2014 年 83 巻 2 号 p. 142-148
2014 年 83 巻 2 号 p. 142-148
This study analyzes and clarifies the eco-physiological traits of high-yielding strawberry (Fragaria ×ananassa Duch.) ‘Benihoppe’ focusing on its dry matter production, total nitrogen content, bleeding rate, mineral nutrient concentration in bleeding fluid, and fine root respiration rate in comparison with those of the cultivar ‘Sachinoka’. The results show that ‘Benihoppe’ has three beneficial characteristics. The first characteristic is higher primary and fine root dry matter and less root rotting during the cold season. The second is superior root function that includes high root activity and a well-maintained root system. The third is a high ability to absorb more NO3− and preferentially partition NO3− to the leaves, crowns, and fine roots. Thus, with these three beneficial functions, ‘Benihoppe’ may produce more dry matter and fruits than ‘Sachinoka’.
During the past five years, strawberry (Fragaria × ananassa) yield and its planted area have continued to decrease (Morishita, 2011). Furthermore, due to oil price hikes, farmers have not been able to sufficiently heat their greenhouses in winter, which has disadvantaged their strawberry production; therefore, it is necessary to breed new cultivars with higher-yielding capacity under unfavorable conditions. A strawberry cultivar, ‘Benihoppe’, which is a progeny resulting from a cross between ‘Akihime’ and ‘Sachinoka’, was bred in Shizuoka Prefecture in 2002. Takeuchi et al. (1999) described that ‘Benihoppe’ possesses the special trait of producing more than 600 grams of fruit per plant per season, and its fruit yield is higher than parental cultivars. Therefore, it may be useful breeding material for future variety improvement programs. However, so far there has been no report on the eco-physiological analysis of the high-yielding capacity of ‘Benihoppe’.
Therefore, we analyzed the high-yielding strawberry cultivar ‘Benihoppe’, focusing on its dry matter production, growth analysis, and leaf photosynthetic rate compared to ‘Toyonoka’, which is not a high-yielding cultivar, in an effort to breed a high-yielding strawberry for the future (Mochizuki et al., 2013). Our results suggested that total dry matter is more important than the harvest index for producing high-yielding ‘Benihoppe’ plants. Efficient dry matter production in the critical short-day and cold-weather season is associated with this cultivar’s large leaf area index and upright petioles, which allow solar radiation to penetrate the plant canopy. In addition, decreasing root dry matter in ‘Benihoppe’ was not statistically different from ‘Toyonoka’, although it was lower than that of ‘Toyonoka’. Furthermore, we observed that ‘Benihoppe’ had more bleeding from leaf hydropores than other cultivars during the cold season. Therefore, since ‘Benihoppe’ seems to possess very active roots that can absorb water under such conditions, analysis of high-yielding ‘Benihoppe’ strawberry plants focusing not only on seasonal changes of root dry matter but also on root function is needed.
Generally, the mechanisms involved in water absorption fall into two patterns, active and passive water absorption (Kramaer and Boyer, 1995). Active water absorption occurs in slowly transpiring plants and is based on root pressure, while passive water absorption occurs in rapidly transpiring plants. Therefore, the phenomenon observed when bleeding occurs from leaf hydropores is considered to be caused by high root pressure. Water uptake affects the ion concentration gradient created between root tips and soil when plants actively absorb ions into their xylem (Schurr, 1998). Root activity can be evaluated by measuring the bleeding rate based on active water absorption (Morita et al., 2000); thus, the bleeding rate of many crops, e.g. rice and soybean, has been evaluated to determine root activity (Ansari et al., 2004; Ma et al., 2005; Yamaguchi et al., 1995). In particular, the high-yielding rice cultivar ‘Akenohoshi’ has been reported to possess special traits that result in high yield, such as having little resistance to water flow in roots, a strong ability to absorb water, and being capable of decreasing the photosynthetic rate of leaves during leaf senescence (Jiang et al., 1988). In addition, root activity can be evaluated by measuring plant hormones and ion concentrations in bleeding fluids in cucumber (Tachibana, 1988), eggplant (Kato and Lou, 1989), watermelon (Yamasaki et al., 1994), and sweet potato (Sugiyama, 1989); thus, bleeding seems to affect root activity and plant vigor.
