traits of crops. However, few useful genes have been identified from bud mutants because of the difficulties in fruit tree research mentioned above. Recent advances in DNA sequencing and “-omics” technologies have enabled the comprehensive comparison of genomes, transcripts, proteins, and metabolites between mutant and wild-type branches. These approaches allow the identification of the responsible genes of bud mutations and accelerate bud mutation analyses. Although few “-omics” approaches have been applied to bud mutation research, transcriptome and proteome analyses of bud mutations of sweet orange that accumulated high lycopene were successfully used to identify key genes and proteins for lycopene accumulation (Liu et al., 2007, 2009; Xu et al., 2009, 2010).
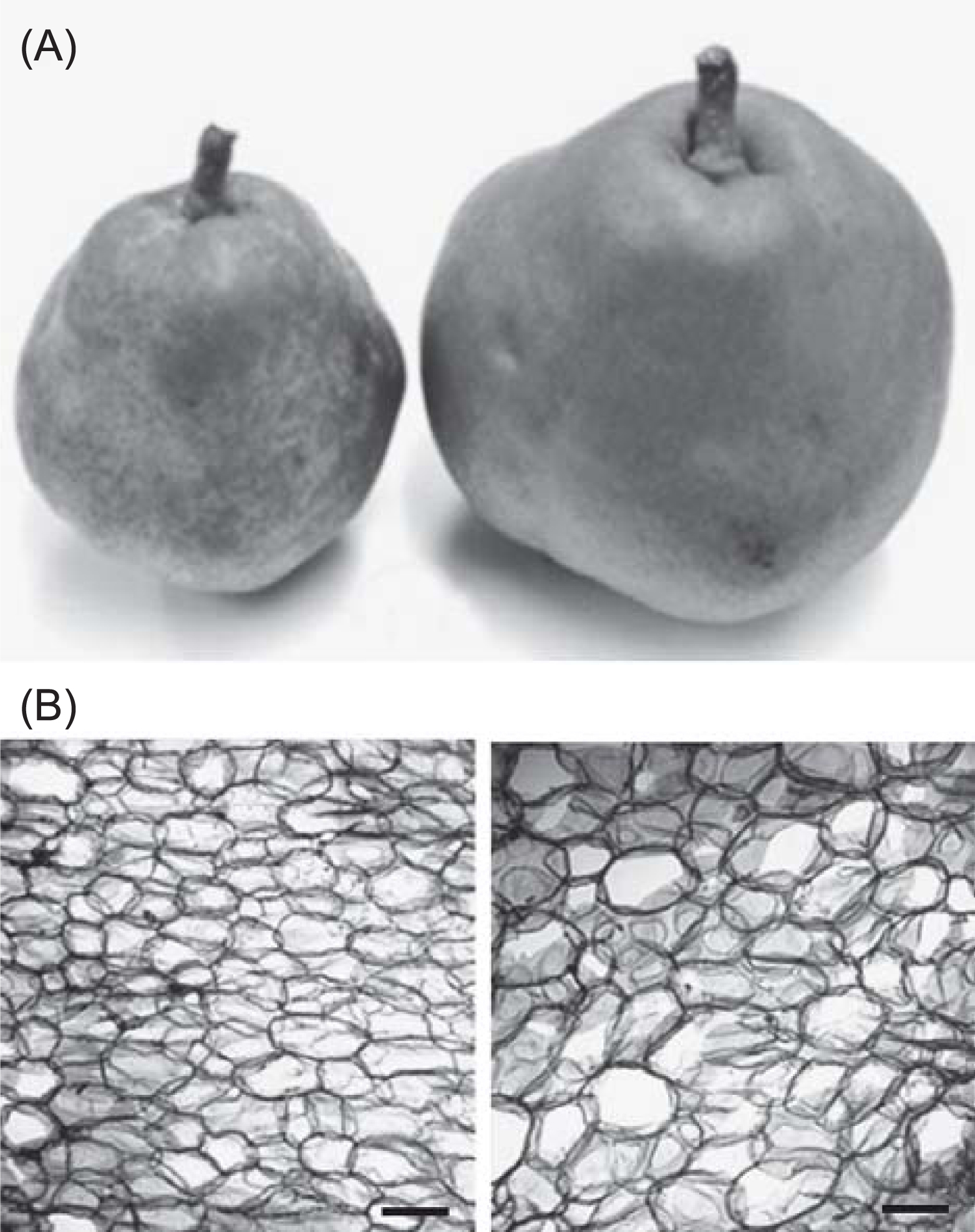
In this study, we focused on Giant La France (GLaF), a bud mutant (mutant branch) of the European pear (Pyrus communis L.) cultivar ‘La France’. GLaF sets 700 g to 820 g of fruits while wild-type branches set 400 g to 440 g of fruits (Isuzugawa et al., unpublished data) (Fig. 1A). The Brix value of GLaF fruits is similar to that of fruits from wild-type branches. In GLaF fruits, no significant change in cell number was observed, but the cell size was larger than that in wild-type fruit (Fig. 1B). Normal ‘La France’ is diploid and the ploidy level of its fruit flesh is 2C. Interestingly, the ploidy of GLaF fruit flesh at harvest time is only 4C, although those of the other floral organs, leaves, and seeds are 2C (Isuzugawa et al., 2005). These observations suggest that DNA reduplication occurs specifically in the flesh of GLaF fruits. Accordingly, GLaF may be useful for studying the mechanisms of fruit enlargement and DNA reduplication. Endoreduplication is observed in many fruits; however, endoreduplication has not been observed in apple or pear (Chevalier et al., 2011; Harada et al., 2005). It is likely that one or several gene mutations occurred in a branch of GLaF and caused DNA reduplication in fruit flesh, leading to increased fruit cell size and fruit size. Endoreduplication and endomitosis are known to induce an organ-specific increase in the amount of DNA (Seguí-Simarro and Nuez, 2008). These phenomena are speculated to be involved in DNA reduplication in GLaF.
In this study, we performed flow cytometric analysis to identify when DNA reduplication occurs in GLaF, and we also performed microarray analysis to identify the mutated gene and differentially expressed genes in GLaF.
Materials and Methods
Plant material
Pyrus communis ‘La France’ flower buds were harvested 1 week before full bloom from a tree in a farmer’s orchard in Kaminoyama, Yamagata prefecture, Japan. GLaF flower buds were harvested from the bud mutant branch of the same tree. For flow cytometric analysis, the harvested flower buds were cooled immediately on ice and kept at 4°C until used. For laser microdissection (LM), the lower parts were cut from the buds and immediately immersed in a fixative [75% (v/v) ethanol/25% (v/v) acetic acid] in a glass vial and fixed under a vacuum for 5 min. The vacuum infiltration step was performed three times with fresh fixative. Samples were left in the fixative overnight at 4°C.
Flow cytometric analysis
Flow cytometric analyses were performed as described by the CyStain UV Precise P (Partec GmbH, Munster, Germany) manufacturer’s protocol. The nucleus was extracted by chopping up the lower part of the flower bud, including the receptacle, in 0.2 mL extraction buffer (Partec GmbH). After 10 min the suspension was filtered through a nylon membrane filter (50-μm mesh; Partec GmbH) and 1 mL staining solution was added. The nuclear suspensions were analyzed with a Partec-PA1 flow cytometer (Partec GmbH). A total of 14,000 counts were collected.
Laser microdissection
Paraffin embedding and preparation of paraffin-embedded sections were performed as described by Takahashi et al. (2010). In brief, the lower parts of flower buds in fixative were dehydrated in a graded ethanol series and then embedded in Paraplast Xtra (Fisher Scientific, Pittsburgh, PA, USA), sectioned to a thickness of 12 μm with a Leica RM2135 rotary microtome (Leica Microsystems, Wetzlar, Germany), and mounted on PEN membrane glass slides (Molecular Devices, Sunnyvale, CA, USA) for LM. To remove the paraffin, slides were immersed twice in Histoclear II (National Diagnostics, Atlanta, GA, USA) for 5 min and then air-dried completely at room temperature. LM was performed using the Veritas Laser Microdissection System LCC1704 (Molecular Devices). The target cells that fused to the CapSure Macro LCM cap (Molecular Devices) were collected by removing the cap from the tissue section. The lower part of a flower bud, which includes the receptacle, was used for each LM experiment, RNA isolation, and microarray analysis.
