2014 年 83 巻 3 号 p. 222-228
2014 年 83 巻 3 号 p. 222-228
In order to improve the use efficiency of a cover crop, hairy vetch (Vicia villosa R., HV), and supplemental chemical N fertilizer, N release and uptake patterns from HV, fast-release N fertilizer (Fast), and slow-release N fertilizer (Slow) in fresh market tomato (Solanum lycopersicum L.) production were investigated using the 15N-labeling method. In the incubation of soil-added N at two mix rates, 20% Fast + 80% Slow (FS) and 100% Slow (S), a large amount of inorganic N, mainly NH4+-N, was released by FS in 4 weeks. Tomato ‘House momotaro’ was grown in 1/2000 a Wagner pots incorporating such N fertilizer and 15N-labeled HV residue (30 g DW/pot, about 200 kg N·ha−1). Plant biomass in tomato grown with HV was larger than that grown without HV. HV-derived N (Ndfhv) was taken up by the tomatoes mainly until 4 weeks after transplant (WAT). The uptake amount of Ndfhv was the same in the pot with HV-FS and HV-S. The rate of N uptake derived from HV to total N uptake in tomato plants (%Ndfhv) was 43% in HV-S, higher than that in HV-FS (34%) in 4 WAT; however, such a difference disappeared after 4 WAT. N uptake by tomato plants continued until 12 WAT. Based on these results, HV acted as a fast-release fertilizer. There was competition in N uptake between chemical fertilizer N and HV-released N in the early stage of tomato cultivation. A large amount of chemical fertilizer tended to suppress the uptake of Ndfhv. N uptake by tomato plant continued until the late stage. These results can be applied to establish a suitable combination of HV and chemical fertilizer for tomato production.
Vegetable crops are usually harvested in their growing period. In intensive greenhouse vegetable production, a large amount of nutrients, mainly N, have to be applied in order to maintain vigorous plant growth and to gain sufficient yield. However, excessive N input causes extra N accumulation in the soil (Hao et al., 2009), resulting in environmental pollution, such as nitrate leaching and greenhouse gas emission (McNeal et al., 1995). For sustainable greenhouse production, reduction of fertilizer addition and proper application are required. A large quantity of fertilizer may not necessarily increase the yield of some crops (Abdul-Baki et al., 1997; Elia and Conversa, 2012).
In crop production, fertilizer management systems have been developed for proper N application in Japan. For tomato production, additional fertilizer dressing combined with the diagnosis of nutrient conditions is often carried out in production areas (Sakaguchi et al., 2004). On the other hand, slow-release fertilizer is sometimes applied at the beginning of tomato production to reduce the labor required for fertilizing. These fertilizer management methods contribute to proper nutrient application in tomato production.
However, for sustainable tomato production in greenhouses or plastic houses, soil properties including physical and biological conditions have to be improved step by step in addition to proper N fertilization. Planting a cover crop is one of the biological tools used for sustainable crop production, which can not only supply N to the main crop (Ruffo and Bollero, 2003), but also has advantages such as increasing organic soil carbon (Araki et al., 2009), thereby improving soil physical properties (Blevins and Frye, 1993), and so on. Among the various kinds of cover crops, hairy vetch (Vicia villosa R.; HV), a legume crop, is reported to be an important species with various advantages, such as abundant N supply due to its effective biological nitrogen fixation, adaptability to low temperatures, resistance to pests, delaying senescence, covering ground surface effectively, and fitness for vegetable production, particularly in rotation with tomatoes (Acosta et al., 2011; Kumar et al., 2005; Seo et al., 2006). Araki et al. (2009) also reported that utilizing HV could reduce chemical N fertilizer addition to half of the recommended amount without yield reduction in tomato production. These results showed that HV has potential as an alternative to chemical N fertilizers.
The N release pattern from organic fertilizer has to be synchronized with crop demands for proper application when organic fertilizer is planned as a substitute for chemical fertilizer (Kramer et al., 2002). Yaffa et al. (2000) have shown the synchronization of tomato N uptake and N release from cover crops; tomato N uptake increased after the increase of soil inorganic N, followed by a decline. On the other hand, Kumar et al. (2001) reported that because N release from white clover residues was faster under rotary hoeing treatment, it was not synchronized with the N demand of wheat during its early growth period and resulted in minimum N benefits. In the present paper, our aim is to clarify the N supply pattern when HV is applied with chemical fertilizer to establish a fertilizer control method in tomato production.