Root activity can also be evaluated while measuring the root respiration rate because active water absorption occurs when roots are respiring rapidly (Kotula and Steudle, 2009). Plants actively absorb nutrients against an ion concentration gradient between the root tips and soil. The ATP energy used to absorb nutrients from the soil is produced by root respiration. Therefore, some factors need to be measured, e.g. bleeding rate, ion concentrations in bleeding fluids, and the root respiration rate, when evaluating root activities in strawberry. This study clarifies the eco-physiological traits of high-yielding ‘Benihoppe’ by focusing on its root dry matter, nitrogen content in plants, bleeding rate, ion concentrations in bleeding fluid, and fine root respiration rate in comparison with ‘Sachinoka’.
In this study, two strawberry cultivars were used, namely the high-yielding ‘Benihoppe’, and ‘Sachinoka’ as the contrast cultivar. Their growing season is 6 months from the end of September to the end of March. This research was conducted for 2 years from 2010 to 2011. At the end of August, plantlets were cut from mother stocks and planted in the nursery. At the end of September, young plants were transferred to containers (29 cm width × 75 cm length × 23.5 cm depth; 28 liter volume), 25 cm apart in rows and with 15 cm interrow spaces. Planting density was 4.4 plants·m−2. Plants were grown by forcing culture in the greenhouse at Tokyo University of Agriculture and Technology, Fuchu, Tokyo, Japan. A mixture of Kanuma soil (weathered pumice stone), vermiculite, and peatmoss in a 1:1:1 volumetric ratio was used as soil substrate, with caustic lime as base fertilizer at the rate of 2 grams per 10 liters of soil. The strawberry plants were fertigated with 50% Otsuka-A Nutrient Solution (Otsuka AgriTechno Co. Ltd., Tokyo, Japan), with electric conductivity (EC) of 0.6 mS·cm−1 before anthesis and EC 1.2 mS·cm−1 in the harvesting period and pH 6.0, at a drain rate of approximately 30%. In the middle of October, the side windows of the greenhouse were closed for warmth, and fluorescent light was supplied from 17:00 to 21:00 and from 1:00 to 2:00. At the beginning of November, plastic curtains were drawn and a heater was installed to maintain the minimum temperature in the greenhouse at 5°C.
1. Changes in total dry matter and root dry matter in strawberry plantsDestructive sampling was conducted for ‘Benihoppe’ and ‘Sachinoka’ plants in the 2010 growing season. Five plants each of ‘Benihoppe’ and ‘Sachinoka’ were harvested on September 27, November 28, December 23, and February 13. Plants were separated into primary roots, fine roots, crowns, petioles, leaves, peduncles, and fruits after washing roots. Sieves with 3.0 and 0.3 mm mesh were used to separate the roots from the soil substrate. The soil substrate was removed from the sieves by flushing with water, and the roots remaining on the 3.0 mm mesh were collected using tweezers. In this study, we divided the roots into two groups; the primary root from the crown and the fine root from the primary root. This procedure took 3 to 5 hours per plant, and was performed alternately for each cultivar. Thereafter, they were dried at 80°C for 72 hours with a circulation drier and weighed after cooling to room temperature in a desiccator.
2. Changes in total nitrogen content in each plant partTotal nitrogen content of dry matter from the sample plants was measured. Dry matter from each plant part was ground using a grinding machine (Power grinder T-351; King Tool Co. Ltd., Taipei, Republic of China). A 250 mg fine sample was used for measuring total nitrogen content using an NC analyzer (NC-220F; Fujiwara Scientific Co. Ltd., Tokyo, Japan). Measuring was conducted twice for multiple samples of each plant part from each plant.
3. Changes in bleeding rateFive plants each of ‘Benihoppe’ and ‘Sachinoka’ were measured for their bleeding rate on September 27, November 28, December 23, and February 13. Each plant was cut immediately above the junction of the shoot and root systems, cotton was placed on the cut end, and this was covered by plastic film at 6:00. At 11:00, the cotton was weighed to measure the bleeding rate.