Microarray analysis
Total RNA was extracted from a receptacle isolated by LM with a PicoPure RNA isolation kit (Molecular Devices), and quantified with a Quant-iT RiboGreen RNA reagent kit (Invitrogen, San Diego, CA, USA). Quality of the total RNA was assessed using a RNA 6000 Pico Kit on an Agilent 2100 bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). RNA integrity was assessed as RNA integrity number (RIN) calculated with 2100 Expert software (version B.02.02, eukaryote total RNA pico mode; Agilent Technologies), and total RNA, with an RIN of > 5.0, was used for microarray analysis.
Reverse transcription of mRNA in the total RNA samples (20 ng each) and synthesis of labeled cRNA was performed using the Low Input Quick Amp Labeling Kit, one color (Agilent Technologies) according to the manufacturer’s instructions. The labeled cRNA was hybridized to the Japanese pear custom 44K oligo microarray (GPL13124) using the Gene Expression Hybridization kit (Agilent Technologies) as a hybridization buffer at 65°C for 17 h in a hybridization chamber. The oligoarray was designed from the 11,540 representative ESTs from 24,945 sequences collected mainly from 11 Japanese pear ‘Housui’(syn. ‘Hosui’) cDNA libraries (leaf bud, leaf, flower bud, flower before opening, flower at full bloom, fruitlet at three developmental stages, immature fruit, fruit at optimum maturity for eating, and over-ripened fruit) (Nishitani et al., 2009).
After hybridization, the array slides were washed with Gene Expression Wash Buffer (Agilent Technologies) and the dried slides were scanned with an Agilent microarray scanner (G2505C; Agilent Technologies). Raw scan data were captured using Agilent Feature Extraction software (version 10.7.1.1; Agilent Technologies). Three biological replications were analyzed using three different pools of flower buds.
Microarray data analysis
The dataset were normalized using the Subio Platform (Subio Inc., Tokyo, Japan), by log-based transformation and global normalization. A t-test was used to estimate the P values of the observed differences between the normalized intensities.
For gene annotation, probe sequences were matched to coding sequences (CDSs) of the Chinese pear (v1.0, data name: pear.gene.cds) obtained from Pear Genome Project (http://peargenome.njau.edu.cn:8004/, August 27, 2013) and apple (v1.0, data name: Consensus gene model CDSs) obtained from GDR (http://www.rosaceae.org/, August 27, 2013) (Jung et al., 2008) by BLASTN similarity searches using the EST sequences used for designing the Japanese pear custom oligoarray (GPL13124) (Nishitani et al., 2010, 2012) as a query with maximum E value of 1e–30. The Arabidopsis genes most similar to the Chinese pear or apple predicted CDSs were searched by the BLASTX program using apple or Chinese pear CDSs above as the query and Arabidopsis protein sequences (TAIR10_pep_20101214 updated on 08/23/2011) as the database. Japanese pear EST sequences were classified according to the MIPS Functional Catalogue database (Ruepp et al., 2004) using matched Arabidopsis genes as queries.
Results
Flow cytometric analysis
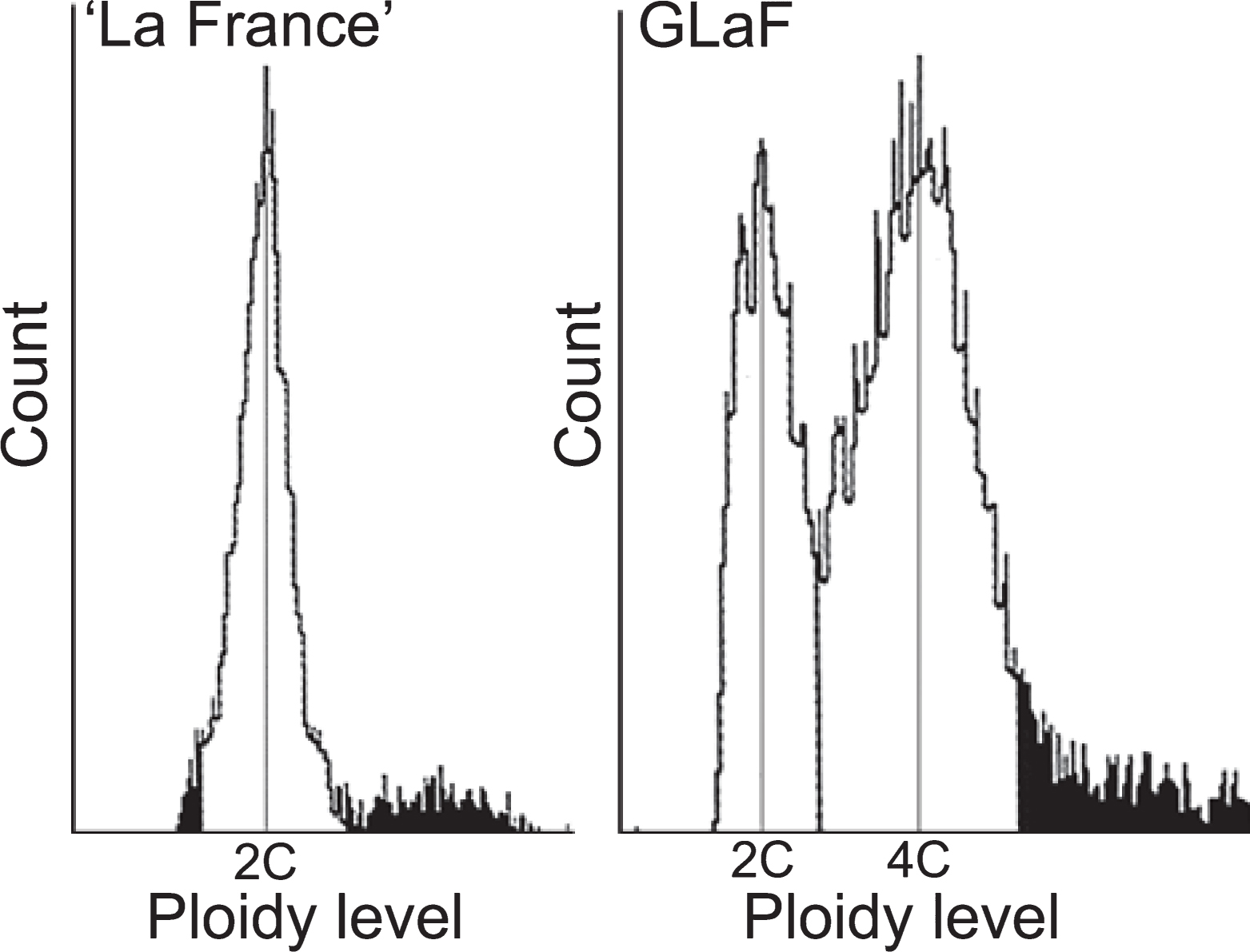
Although the ploidy of ‘La France’ fruit at harvest time is only 2C and the ploidy of GLaF fruit is only 4C (Isuzugawa et al., 2005), it is unclear at what stage the ploidy of GLaF fruit increases. Thus, the ploidy level in the lower part of the flower bud, which includes the ovary and the receptacle (young fruit flesh tissue) 1 week before full bloom, which is before active cell division at the receptacle, was determined by flow cytometric analysis. The ploidy of ‘La France’ was only 2C and that of GLaF was both 2C (35%) and 4C (65%) (Fig. 2). This result showed that DNA reduplication in GLaF fruit had already occurred by 1 week before flowering. To determine gene expressions related to DNA reduplication in GLaF fruit, we decided to use flower buds that were sampled 1 week before full bloom for microarray analysis.