The 15N-labeling method is suitable for the investigation of crop N uptake derived from cover crops. Earlier studies found that N uptake by subsequent crops such as tomato, rice etc., was 6–56% of N applied by 15N-labeled cover crops such as HV and ryegrass (Asagi and Ueno, 2009; Bergström and Kirchmann, 2004; Doane et al., 2009; Harris et al., 1994; Seo et al., 2006; Thönnissen et al., 2000a), and the efficiency differed depending on the subsequent crop species, cover crop species, or cultivation conditions.
In our previous study using the 15N-labeling method (Sugihara et al., 2013), it was clarified that HV-derived N was taken up by tomato plants mainly until 4 weeks after transplant in the early growth period and was more effective when the chemical N fertilizer was reduced in tomato cultivation. In the present paper, when HV was incorporated into soil with or without fast-release N fertilizer, the dynamics of N derived from HV was investigated by pot examination. These results can be used to design the optimum combination of a chemical fertilizer and cover crop.
This experiment was conducted at the Experimental Farm, Field Science Center for Northern Biosphere, Hokkaido University, Sapporo, Japan in 2012 and included 2 investigations: 1) soil incubation experiment; investigation of N release patterns from fast-release N fertilizer and slow-release N fertilizer in a laboratory experiment, and 2) tomato cultivation experiment; investigation of N dynamics in tomato-planted pots administered chemical N fertilizer and HV residue in plastic houses. In the latter experiment, to evaluate the content of N derived from HV mineralization and from soil and chemical N fertilizer in the soil of the plot, tomato-free pots were also prepared. The soil of the Experimental Farm, Hokkaido University, was used in the present examination. The used soil was Andisol and its texture was sandy loam (SL) containing 4.4 mg NH4+-N, 0.6 mg NO3−-N, 149 mg K2O and 124 mg P2O5 (Bray No. 2 method) per 100 mg dry soil in a soil analysis before the examination. The content of soil carbon and N was 3.5% and 0.3%, respectively.
1) Soil incubation experiment in laboratoryIn order to check the N release patterns from fast-release N fertilizer (Fast) (Ammonium sulfate; JFE Chemical Corporation, Tokyo, Japan) and slow-release N fertilizer (Slow) (LP-S100 40%-N; JCAM AGRI. Co., Ltd., Tokyo, Japan), a soil incubation experiment was conducted in an incubator. Ten grams of dried soil sample was placed in a 100 mL plastic bottle, and then N fertilizer was applied. Three treatments were prepared: (1) Fast + Slow: nitrogen fertilizer applied at a rate of 166 mg/bottle (240 kg N·ha−1) containing Fast for 20% of total N (33 mg/bottle) and Slow for 80% of total N (133 mg/bottle), (2) Slow-only: 166 mg/bottle containing 100% Slow, and (3) N0: only soil without any fertilizer (Table 1). After fertilization, distilled water was added up to 60% of maximum water-holding capacity and the bottles were capped loosely to provide air. Incubation was performed at 25°C. Before incubation, the total weight of the bottle was measured, and if it decreased during the incubation period, distilled water was added once a week. Incubated soil was collected after 1, 2, and 4 weeks’ incubation with 5 replications, and dried and sieved through a 2 mm mesh. Soil inorganic N was determined by a soil analyzer (ZA-II; National Federation of Agricultural Cooperative Associations, Tokyo, Japan). NH4+-N and NO3−-N were measured by the indophenol method and alkali reduction-diazotization method, respectively.

Experiment design.
Seeds of hairy vetch (‘Mamesuke’; Snow Brand Seed Co., Ltd., Hokkaido, Japan) were sown in sowing boxes (42 × 66 × 21 cm) on March 10, 2012 and grown in a plastic house. After germination, 100 mL of 15NH4Cl (98 atom%, 0.5 g·L−1) was added to each sowing box every week, for a total of 10 times. The above-ground and underground biomasses were harvested at the flowering stage on May 31, 2012. Roots were washed under running water to remove the soil, and thin roots were collected by a net with small mesh. Shoots and roots of HV were separated, dried naturally, and chopped into 5 cm pieces.
HV and fertilizer applicationN concentrations in HV shoots and roots were measured as 3.56% and 2.37%, and the applied dry weights (DW) were 25 g and 5 g, respectively; therefore, the total amount of N in the HV added to the Wagner pots was 1008 mg per pot (ca 200 kg N·ha−1). The N isotopic ratio of shoots and roots in HV was 0.91 and 0.77 atom% excess, respectively. The isotopic ratio of total HV, mixed shoots and roots was considered to be 0.89 atom% excess, as calculated from the ratio of N content in shoots and roots.