4. Comparison of mineral nutrient concentrations in bleeding fluidOn November 26, 2011, three plants each of ‘Benihoppe’ and ‘Sachinoka’ were used for measuring the mineral nutrient concentrations in the bleeding fluid using an ion chromatograph system (HIC-SP; Shimadzu Co. Ltd., Kyoto, Japan). Cation levels were measured under baseline conditions in a Shim-pack IC-C4 analytical column (Shimadzu Co. Ltd.), guard column Shim-pack IC-GC3 II (Shimadzu Co. Ltd.), dilution rate 50-fold, mobile phase 2.5 mM oxalic acid, flow 1.0 mL·min−1, and oven temperature 40°C. Anion levels were measured under baseline conditions in a Shim-pack IC-SA2 analytical column (Shimadzu Co. Ltd.), guard column Shim-pack IC-SA2G (Shimadzu Co. Ltd.), dilution rate 500-fold, mobile phase 12 mM NaHCO3/0.6 mM Na2CO3, flow 1.0 mL·min−1, and oven temperature 30°C.
5. Comparison of fine root respiration rateThe fine root respiration rate was measured for nine plants each of ‘Benihoppe’ and ‘Sachinoka’ using an oxygen electrode (Oxytherm; Hansateck Co. Ltd., Norfolk, UK) on December 10, 2010 and February 17, 2011. After collecting fine roots from plants as above, they were dabbed with a paper towel. From each plant, 50 mg FW (acceptable range was 49.9–50.1 mg) of healthy fine roots were put into the chamber of the oxygen electrode. Two minutes later the dissolved oxygen level was recorded for 1 min as root respiration.
No difference in total dry matter was found between ‘Benihoppe’ and ‘Sachinoka’ on September 27, while on and after November 28, total dry matter of ‘Benihoppe’ was higher than that of ‘Sachinoka’ (Fig. 1A). Also, total root dry matter of ‘Benihoppe’ was lower than that of ‘Sachinoka’ on September 27, and no significant difference was observed between these two cultivars in root dry matter on November 28 and December 23 (Fig. 1B). However, total root dry matter of ‘Benihoppe’ was significantly higher than that of ‘Sachinoka’ on February 13. Although primary root dry matter of ‘Benihoppe’ was lower than that of ‘Sachinoka’ from September 27 to December 23, there was no difference in primary root dry matter between two cultivars on February 13 (Fig. 1C). In addition, fine root dry matter of ‘Benihoppe’ was higher than that of ‘Sachinoka’ after December 23 (Fig. 1D). Therefore, increasing the total root dry matter of ‘Benihoppe’ during the winter season from December 23 to February 13 was reflected in the increased primary and fine root dry matter, while in ‘Sachinoka’, total root dry matter did not increase during that time. In particular, some roots of ‘Sachinoka’ had rotted by February 13 (Fig. 2).

Changes in total dry matter (A), total root dry matter (B), primary root dry matter (C), and fine root drymatter (D) between ‘Benihoppe’ and ‘Sachinoka’ during the whole growing season (2010). Data are the mean (± SD) of five independent measurements. **, * and NS: significance at P = 0.01, 0.05 and non-significance by t-test.

Comparison of root system in ‘Benihoppe’ and ‘Sachinoka’. A: ‘Sachinoka’, B: ‘Benihoppe’ (2010/02/13). Bars = 5 cm. Primary root came out of the crown. Fine root came out of the primary root.