Isolation of receptacle by LM and microarray analysis
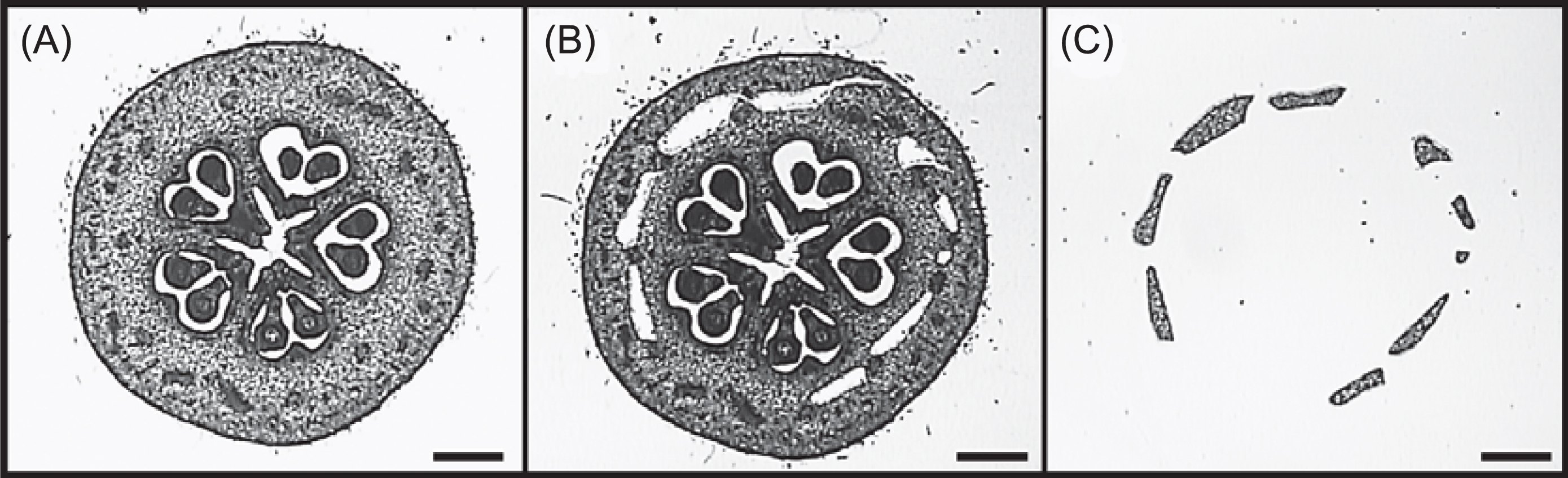
The image of pear flower bud 1 week before full bloom and a diagram of its cross section are shown in Figure 3. Isuzugawa et al. (2005) observed ploidy levels of only 4C in flesh (receptacle) of GLaF fruit at harvest time, 2C and 4C in the core (ovary and part of the receptacle) of the fruit, and 2C in seeds. Therefore, to identify genes involved in DNA reduplication, we isolated RNA samples from isolated receptacles without contamination by other organs and used them for microarray analysis. Because the flower bud is too small for unaided isolation of the receptacle, we used LM (Nakazono et al., 2003; Nelson et al., 2006) to isolate pure receptacles of GLaF and ‘La France’ from flower buds 1 week before full bloom (Fig. 4).

The RNA samples extracted from the LM-isolated receptacles were labeled with Cy3 dye and the labeled complementary RNAs (cRNAs) were then used to perform one-color microarray analysis using Japanese pear custom oligoarrays. The microarray experiment was repeated three times using RNA from receptacles isolated from 2–3 flower buds in each experiment.
Apple and Chinese pear CDSs predicted from the genome sequence (Jung et al., 2008; Wu et al., 2012) were used as a reference to annotate Japanese pear ESTs because apple and Chinese pear are genetically closely related to Japanese pear. BLASTN similarity searches were used to obtain the most homologous apple and Chinese pear CDSs for each Japanese pear ESTs, yielding 6,996 apple CDSs with high similarity (E < 1e–30) to 9,949 Japanese pear ESTs, and 6,559 Chinese pear CDSs with high similarity (E < 1e–30) to 9,711 Japanese pear ESTs. The most homologous Arabidopsis genes were searched for apple and Chinese pear CDSs by BLASTX similarity searches. The obtained Arabidopsis genes were almost the same between apple and Chinese pear CDSs. Because slightly more Japanese pear ESTs were annotated when using apple CDSs, we used apple CDSs as a reference.
We selected probes whose Cy3 intensities after normalization were more than 1.5 times higher or lower in GLaF than in ‘La France’ (t-test, P < 0.05). If more than one EST was highly similar (E < 1e–30) to one apple CDS, they were assumed to originate from the same gene and the probe with the lowest P value was taken. By these criteria, 277 genes were up-regulated and 122 genes were down-regulated in GLaF. Among up-regulated genes, 5 genes showed a change more than 4 times higher in GLaF. Among down-regulated genes, 1 gene was more than 4 times lower (Table 1). NFR1_26_59 and NFW2_26_58 did not show enough similarity to Arabidopsis genes; however, NFR1_26_59 showed similarity to an uncharacterized protein of Vitis vinifera in UniProtKB (F6I554) and NFW2_26_58 showed similarity to an anion transporter of Chlamydomonas reinhardtii in UniProtKB (Q75NZ3). A homolog of NLE2_07_80 (β-D-xylosidase 4) was previously reported as the gene encoding secondary cell wall metabolism in Arabidopsis (Goujon et al., 2003). However, functions of the other homologs of highly up-or down-regulated genes have not been reported previously.
Table 1.
Genes showing more than 4 times higher or lower expression in GLaF with
t-test
P < 0.05.
| Pear EST name |
Accession |
GLaF/
‘La France’ |
P value |
AGI code |
Protein name |
| Up-regulated in GLaF |
| NFR3_24_91 |
HX753237 |
5.301 |
0.042 |
AT1G62510 |
Bifunctional inhibitor/lipid-transfer protein/seed storage 2S albumin-like protein |
| NFW2_26_58 |
HX764034 |
4.610 |
0.013 |
|
|
| NFR1_26_59 |
HX747860 |
4.589 |
0.047 |
|
|
| TUNO2_A07 |
FY998776 |
4.527 |
0.006 |
AT5G18520 |
Putative lung seven transmembrane receptor |
| NLE2_07_80 |
HX767325 |
4.237 |
0.047 |
AT5G64570 |
β-D-xylosidase 4 |
| Down-regulated in GLaF |
| NFW3_10_21 |
HX765716 |
0.181 |
0.034 |
AT1G66920 |
Receptor serine/threonine kinase PR5K, putative |
To categorize genes according to their functions, we classified the genes according to the MIPS Functional Catalogue database (Ruepp et al., 2004) using best hit Arabidopsis genes. Genes whose expression was up- or down-regulated in GLaF were categorized by subcellular localization (Table 2). Among up-regulated genes in GLaF, “Cytoskeleton” and “Nucleus” were significantly enriched (one-sided binomial test, P < 0.01). Because DNA reduplication occurs within the nucleus, and the cytoskeleton plays indispensable roles in cell division and nuclear division, next we considered the up- and down-regulated genes in GLaF classified into the “Nucleus” or “Cytoskeleton” categories.
Table 2.
Classification of differentially expressed genes between GLaF and ‘La France’ receptacles 1 week before full bloom stage according to subcellular localization categories of the MIPS Functional Catalogue.