The tomatoes were cultivated in a 1/2000 a Wagner pot containing Andisol soil supplemented with HV and chemical fertilizer. There were 3 main sources of nitrogen in this experiment: chemical fertilizer, soil, and HV. Dried 5 cm pieces of 15N-labeled HV were incorporated into each pot containing 240 kg N·ha−1 chemical fertilizer. Fast-release fertilizer (Fast) and slow-release fertilizer (Slow) were used as N fertilizer and two mix rates of these N fertilizers were prepared, 20% Fast + 80% Slow (FS) or 100% Slow (S), by the presence and absence of Fast. As a control, 240 kg N·ha−1 of N fertilizer treatment without HV was prepared (Control: C). Thus, four treatments, presence and absence of HV and Fast (HVFS, HV-S, C-FS, C-S), were prepared, and the effects of fast-release fertilizer utilization on HV-derived N uptake by tomatoes were investigated. This experiment was carried out with 5 replications of each treatment, so 20 pots were prepared for each treatment, except for the control, to collect whole tomato plants 4 times (details described later).
Tomato cultivationSeeds of a fresh marketable tomato ‘House Momotaro’ (Takii & Co., Ltd., Kyoto, Japan) were sown in sowing boxes in a plastic house on March 26, 2012, and seedlings were grafted with a root stock cultivar ‘Anchor T’ (Takii & Co., Ltd.) on April 19, 2012. The grafted seedlings were transferred into plastic pots, 12 cm in diameter. These seedlings were transplanted into a 1/2000 a Wagner pot on June 8, 2012. Before tomato transplanting, 15N-labeled HV was incorporated into the soil in each pot except for the C-FS and C-S treatment. In every plot, 200 kg P2O5 by fused magnesium phosphate (Hinode Chemical Industry Co., Ltd., Kyoto, Japan) and 200 kg K2O by potassium sulfate (Hokuren Federation of Agricultural Cooperative Associations, Hokkaido, Japan) were added per ha. Watering was conducted so as not to dry the soil surface. Weeds were removed by hand. Pesticides and fungicides were applied 4 times. Fruits were collected from 1 to 6 fruits clusters, and only 3 fruits were allowed to set in each fruit cluster.
In order to estimate the N release pattern from HV, fertilizer, or soil under the cultivation conditions, another 5 pots were prepared in the same way without transplanting tomatoes (tomato-free pots) in HV-FS and HV-S, respectively (Table 1). These tomato-free pots were placed in the same plastic house as tomato-planted pots, and watering was performed at the same rate as for tomato-planted pots.
Sample collectionIn order to investigate the time course effect of HV and fertilizer application, 5 whole tomato plants including leaves, stems, mature and immature fruit, and roots in each treatment were collected 4 times, at 2, 4, 8, and 12 weeks after transplant (WAT). The soil in the pot was well mixed, and one soil sample in each pot was collected when the whole plant was collected. In the C-FS and C-S treatment, whole tomato plants were harvested at only 12 WAT. The tomato-free pot soil was sampled 7 times, at 1, 2, 4, 6, 8, 10, and 12 WAT, respectively. Samples were taken from 2 points in each pot and then mixed. Hence, one soil sample was prepared in each pot, and five samples were collected in each treatment.
Chemical analysisAfter measuring the fresh weight of the fruits including both mature and immature, tomato fruits and plants were dried in a circulation oven at 90°C and 60°C for 4 and 2 days, respectively. DW of tomato plants including fruits was measured, and the dried samples were finely ground. Soil samples were also dried at 60°C and sieved through 2 mm mesh. Total N concentration and the abundance of 15N in the powdered samples of the plants and soils were determined using an elemental analyzer (EA1110; Thermo Fisher Scientific Inc., Waltham, MA, USA) coupled to a Finnigan MAT252 isotope ratio mass spectrometer via a Con Flo 2 split interface (Thermo Fisher Scientific Inc.). Soil samples from both tomato-planted pots and tomato-free pots were also used for nitrate N analysis with the same soil analyzer as used for soil incubation experiments.
CalculationThe rate of plant N uptake derived from HV to total N uptake in tomato plants (%Ndfhv) can be calculated from Eq. (1).
| (1) |
| (2) |
The uptake amount of N derived from soil and fertilizer (Ndfsf) can be calculated from Eq. (3).
| (3) |
The values are the mean of five replications. Significant differences of the mean values were calculated using t-test, except for soil inorganic N in incubation examination, fruit fresh weight, whole plant dry weight at 12 WAT, and N uptake at 12 WAT, for which significant differences were calculated using Tukey’s test.