No significant difference in total nitrogen content between ‘Benihoppe’ and ‘Sachinoka’ was observed on September 27 because of nitrogen discontinuation for flower bud induction (Table 1). However, total nitrogen contents in leaf, crown, and fine root of ‘Benihoppe’ were higher than those of ‘Sachinoka’ after November 28
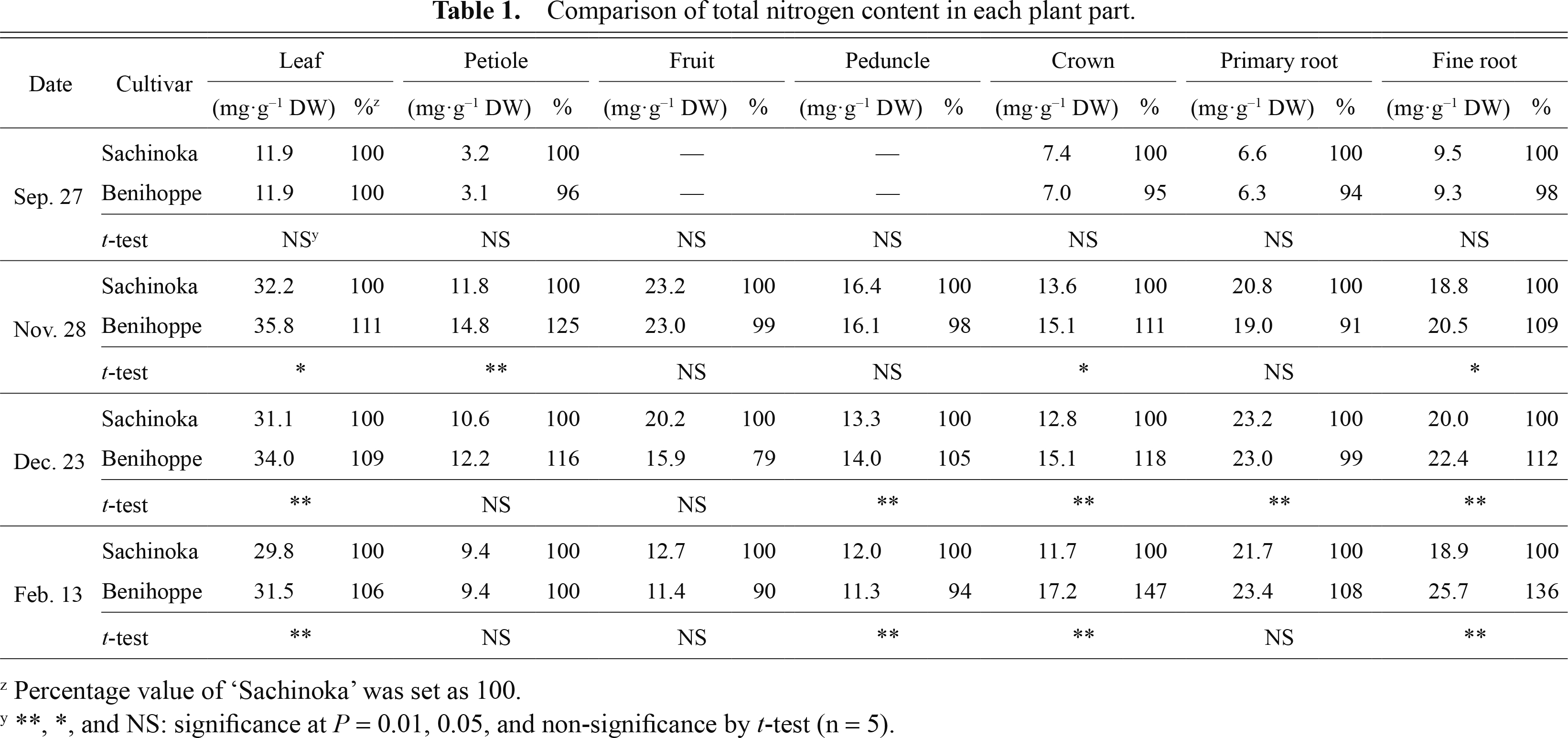
Comparison of total nitrogen content in each plant part.
Except for September 27, the bleeding rate of ‘Benihoppe’ was higher than that of ‘Sachinoka’ during the entire growing season (Fig. 3). In particular, the bleeding rate of ‘Sachinoka’ decreased from 0.31 g·hr−1 on December 23 to 0.10 g·hr−1 on February 13, while that of ‘Benihoppe’ increased from 0.49 g·hr−1 to 0.66 g·hr−1. Although the bleeding rates per unit of fine root dry matter from September 27 to December 23 of ‘Benihoppe’ ranged between 0.31 g·hr−1·g−1 DW and0.35 g·hr−1·g−1 DW, those of ‘Sachinoka’ ranged between 0.08 g·hr−1·g−1 DW and 0.14 g·hr−1·g−1 DW, which was about half the mass of ‘Benihoppe’ (Fig. 4). In addition, the bleeding rate per unit of fine root dry matter in ‘Benihoppe’ and ‘Sachinoka’ decreased 21% and 54%, respectively, on February 13.