P values were calculated by the one-sided binomial test.
| MIPS Functional Categories (Category number) |
Entire array |
Up-regulated genes in GLaF |
Down-regulated genes in GlaF |
| % coverage |
% coverage |
Enrichment |
P value |
% coverage |
Enrichment |
P value |
| Cell wall (70.01) |
1.89% |
3.97% |
2.10 |
1.73E − 02 |
3.33% |
1.76 |
1.92E-01 |
| Eukaryotic plasma membrane/membrane attached (70.02) |
2.36% |
4.69% |
1.99 |
1.54E − 02 |
1.67% |
0.71 |
7.78E-01 |
| Cytoplasm (70.03) |
8.48% |
7.22% |
0.85 |
8.02E − 01 |
2.50% |
0.29 |
9.98E − 01 |
| Cytoskeleton (70.04) |
1.06% |
5.42% |
5.11 |
3.90E − 07 |
0.00% |
0.00 |
1.00E + 00 |
| Centrosome (70.05) |
0.00% |
0.00% |
— |
— |
0.00% |
— |
— |
| Cell junction (70.06) |
0.03% |
0.00% |
0.00 |
1.00E + 00 |
0.00% |
0.00 |
1.00E + 00 |
| Endoplasmic reticulum (70.07) |
1.86% |
2.89% |
1.55 |
1.40E − 01 |
0.83% |
0.45 |
8.95E − 01 |
| Golgi (70.08) |
0.69% |
1.08% |
1.57 |
3.00E − 01 |
0.00% |
0.00 |
1.00E + 00 |
| Intracellular transport vesicles (70.09) |
0.59% |
0.72% |
1.22 |
4.83E − 01 |
0.00% |
0.00 |
1.00E + 00 |
| Nucleus (70.10) |
9.16% |
15.16% |
1.66 |
8.78E − 04 |
8.33% |
0.91 |
6.69E − 01 |
| Mitochondrion (70.16) |
11.88% |
13.72% |
1.15 |
1.95E − 01 |
11.67% |
0.98 |
5.70E − 01 |
| Peroxisome (70.19) |
0.54% |
0.36% |
0.67 |
7.79E − 01 |
0.00% |
0.00 |
1.00E + 00 |
| Endosome (70.22) |
0.49% |
0.72% |
1.47 |
3.90E − 01 |
0.83% |
1.69 |
4.43E − 01 |
| Vacuole or lysosome (70.25) |
0.31% |
0.36% |
1.16 |
5.82E − 01 |
0.00% |
0.00 |
1.00E + 00 |
| Plastid (70.26) |
13.31% |
10.47% |
0.79 |
9.34E − 01 |
10.83% |
0.81 |
8.24E − 01 |
| Extracellular/secretion proteins (70.27) |
0.30% |
0.00% |
0.00 |
1.00E + 00 |
4.17% |
13.90 |
3.49E − 05 |
On the other hand, among the down-regulated genes in GLaF, “Extracellular/secretion protein” was significantly enriched. Genes categorized into “Extracellular/secretion protein” are involved in cell wall regulation or stress response, but not in cell division or the cell cycle. Three cell wall-regulating genes and two stress response genes were included in this category (Table 3), and there are no cell division or cell cycle-related genes according to previous reports. However, because cell wall regulation is involved in cytokinesis through cell plate formation (Assaad, 2001), the possibility that these genes are involved in DNA reduplication is not excluded.
Table 3.
Extracellular/secretion protein coding genes that were differentially expressed between GLaF and ‘La France’ receptacles 1 week before full bloom. Shown are those at least 1.5-fold down-regulated in GLaF and with
t-test
P < 0.05. There was no up-regulated gene.
| Pear EST name |
Accession |
GLaF/
‘La France’ |
P value |
AGI code |
Protein name |
| AB236427.1 |
AB236427 |
0.514 |
0.044 |
AT2G02990 |
Ribonuclease 1 |
| AB093029.1 |
AB093029 |
0.490 |
0.008 |
AT2G40610 |
Expansin-A8 |
| NFR4_05_58 |
HX754137 |
0.659 |
0.023 |
AT2G40610 |
Expansin-A8 |
| NLE2_27_89 |
HX768908 |
0.485 |
0.045 |
AT5G20630 |
Germin-like protein subfamily 3 member 3 |
Among up- or down-regulated genes classified into “Nucleus” (Table 2), genes subcategorized into “Cell cycle”, “DNA synthesis and replication”, “Proteasomal degradation”, and “Transcription” were expected to be related to DNA reduplication. These genes are described in Table 4.
Table 4.
Nucleus-localized genes that were differentially expressed between GLaF and ‘La France’ receptacles 1 week before full bloom. Shown are those at least 1.5-fold up-regulated in GLaF and with
t-test
P < 0.05.
| Pear EST name |
Accession |
GLaF/
‘La France’ |
P value |
AGI code |
Protein name |
| Cell cycle |
| NFR3_07_31 |
HX751692 |
2.396 |
0.006 |
AT2G26760 |
Cyclin-B1-4 |
| NFR3_21_11 |
HX752892 |
2.171 |
0.042 |
AT1G15570 |
Cyclin-A2-3 |
| NFR3_23_70 |
HX753126 |
1.663 |
0.028 |
AT3G12380 |
Actin-related protein 5 |
| NFR1_11_72 |
HX746556 |
1.536 |
0.014 |
AT2G37630 |
Transcription factor AS1 |
| DNA synthesis and replication |
| NFR6_21_64 |
HX760483 |
2.804 |
0.010 |
AT2G29570 |
Prolifelating cell nuclear antigen 2 |
| NFR3_30_15 |
HX753707 |
2.531 |
0.014 |
AT2G37560 |
Origin recognition complex subunit 2 |
| NFR2_11_50 |
HX749160 |
2.292 |
0.015 |
AT2G31270 |
CDT1-like protein |
| Proteasomal degradation |
| NFR2_10_35 |
HX749069 |
1.839 |
0.042 |
AT4G38630 |
26S proteasome non-ATPase regulatory subunit 4 |
| NFR6_12_89 |
HX759859 |
1.810 |
0.035 |
AT5G19990 |
26S protease regulatory subunit 8 homolog A |
| NFW3_01_10 |
HX764930 |
1.658 |
0.017 |
AT5G42790 |
Proteasome subunit α type-1-A |
| NFR6_29_18 |
HX761036 |
1.586 |
0.024 |
AT5G58290 |
26S protease regulatory subunit 6B homolog |
| NFR1_18_90 |
HX747162 |
1.563 |
0.003 |
AT4G29040 |
26S proteasome regulatory subunit 4 homolog A |
| NFR3_25_37 |
HX753276 |
1.546 |
0.032 |
AT1G29150 |
26S proteasome regulatory subunit N6 |
| Transcription |
| NFW2_02_62 |
HX762219 |
3.398 |
0.022 |
AT2G36530 |
Bifunctional enolase 2/transcriptional activator |
| NFR2_01_62 |
HX748474 |
2.883 |
0.012 |
AT5G25190 |
Ethylene-responsive transcription factor ERF003 |
| NFW2_10_12 |
HX762786 |
2.088 |
0.042 |
AT1G75390 |
Basic leucine-zipper 44 |
| NFR1_17_69 |
HX747053 |
1.970 |
0.014 |
AT1G59750 |
Auxin response factor 1 |
| NFW1_06_80 |
HX761629 |
1.907 |
0.022 |
AT2G40260 |
Myb family transcription factor |
| NLE1_06_04 |
HX766203 |
1.756 |
0.033 |
AT3G15270 |
Squamosa promoter-binding-like protein 5 |
| NFR3_05_56 |
HX751539 |
1.694 |
0.028 |
AT2G26150 |
Heat stress transcription factor A-2 |
| NFR2_23_17 |
HX750107 |
1.601 |
0.027 |
AT3G52910 |
Growth-regulating factor 4 |
| NFR1_21_85 |
HX747431 |
1.571 |
0.019 |
AT5G65670 |
Indole-3-acetic acid inducible 9 |
| NFR3_05_77 |
HX751558 |
1.548 |
0.013 |
AT1G79890 |
Chromosome transmission fidelity protein 1 |
| NFW2_28_50 |
HX764199 |
1.517 |
0.048 |
AT4G36920 |
Floral homeotic protein APETALA 2 |
Genes involved in cell cycle regulation are closely related to DNA polyploidization events such as endoreduplication and endomitosis (Joubès and Chevalier, 2000). Both endoreduplication and endomitosis lack mitosis entirely or partially. Joubès and Chevalier (2000) reported that the gene expression of mitotic cyclin decreased during endoreduplication. Among the genes classified into “Nucleus” (Table 2), 4 were subclassified into “Cell cycle”. NFR3_07_31 (Cyclin-B1-4), NFR3_21_11 (Cyclin-A2-3), NFR3_ 23_70 (Actin-related protein 5) and NFR1_11_72 (Transcription factor AS1) were up-regulated. Among these genes, the tobacco homolog of NFR3_07_31 (Cyclin-B1-4) induces endomitosis (Weingartner et al., 2004) and the Arabidopsis homolog of NFR3_21_11 (Cyclin-A2-3) negatively regulates endoreduplication (Boudolf et al., 2009; Imai et al., 2006).
Genes subclassified into “DNA synthesis and replication” were chosen because DNA synthesis and replication are necessary for DNA reduplication observed in GLaF fruit. NFR6_21_64 (Proliferating cell nuclear antigen 2), NFR3_30_15 (Origin recognition complex 2), and NFR2_11_50 (CDT1-like protein) were up-regulated in GLaF, whereas no gene in this category was down-regulated. The Arabidopsis homolog of NFR2_11_50 (CDT1-like protein) AtCDT1a is reported to regulate the endocycle number positively in endoreduplication (Castellano et al., 2004).
Proteasomes are extremely large protein complexes responsible for the degradation of ubiquitinated proteins. They are active in various biological processes including the cell cycle (Castellano et al., 2004; Kurepa et al., 2009). Sako et al. (2010) reported that in Arabidopsis, loss of function of a proteasome subunit increases endoreduplication levels. Accordingly, we considered the possibility that DNA reduplication in GLaF is caused by change in the gene expression level of a proteasome subunit. Six up-regulated genes were subclassified into the “Proteasomal degradation” category (Table 4). NFR1_18_90 (26S proteasome regulatory subunit 4 homolog A) is one of the up-regulated genes, and the null mutant of its Arabidopsis homolog shows a high frequency of endoreduplication (Sako et al., 2010).
It is possible that transcription factors cause DNA reduplication by regulating downstream gene expression. In Arabidopsis, the transcription factors MYB3R1 and MYB3R4 regulate the expression of cell cycle-related genes expressed in the G2/M phase such as CYCB2 and KNOLLE (Haga et al., 2007). The Arabidopsis double null mutant myb3r1 myb3r4 showed incomplete cytokinesis and polykaryocytes (Haga et al., 2007). Twenty up-regulated genes and 4 down-regulated genes were subcategorized into “Transcription” (Table 4). Of these genes, NFW1_06_80 (Myb family transcription factor) belongs to the MYB family. However, this gene and their homologs have not been reported to regulate the cell cycle.
Genes encoding cytoskeleton-related proteins
Some of the genes assigned to the “Cytoskeleton” category may regulate the division and transfer of the chromosomes, nucleus, and cytosol in mitosis, such that disruption of their expression causes DNA reduplication. Fifteen up-regulated and non-down-regulated genes were classified into “Cytoskeleton” and subclassified into “Cell cycle”, “Actin cytoskeleton”, “Microtubule cytoskeleton” and “Others” (Table 5). Among the genes subclassified into “Cell cycle”, NFR3_23_70 (Actin-related protein 5) was also listed in “Nucleus” in Table 2. The Arabidopsis homolog of NFR3_14_05 (Protein MOR1), MOR1 is required for cytokinesis, and a MOR1 mutant shows a cytokinesis-defective phenotype (Twell et al., 2002).
Table 5.
Cytoskeleton-related protein coding genes that were differentially expressed between GLaF and ‘La France’ receptacles 1 week before full bloom. Shown are those at least 1.5-fold up-regulated in GLaF and with
t-test
P < 0.05. There was no down-regulated gene.
| Pear EST name |
Accession |
GLaF/
‘La France’ |
P value |
AGI code |
Protein name |
| Cell cycle |
| NFR3_14_05 |
HX752291 |
1.752 |
0.012 |
AT2G35630 |
Protein MOR1 |
| NFR3_23_70 |
HX753126 |
1.663 |
0.028 |
AT3G12380 |
Actin-related protein 5 |
| Actin cytoskeleton |
| NFR4_04_50 |
HX754063 |
1.750 |
0.044 |
AT3G12110 |
Actin-11 |
| NFR3_01_55 |
HX751189 |
1.619 |
0.034 |
AT3G12110 |
Actin-11 |
| NFR5_09_54 |
HX755935 |
1.586 |
0.021 |
AT3G53750 |
Actin-3 |
| TUSSH48 |
FY998137 |
1.580 |
0.032 |
AT5G09810 |
Actin-7 |
| Microtubule cytoskeleton |
| NLE2_15_44 |
HX767932 |
2.107 |
0.010 |
AT5G23860 |
Tubulin β-8 chain |
| NFR1_04_13 |
HX745973 |
1.909 |
0.017 |
AT1G50010 |
Tubulin α-2 chain |
| NFR4_06_64 |
HX754222 |
1.883 |
0.015 |
AT4G14960 |
Tubulin α-6 chain |
| NFR6_28_14 |
HX760952 |
1.759 |
0.019 |
AT1G50010 |
Tubulin α-2 chain |
| Others |
| NFW3_10_74 |
HX765764 |
2.335 |
0.015 |
AT2G22610 |
Di-glucose binding protein with Kinesin motor domain |
| NFR1_08_64 |
HX746301 |
2.156 |
0.023 |
AT2G38720 |
65-kDa microtubule-associated protein 5 |
| NFW2_18_45 |
HX763439 |
2.049 |
0.045 |
AT4G14330 |
Phragmoplast-associated kinesin-related protein 2 |
| NFW2_11_75 |
HX762922 |
1.930 |
0.015 |
AT2G47500 |
Putative kinesin |
| NLE1_10_86 |
HX766606 |
1.579 |
0.034 |
AT1G13180 |
Actin-related protein 3 |
Discussion
GLaF is a mutant branch that occurs in a ‘La France’ pear tree and its fruit size and fruit cell size are larger than those of ‘La France’ branches (Fig. 1). The ploidy of a GLaF fruit flesh cell at harvest time is 4C (Isuzugawa et al., 2005), whereas that of a ‘La France’ fruit flesh cell is 2C. Such a ploidy increase to 4C was not observed in the leaves or seeds of the GLaF branch, whose ploidy is 2C (Isuzugawa et al., 2005). From these observations, it appears that the mutation occurring in the GLaF branch causes DNA reduplication specifically in fruit flesh so that the fruit flesh cell size increases. First we performed flow cytometric analysis to estimate when DNA reduplication occurs. As a result, cells of 4C ploidy, namely DNA reduplicated cells, were observed in 65% of GLaF fruit 1 week before full bloom (Fig. 2). The major part of fruit cells had already completed DNA reduplication before active cell division; this is much earlier than general endoreduplication. Generally, once a cell enters the endoreduplication cycle, there is no way back to the mitotic cell cycle and the cell loses division competency (Joubès and Chevalier, 2000; Larkins et al., 2001; Seguí-Simarro and Nuez, 2008). Because cell number reduction was not observed in GLaF (Isuzugawa et al., 2005), we suggest that DNA reduplication occurring in GLaF is a consequence of endomitosis rather than endoreduplication.
However, it is possible that one round of endoreduplication occurred in GLaF and then reverted to a mitotic cell cycle. Apple ‘Grand Gala’, which is a bud mutant of ‘Gala’, shows a similar phenomenon to GLaF. In ‘Grand Gala’, fruit-specific DNA reduplication is observed 2 days before full bloom, and it is suggested that DNA reduplication occurs by one cycle of endoreduplication (Malladi and Hirst, 2010). In ‘Grand Gala’, higher cell production rates around bloom could compensate for the reduction of division competent cells due to endoreduplication (Malladi and Hirst, 2010). This scenario could be applied to DNA reduplication in GLaF. To determine which type of DNA reduplication occurred in GLaF, it is necessary to perform cytological analysis.