Comparison of bleeding rate between ‘Benihoppe’ and ‘Sachinoka’ during the whole growing season. Data are the mean (± SD) of five independent measurements. ** and NS: significance at P = 0.01 and non-significance by t-test.
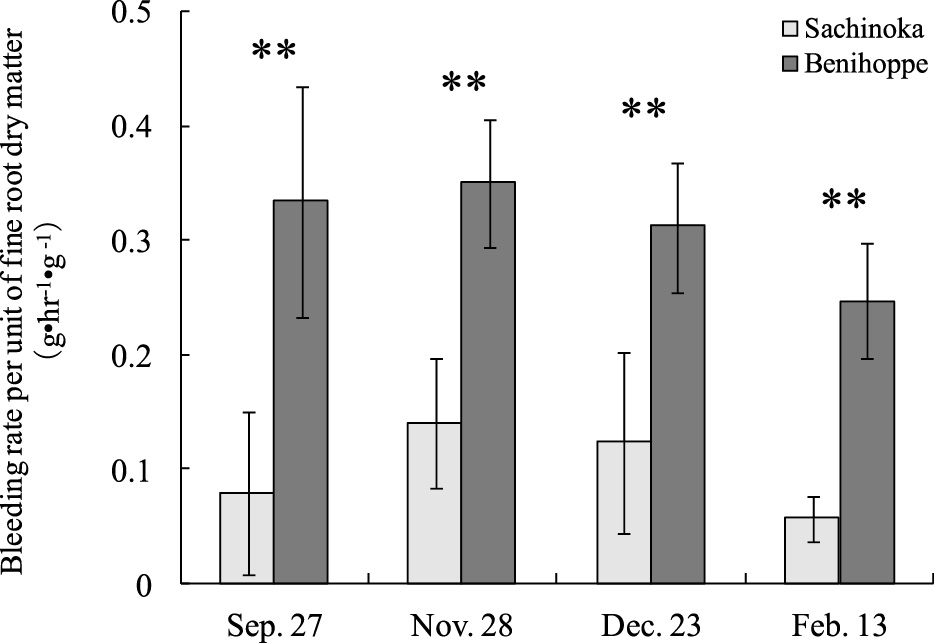
Comparison of bleeding rate per unit of fine root dry matter between ‘Benihoppe’ and ‘Sachinoka’ during the whole growing season. Data are the mean (± SD) of five independent measurements. **: significance at P = 0.01 by t-test.
For ‘Benihoppe’, the NO3−, PO43−, K+, and Ca2+ concentrations in bleeding were 949.9 mg·L−1, 352.4 mg·L−1, 3198.4 mg·L−1, and 284.4 mg·L−1, respectively, and for ‘Sachinoka’, the concentrations were 686.2 mg·L−1, 414.7 mg·L−1, 2683.0 mg·L−1, and 209.5 mg·L−1, respectively (Table 2). NO3−, K+, and Ca2+ concentrations in bleeding fluid of ‘Benihoppe’ were significantly higher than those of ‘Sachinoka’.

Comparison of mineral concentrations in bleeding fluid between ‘Benihoppe’ and ‘Sachinoka’.
The fine root respiration rate of ‘Benihoppe’ on December 10 and February 17 was 5.8 nmol O2·g−1 FW·s−1 and 5.2 nmol O2·g−1 FW·s−1, respectively, while that of ‘Sachinoka’ was 3.2 nmol O2·g−1 FW·s−1 and 2.6 nmol O2·g−1 FW·s−1, respectively (Fig. 5). Therefore, the fine root respiration rate of ‘Benihoppe’ was significantly higher than that of ‘Sachinoka’.