To identify genes expressed differentially between GLaF and ‘La France’, microarray analysis was performed. To eliminate contamination with tissues other than the receptacle, because the core part (ovary and part of the receptacle) of GLaF fruit contains both 2C and 4C cells and seeds contain 2C cells (Isuzugawa et al., 2005), RNA from the LM-isolated receptacle 1 week before full bloom (Fig. 4) was used. From the microarray results, 277 up-regulated genes and 120 down-regulated genes (more than 1.5 times higher or lower in GLaF than ‘La France’, t-test P < 0.05) were extracted as differentially expressed genes. Six genes showed more than 4 times higher or lower expression in GLaF (Table 1). However, no gene was related closely to DNA reduplication among these 6 genes.
Classification of the differentially expressed genes according to the MIPS Functional Catalogue (Ruepp et al., 2004) revealed that genes categorized into subcellular localization categories “Nucleus” and “Cytoskeleton” were significantly enriched in the up-regulated genes (Table 2). This observation is to be expected because the DNA reduplication of GLaF fruit is an event that occurs in the nucleus, and nucleoproteins and cytoskeletal proteins are intimately involved in cell cycle and cell division processes. Up- or down-regulated genes in the “Nucleus” category (Table 2) were subclassified and the subcategories closely associated with DNA reduplication, “Cell cycle”, “DNA synthesis and replication”, “Proteasomal degradation”, and “Transcription” were selected (Table 4). All of the genes in these subcategories were up-regulated in GLaF. “Cell cycle” was selected, because endoreduplication and endomitosis is caused by disruption of the cell cycle. “DNA synthesis and replication” was selected because DNA replication is a necessary step in the generation of a DNA-reduplicated cell. “Proteasomal degradation” was selected because loss of function of a proteasome subunit increases endoreduplication through degradation of cyclin proteins (Genschik et al., 1998; Sako et al., 2010). “Transcription” was selected because some transcription factors regulate the cell cycle by regulating cell cycle-gene expression (Haga et al., 2007). In these subcategories, we found genes whose homologs have been reported to be involved in DNA reduplication, namely NFR3_07_31 (Cyclin-B1-4) and NFR3_21_11 (Cyclin-A2-3) in “Cell cycle”, NFR1_18_90 (26S proteasome regulatory subunit 4 homolog A) in “Proteasomal degradation”, and NFR2_11_50 (CDT1-like protein) in “DNA synthesis and replication”.
A homolog of the gene NFR3_07_31 (Cyclin-B1-4), which was up-regulated in GLaF, is involved in endomitosis. Tobacco cells expressing nondegradable cyclin B1, whose destruction box was modified, experienced endomitosis and showed doubled DNA concentration (Weingartner et al., 2004). It is accordingly possible that DNA reduplication in the receptacle cells of GLaF was caused by up-regulation of NFR3_07_31 (Cyclin-B1-4). Previously, in addition to tobacco CYCB1 (Weingartner et al., 2004), rice CDKB2, and Arabidopsis GIG1/OSD1 were identified as endomitosis causative genes (Iwata et al., 2011; Endo et al., 2012). It is important to confirm their expression levels; however, CDKB2 and GIG/OSD gene probes were not installed on the microarray.
Among the upregulated genes in GLaF, both positive and negative regulators of endoreduplication were found. Homologs of NFR3_21_11 (Cyclin-A2-3) and NFR1_18_90 (26S proteasome regulatory subunit 4 homolog A) were reported to be negative regulators of endoreduplication (Castellano et al., 2001, 2004; Imai et al., 2006; Sonoda et al., 2009). In Arabidopsis, a null mutation of RPT2a, a gene encoding 26S proteasome regulatory subunit 4 homolog A, or CYCA2-3 stimulated endoreduplication (Imai et al., 2006; Sako et al., 2010; Sonoda et al., 2009). Furthermore, mitotic cyclins, including B1-type and A2-type cyclins, are down-regulated when endoreduplication occurs (Joubès and Chevalier, 2000). Accordingly inhibition of endoreduplication is expected from up-regulation of NFR3_21_11 (Cyclin-A2-3) and NFR1_18_90 (26S proteasome regulatory subunit 4 homolog A). These results suggest that DNA reduplication observed in GLaF was different from endoreduplication. On the other hand, next to these endoreduplication suppressing genes, NFR2_11_50 (CDT1-like protein), a homolog of the DNA replication licensing factor CDT1a, which contributes to increasing the endocycle number in Arabidopsis (Castellano et al., 2001, 2004), was up-regulated in GLaF. Although our transcriptome analysis suggests a mechanism that is divergent from endoreduplication, the occurrence of some endoreduplication stimulating genes (such as CDT1) is still expected to contribute to DNA reduplication in GLaF. The cell cycle is regulated by complicated and precise interactions among large numbers of cell cycle-regulating proteins. Either an increase or decrease in the expression of genes encoding these proteins may destabilize the cell cycle and lead to DNA reduplication by abnormal cell division.
NFR3_23_70 (Actin-related protein 5), which is a homolog of Arabidopsis ARP5, was also up-regulated in GLaF. ARP5 encodes a subunit of the chromatin-remodeling complex, which is required for the exchange of histone-subunit isovariants in nucleosomes (Chen and Shen, 2007; Kandasamy et al., 2009). ARP-dependent chromatin remodeling activity regulates the cell cycle and endocycle by directly priming the expression of transcription factors and cell cycle signaling proteins (Meagher et al., 2009). An Arabidopsis null mutant of ARP5 showed a moderately dwarfed phenotype and all organs were smaller than the wild type (Kandasamy et al., 2009). Zhang et al. (2011) performed cDNA-amplified fragment length polymorphism analysis for Chinese pear ‘Nanguoli’ and its bud mutant ‘Da Nanguoli’, whose fruit size is larger than that of ‘Nanguoli’. A gene encoding ARP was identified by cDNA-amplified fragment length polymorphism analysis and quantitative real-time PCR analysis as a candidate gene for the large-fruit mutation and as a differentially expressed gene between ‘Nanguoli’ and ‘Da Nanguoli’. The above observations support the hypothesis that up-regulation of NFR3_23_70 (Actin-related protein 5) contributes to increasing cell size in GLaF fruit.
Up-regulated genes classified into “Cytoskeleton” are listed in Table 5. No down-regulated gene in this category was identified. Many up-regulated genes encode actin or tubulin elements of the cytoskeleton. Cytoskeleton functions in the movement of organelles and chromosomes during cell division. Colchicine inhibits tubulin polymerization and causes DNA reduplication (Eigsti, 1938; Levan, 1938). The occurrence of nuclear fusion, which generates doubled DNA cells, is closely related to the movement of actin filaments (Seguí-Simarro and Nuez, 2008; Shim et al., 2006). Based on these ideas, it may be speculated that up-regulation of genes encoding actin and tubulin genes affects cell division, particularly nuclear division, leading to the DNA reduplication observed in GLaF fruit.
NFR3_23_70 (Actin-related protein 5) and NFR3_14_05 (Protein MOR1) were up-regulated in GLaF and classified into “Cytoskeleton” and “Cell cycle”. NFR3_23_70 (Actin-related protein 5) has been discussed above. The Arabidopsis knockdown mutant of MOR1, the homolog of NFR3_14_05 (Protein MOR1), shows a cytokinesis-defective phenotype in pollen and increases the proportion of DNA reduplicated cells (Twell et al., 2002). NFR3_14_05 (Protein MOR1) was not down-regulated but up-regulated in the GLaF receptacle so that the phenomenon in Arabidopsis MOR1 mutant cannot apply to GLaF. However, it is still possible that a change in NFR3_14_05 (Protein MOR1) expression induced abnormal cell division, leading to the DNA reduplication observed in GLaF.
In this study, we found DNA reduplication of GLaF 1 week before full bloom (Fig. 2) and identified genes expressed differently between GLaF and ‘La France’ in the receptacle 1 week before full bloom. We assumed that the DNA reduplication observed in GLaF fruit is due to endomitosis, rather than endoreduplication. In this connection, the upregulation of NFR3_07_31 (Cyclin-B1-4) whose tobacco homolog was involved in endomitosis (Weingartner et al., 2004), is noteworthy. However, it is possible that a proportion of cells underwent one round of endoreduplication while division competent cells resumed the mitotic cycle at full bloom. Therefore, endoreduplication-related gene homologues such as NFR3_21_11 (Cyclin-A2-3), NFR2_11_50 (CDT1-like protein) and NFR1_18_90 (26S proteasome regulatory subunit 4 homolog A) were also notable.
However, it was also important that a probe for the gene responsible for GLaF mutation was not present on the Japanese pear oligoarray. Therefore, we performed next-generation sequencing analysis on GLaF and ‘La France’ receptacles to identify sequence information and to perform differential gene expression analysis (Nashima et al., submitted). Further studies may shed more light on the DNA reduplication mechanism in GLaF and identify the gene responsible for GLaF mutation.
Literature Cited
- Assaad, F. F. 2001. Plant cytokinesis. Exploring the links. Plant Physiol. 126: 509–516.
- Boudolf, V., T. Lammens, J. Boruc, J. Van Leene, H. Van Den Daele, S. Maes, G. Van Isterdael, E. Russinova, E. Kondorosi, E. Witters, G. De Jaeger, D. Inzé and L. De Veylder. 2009. CDKB1;1 forms a functional complex with CYCA2;3 to suppress endocycle onset. Plant Physiol. 150: 1482–1493.
- Castellano, M. M., M. B. Boniotti, E. Caro, A. Schnittger and C. Gutierrez. 2004. DNA replication licensing affects cell proliferation or endoreplication in a cell type-specific manner. Plant Cell 16: 2380–2393.
- Castellano, M. M., J. C. del Pozo, E. Ramirez-Parra, S. Brown and C. Gutierrez. 2001. Expression and stability of Arabidopsis CDC6 are associated with endoreplication. Plant Cell 13: 2671–2686.
- Chen, M. and X. Shen. 2007. Nuclear actin and actin-related proteins in chromatin dynamics. Curr. Opin. Cell Biol. 19: 326–330.
- Cheniclet, C., W. Y. Rong, M. Causse, N. Frangne, L. Bolling, J. P. Carde and J. P. Renaudin. 2005. Cell expansion and endoreduplication show a large genetic variability in pericarp and contribute strongly to tomato fruit growth. Plant Physiol. 139: 1984–1994.
- Chevalier, C., M. Nafati, E. Mathieu-Rivet, M. Bourdon, N. Frangne, C. Cheniclet, J. P. Renaudin, F. Gévaudant and M. Hernould. 2011. Elucidating the functional role of endoreduplication in tomato fruit development. Ann. Bot. 107: 1159–1169.
- Cong, B., L. S. Barrero and S. D. Tanksley. 2008. Regulatory change in YABBY-like transcription factor led to evolution of extreme fruit size during tomato domestication. Nat. Genet. 40: 800–804.
- Cong, B., J. Liu and S. D. Tanksley. 2002. Natural alleles at a tomato fruit size quantitative trait locus differ by heterochronic regulatory mutations. Proc. Natl. Acad. Sci. USA 99: 13606–13611.
- D’Amato, F. 1984. Role of polyploidy in reproductive organs and tissues. P. 519–566. In: B. M. Johri (ed.). Embryology of Angiosperms. Springer-Verlag, New York.
- Eigsti, O. J. 1938. A cytological study of colchicine effects in the induction of polyploidy in plants. Proc. Natl. Acad. Sci. USA 24: 56–63.
- Endo, M., S. Nakayama, C. Umeda-Hara, N. Ohtsuki, H. Saika, M. Umeda and S. Toki. 2012. CDKB2 is involved in mitosis and DNA damage response in rice. Plant J. 69: 967–977.
- Frary, A., T. C. Nesbitt, S. Grandillo, E. Knaap, B. Cong, J. Liu, J. Meller, R. Elber, K. B. Alpert and S. D. Tanksley. 2000. fw2.2: a quantitative trait locus key to the evolution of tomato fruit size. Science 289: 85–88.
- Furuta, O., T. Imai, T. Miyoshi, N. Yatsumi, S. Ueki, S. Hayashi and S. Hiragi. 1980. Properties of Japanese pear ‘Osa-Nijisseiki’. J. Japan. Soc. Hort. Sci. 49 (Suppl. 2): 70–71 (In Japanese).
- Genschik, P., M. C. Criqui, Y. Parmentier, A. Derevier and J. Fleck. 1998. Cell cycle-dependent proteolysis in plants. Identification of the destruction box pathway and metaphase arrest produced by the proteasome inhibitor mg132. Plant Cell 10: 2063–2076.
- Gonzalez, N., F. Gévaudant, M. Hernould, C. Chevalier and A. Mouras. 2007. The cell cycle-associated protein kinase WEE1 regulates cell size in relation to endoreduplication in developing tomato fruit. Plant J. 51: 642–655.
- Goujon, T., Z. Minic, A. El Amrani, O. Lerouxel, E. Aletti, C. Lapierre, J. P. Joseleau and L. Jouanin. 2003. AtBXL1, a novel higher plant (Arabidopsis thaliana) putative beta-xylosidase gene, is involved in secondary cell wall metabolism and plant development. Plant J. 33: 677–690.
- Haga, N., K. Kato, M. Murase, S. Araki, M. Kubo, T. Demura, K. Suzuki, I. Müller, U. Voss, G. Jürgens and M. Ito. 2007. R1R2R3-Myb proteins positively regulate cytokinesis through activation of KNOLLE transcription in Arabidopsis thaliana. Development 134: 1101–1110.
- Harada, T., W. Kurahashi, M. Yanai, Y. Wakasa and T. Satoh. 2005. Involvement of cell proliferation and cell enlargement in increasing the fruit size of Malus species. Sci. Hortic. 105: 447–456.
- Imai, K. K., Y. Ohashi, T. Tsuge, T. Yoshizumi, M. Matsui, A. Oka and T. Aoyama. 2006. The A-type cyclin CYCA2;3 is a key regulator of ploidy levels in Arabidopsis endoreduplication. Plant Cell 18: 382–396.
- Isuzugawa, K., K. Ikeda and K. Nishimura. 2005. Morphological and ploidy analysis of large fruited mutant in European pear. Breeding Res. 7 (Suppl. 1, 2): 423 (In Japanese).
- Iwata, E., S. Ikeda, S. Matsunaga, M. Kurata, Y. Yoshioka, M. C. Criqui, P. Genschik and M. Ito. 2011. GIGAS CELL1, a novel negative regulator of the anaphase-promoting complex/cyclosome, is required for proper mitotic progression and cell fate determination in Arabidopsis. Plant Cell 12: 4382–4393.
- Joubès, J. and C. Chevalier. 2000. Endoreduplication in higher plants. Plant Mol. Biol. 43: 735–745.
- Jung, S., M. Staton, T. Lee, A. Blenda, R. Svancara, A. Abbott and D. Main. 2008. GDR (Genome Database for Rosaceae): integrated web-database for Rosaceae genomics and genetics data. Nucleic Acids Res. 36: D1034–1040.
- Kandasamy, M. K., E. C. McKinney, R. B. Deal, A. P. Smith and R. B. Meagher. 2009. Arabidopsis actin-related protein ARP5 in multicellular development and DNA repair. Dev. Biol. 335: 22–32.
- Kurepa, J., S. Wang, Y. Li, D. Zaitlin, A. J. Pierce and J. A. Smalle. 2009. Loss of 26S proteasome function leads to increased cell size and decreased cell number in Arabidopsis shoot organs. Plant Physiol. 150: 178–189.
- Larkins, B. A., B. P. Dilkes, R. A. Dante, C. M. Coelho, Y. M. Woo and Y. Liu. 2001. Investigating the hows and whys of DNA endoreduplication. J. Exp. Bot. 52: 183–192.