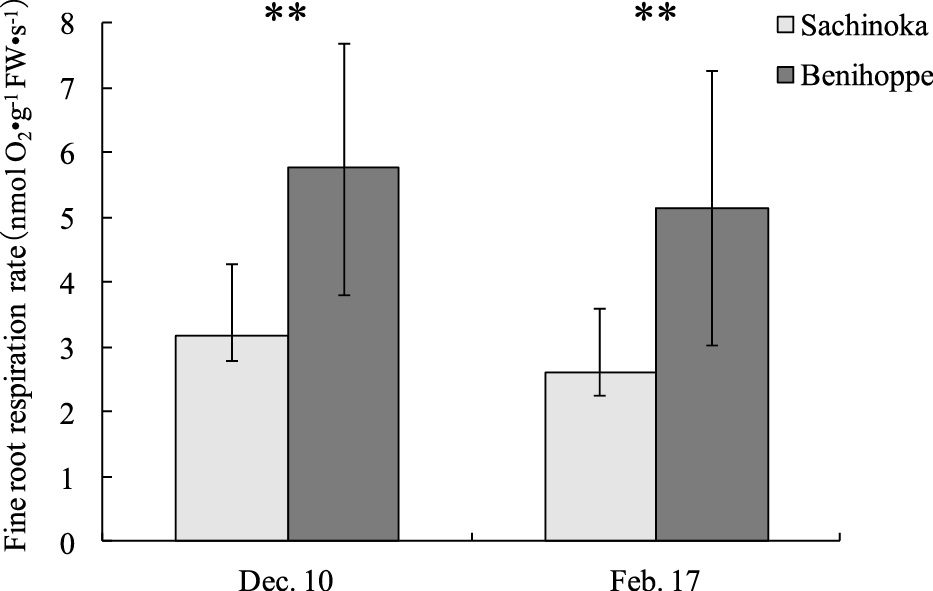
Comparison of fine root respiration rate between ‘Benihoppe’ and ‘Sachinoka’. Data are the mean (± SD) of nine independent measurements. **: significance at P = 0.01 by t-test.
Total dry matter of ‘Benihoppe’ was higher than that of ‘Sachinoka’ on and after November 28 (Fig. 1A), showing similar results to our previous study (Mochizuki et al., 2013). In addition, primary root dry matter of ‘Sachinoka’ was higher than that of ‘Benihoppe’ from September 27 to December 23 (Fig. 1C), while fine root dry matter of ‘Benihoppe’ was higher than that of ‘Sachinoka’ on December 23 (Fig. 1D). Furthermore, since ‘Sachinoka’ experienced a higher amount of root rot than ‘Benihoppe’ on February 13 (Fig. 2), total root dry matter and fine root dry matter of ‘Benihoppe’ were higher than those of ‘Sachinoka’ on February 13 (Fig. 1B, D); this showed that the root amount of these cultivars was different.
Total nitrogen content was measured for both cultivars to clarify which plant parts accumulate nitrogen, which is absorbed from roots. Nitrogen content in leaf, crown, and fine root of ‘Benihoppe’ was higher than that of ‘Sachinoka’ (Table 1). Marked differences in nitrogen content between these two cultivars appeared on February 13 (Table 1). Nagashima and Sada (1982) measured the seasonal changes of oxygen and nitrogen absorption in strawberry roots. In this study, there was the same tendency between seasonal changes of oxygen and nitrogen absorption, and there was a positive correlation between oxygen and nitrogen absorption. In addition, some researchers studying rice plants reported that nitrogen content in plants was correlated with the nitrogen concentration in bleeding (Sakaigaichi et al., 2007). Nitrogen absorption varies by variety (Nagahata and Kuroda, 2004), and high-yielding rice cultivars were able to absorb more nitrogen than contrasting cultivars in the tillering stage (Higuchi and Yoshino, 1986). Based on these reports, it may be that ‘Benihoppe’ is able to absorb more nitrogen in cold winter weather than ‘Sachinoka’, enabling it to increase more biomass. Nitrogen content in the leaf, crown, and fine root of ‘Benihoppe’ was higher than that of ‘Sachinoka’ (Table 1) suggesting that the higher nitrogen content of ‘Benihoppe’ may suppress leaf and root senescence, helping the plants to absorb nitrogen during cold winter weather.