- Lee, H. S. 2001. Characterization of carotenoids in juice of red navel orange (Cara Cara). J. Agr. Food Chem. 49: 2563–2568.
- Levan, A. 1938. The effect of colchicine on root mitoses of Allium. Hereditas 24: 471–486.
- Liu, Q., A. Zhu, L. Chai, W. Zhou, K. Yu, J. Ding, J. Xu and X. Deng. 2009. Transcriptome analysis of a spontaneous mutant in sweet orange [Citrus sinensis (L.) Osbeck] during fruit development. J. Exp. Bot. 60: 801–813.
- Liu, Q., J. Xu, Y. Liu, X. Zhao, X. Deng, L. Guo and J. Gu. 2007. A novel bud mutation that confers abnormal patterns of lycopene accumulation in sweet orange fruit (Citrus sinensis L. Osbeck). J. Exp. Bot. 58: 4161–4171.
- Malladi, A. and P. M. Hirst. 2010. Increase in fruit size of a spontaneous mutant of ‘Gala’ apple (Malus × domestica Borkh.) is facilitated by altered cell production and enhanced cell size. J. Exp. Bot. 61: 3003–3013.
- Martre, P., N. Bertin, C. Salon and M. Génard. 2011. Modelling the size and composition of fruit, grain and seed by process-based simulation models. New Phytol. 191: 601–618.
- Mathieu-Rivet, E., F. Gévaudant, C. Cheniclet, M. Hernould and C. Chevalier. 2010. The anaphase promoting complex activator CCS52A, a key factor for fruit growth and endoreduplication in tomato. Plant Signal Behav. 5: 985–987.
- Meagher, R. B., M. K. Kandasamy, E. C. McKinney and E. Roy. 2009. Chapter 5. Nuclear actin-related proteins in epigenetic control. Int. Rev. Cell Mol. Biol. 277: 157–215.
- Nakazono, M., F. Qiu, L. A. Borsuk and P. S. Schnable. 2003. Laser-capture microdissection, a tool for the global analysis of gene expression in specific plant cell types: identification of genes expressed differentially in epidermal cells or vascular tissues of maize. Plant Cell 15: 583–596.
- Nelson, T., S. L. Tausta, N. Gandotra and T. Liu. 2006. Laser microdissection of plant tissue: what you see is what you get. Ann. Rev. Plant Biol. 57: 181–201.
- Nishitani, C., T. Shimizu, H. Fujii, F. Hosaka, S. Terakami, Y. Nakamura, A. Itai, A. Yamaguchi-Nakamura and T. Yamamoto. 2010. Oligoarray analysis of gene expression in ripening Japanese pear fruit. Sci. Hortic. 124: 195–203.
- Nishitani, C., T. Shimizu, H. Fujii, S. Terakami and T. Yamamoto. 2009. Analysis of expressed sequence tags from Japanese pear ‘Housui’. Acta Hort. 814: 645–650.
- Nishitani, C., A. Yamaguchi-Nakamura, F. Hosaka, S. Terakami, T. Shimizu, K. Yano, A. Itai, T. Saito and T. Yamamoto. 2012. Parthenocarpic genetic resources and gene expression related to parthenocarpy among four species in pear (Pyrus spp.) Sci. Hortic. 136: 101–109.
- Ruepp, A., A. Zollner, D. Maier, K. Albermann, J. Hani, M. Mokrejs, I. Tetko, U. Güldener, G. Mannhaupt, M. Münsterkötter and H. W. Mewes. 2004. The FunCat, a functional annotation scheme for systematic classification of proteins from whole genomes. Nucleic Acids Res. 32: 5539–5545.
- Sako, K., Y. Maki, K. K. Imai, T. Aoyama, D. B. Goto and J. Yamaguchi. 2010. Control of endoreduplication of trichome by RPT2a, a subunit of the 19S proteasome in Arabidopsis. J. Plant Res. 123: 701–706.
- Seguí-Simarro, J. M. and F. Nuez. 2008. Pathways to doubled haploidy: chromosome doubling during androgenesis. Cytogenet. Genome Res. 120: 358–369.
- Shim, Y. S., K. J. Kasha, E. Simion and J. Letarte. 2006. The relationship between induction of embryogenesis and chromosome doubling in microspore cultures. Protoplasma 228: 79–86.
- Sonoda, Y., K. Sako, Y. Maki, N. Yamazaki, H. Yamamoto, A. Ikeda and J. Yamaguchi. 2009. Regulation of leaf organ size by the Arabidopsis RPT2a 19S proteasome subunit. Plant J. 60: 68–78.
- Takahashi, H., H. Kamakura, Y. Sato, K. Shiono, T. Abiko, N. Tsutsumi, Y. Nagamura, N. K. Nishizawa and M. Nakazono. 2010. A method for obtaining high quality RNA from paraffin sections of plant tissues by laser microdissection. J. Plant Res. 123: 807–813.
- Tanksley, S. D. 2004. The genetic, developmental, and molecular bases of fruit size and shape variation in tomato. Plant Cell 16: 181–189.
- Twell, D., S. K. Park, T. J. Hawkins, D. Schubert, R. Schmidt, A. Smertenko and P. J. Hussey. 2002. MOR1/GEM1 has an essential role in the plant-specific cytokinetic phragmoplast. Nat. Cell Biol. 4: 711–714.
- Weingartner, M., M. C. Criqui, T. Mészáros, P. Binarova, A. C. Schmit, A. Helfer, A. Derevier, M. Erhardt, L. Bögre and P. Genschik. 2004. Expression of a nondegradable cyclin B1 affects plant development and leads to endomitosis by inhibiting the formation of a phragmoplast. Plant Cell 16: 643–657.
- Wu, J., Z. Wang, Z. Shi, S. Zhang, R. Ming, S. Zhu, M. A. Khan, S. Tao, S. S. Korban, H. Wang, N. J. Chen, T. Nishio, X. Xu, L. Cong, K. Qi, X. Huang, Y. Wang, X. Zhao, J. Wu, C. Deng, C. Gou, W. Zhou, H. Yin, G. Qin, Y. Sha, Y. Tao, H. Chen, Y. Yang, Y. Song, D. Zhan, J. Wang, L. Li, M. Dai, C. Gu, Y. Wang, D. Shi, X. Wang, H. Zhang, L. Zeng, D. Zheng, C. Wang, M. Chen, G. Wang, L. Xie, V. Sovero, S. Sha, W. Huang, S. Zhang, M. Zhang, J. Sun, L. Xu, Y. L, X. Liu, Q. Li, J. Shen, J. Wang, R. E. Paull, J. L. Bennetzen, J. Wang and S. Zhang. 2012. The genome of the pear (Pyrus bretschneideri Rehd.). Genome Res. 23: 396–408.
- Xu, Q., Y. Liu, A. Zhu, X. Wu, J. Ye, K. Yu, W. Guo and X. Deng. 2010. Discovery and comparative profiling of microRNAs in a sweet orange red-flesh mutant and its wild type. BMC Genomics 11: 246.
- Xu, Q., K. Yu, A. Zhu, J. Ye, Q. Liu, J. Zhang and X. Deng. 2009. Comparative transcripts profiling reveals new insight into molecular processes regulating lycopene accumulation in a sweet orange (Citrus sinensis) red-flesh mutant. BMC Genomics 10: 540.
- Yoshioka, S., T. Matsuda, K. Kotobuki, T. Sanada and Y. Ito. 1999. Gamma-ray-induced mutation breeding in fruit trees: Breeding of mutant cultivars resistant to black spot disease in Japanese pear. Jarq-Jpn. Agr. Res. Q. 33: 227–234.
- Zhang, S. J., J. Wu, H. Chen, C. Gu, S. T. Tao, J. Y. Wu and S. L. Zhang. 2011. Identification of differentially expressed genes in a spontaneous mutant of ‘Nanguoli’ pear (Pyrus ussuriensis Maxim) with large fruit. J. Hort Sci. Biotech. 86: 595–602.