We thus measured the bleeding rate and ion concentrations in bleeding fluid to evaluate the ability of the plants to actively absorb water. On and after November 28, the bleeding rate of ‘Benihoppe’ was higher than that of ‘Sachinoka’ (Fig. 3). The bleeding rate per fine root dry matter as well as the NO3− concentration in bleeding from ‘Benihoppe’ was also higher than that of ‘Sachinoka’ (Fig. 4; Table 2). Morita et al. (2000) reported a significant correlation exists between root dry matter and the bleeding rate in maize during the growing season. Furthermore, in rice (Kusutani et al., 2000), tomato (Nakano et al., 2007), and eggplant (Kato and Lou, 1989), the bleeding rates of high-yielding cultivars were higher than those of nomal cultivars used for comparison. Moreover, Yamasaki et al. (1994) grafted water-melon onto bottle gourd and squash and later measured the bleeding rate and ion concentrations in bleeding fluid, finding that, in the watermelon/squash plants, the NO3−, K+, Mg2+, and cytokinin concentrations in bleeding fluids were higher than those of the watermelon/bottle gourd plants and also of non-grafted seedlings throughout the growing season, allowing the watermelon/squash plants to produce a heavier crop load. Considering these reports and our results, ‘Benihoppe’ possessed a strong ability to absorb nutrients and could partition more nitrogen to each plant part, giving it nutritional superiority with its high nitrogen content in each plant part and increased plant vigor.
Also, in rice, high-yielding cultivars have unique traits, such as lower leaf senescence and high leaf photosynthetic rates during the ripening stage that allows the plants to translocate more cytokinin to their stems and leaves (Soejima, 2000). In our previous study, no difference was observed between the maximum leaf photosynthetic rates of ‘Benihoppe’ and ‘Toyonoka’ under various environmental conditions (Mochizuki et al., 2013). Oda and Yanagi (1990) reported no difference among Japanese, American, and European strawberry cultivars in the leaf photosynthetic rate. In addition, in Japan, a limited number of parent cultivars, including ‘Fukuba’, have been repeatedly used for strawberry breeding (Inaba and Yoshida, 2006). Therefore, little difference in the leaf photosynthetic rate is inherited among new strawberry cultivars. However, the leaf senescence rate was not studied in these reports. From these reports, since ‘Benihoppe’ possesses high root activity, it may be able to produce more plant hormones such as cytokinin. Therefore, it seems logical that the highly maintained leaf photosynthetic rate in ‘Benihoppe’ allows it to produce more assimilation products than ‘Sachinoka’. These traits may improve its ability to produce high yields, so further studies focusing on leaf senescence and the plant hormones found in bleeding fluids are needed.
The fine root respiration rate of ‘Benihoppe’ was higher than that of ‘Sachinoka’ (Fig. 5). Blanke and Cooke (2000) reported that very bioactive parts of strawberry plants actively respire. Some genes regulate nitrate transporters that allow plants to absorb nitrogen into their roots (Krouk et al., 2010) and require ATP to absorb and reduce nitrates (Crawford, 1995). Based on these reports, it seems that the roots of ‘Benihoppe,’ which have a high respiration rate and actively absorb water and nutrients from soil, are more effective at absorbing nutrients than roots of ‘Sachinoka’.
In conclusion, we believe that studies of the high-yielding ability of ‘Benihoppe’ should focus on roots as described below. ‘Benihoppe’ develops fine roots more vigorously than ‘Sachinoka’. The fine roots of ‘Benihoppe’ possess a strong ability to actively absorb water during cold winter weather and this trait allows the plants to partition more nitrogen to various plant parts. In particular, a strong ability of plants to partition nitrogen to leaves may help the plants assimilate photosynthetic components such as chlorophyll and maintain a high level of photosynthetic activity. Therefore, these traits can more efficiently produce dry matter and sustain high yields.