It is known that ammonium sulfate releases N immediately after application to soil (Schnier et al., 1987). Soil ammonium N content (NH4+-N) in Fast + Slow was markedly higher than that in Slow-only and N0 from 1 to 4 weeks (Table 2). On the other hand, NH4+-N in Slow-only was equal to that in N0 until 2 weeks, and then increased toward 4 weeks. NH4+-N in N0 maintained a constantly low value during the incubation period. Therefore, NH4+-N in Slow-only became higher than in N0 at 4 weeks.

Inorganic N release in soil incubation experiment.
Soil nitrate N content (NO3−-N) increased until 2 weeks in all treatments, and that in N0 decreased at 4 weeks, whereas that in Fast + Slow and Slow-only continuously increased (Table 2). NH4+-N and NO3−-N in N0 maintained low, while in Slow-only they increased from 4 weeks because LP-S100 fertilizer was used as slow-release fertilizer, which released N from about 4 weeks after application. It was recognized that the used soil did not have the ability to release inorganic N for a long period. Thus, it was confirmed that inorganic N release patterns were mainly differentiated by whether Fast was applied in a soil incubation experiment without HV, and inorganic N became rich just after the application of Fast in the pot examined.
2) Tomato cultivation experiment in plastic house Soil temperature and soil water tensionMean soil temperature at 10 cm depth during the cultivation period was 24.3°C. Soil water tension changed from pF 1.6 to pF 2.5 during the experiment under no hyper-dry or excessive humidification conditions.
Fruit yield and plant dry weight of tomatoThere was no significant difference in fresh tomato yield/plant among treatments with or without both Fast and HV (Table 3). Plant DW increased linearly regardless of the presence or absence of Fast until 8 WAT. At 12 WAT, the DW in HV (HV-FS: 191 g/plant, HV-S: 190 g/plant) were significantly higher than control (C-FS: 143 g/plant, C-S: 139 g/plant). There were no differences in fruit yield, plant DW, N uptake or other characteristics between FS and S at 12 WAT (Tables 3 and 4).

Effects of N fertilizer and HV treatments on whole plant dry weight and total fresh weight of harvested fruits.

Effects of N fertilizer and HV treatments on N uptake by tomato plants.
Ndfhv was already released at 1 WAT in tomato-free pots (Table 5), and the uptake of Ndfhv started soon after tomato transplant. This result corresponded to reports that legume cover crops including HV decompose relatively rapidly due to their low C/N rate (Choi and Daimon, 2008; Hadas et al., 2002; Rosecrance et al., 2000; Thönnissen et al., 2000b; Yano et al., 1994). The authors observed that Ndfhv had already been released at 1 WAT, and the release ceased at 4 WAT in a previous report (Sugihara et al., 2013).

Effects of N fertilizer treatments on changes of soil-remaining N content derived from HV in tomato-free pots.
The amounts of Ndfsf and Ndfhv taken up at 2 WAT were 419–508 mg/plant and 259–326 mg/plant, respectively (Table 4). The amount of Ndfsf in HV-FS was larger than that in HV-S at 2 WAT and 4 WAT. After 8 WAT, there was no difference in the uptake amount of total N, Ndfsf, and Ndfhv between HV-FS and HV-S. Total N and Ndfsf uptake increased linearly to 12 WAT. Total N uptake reached the same amount, 2497 mg/plant in HV-FS and in HV-S at 12 WAT, and these amounts were larger than those of C-FS and C-S. The increase of total N uptake caused an increase of whole plant DW in HV-FS and HV-S.
HV application and incorporation into soil increased Ndfsf and, as a result, increased total N uptake. HV seems to make it possible for the environment to readily take up N, and Blanco-Canqui et al. (2012) reported that cover crops increased the total N pool near the soil surface.
A difference in the N uptake pattern by tomato was recognized between Ndfsf and Ndfhv in HV-incorporated soil. The uptake amount of Ndfhv by tomatoes was 430 mg/plant and 480 mg/plant until 4 WAT in HV-FS and HV-S, respectively, and these accounted for 84% and 89% of the Ndfhv amount taken up until 12 WAT (Table 4). On the other hand, the uptake amount of Ndfsf until 4 WAT was 774 mg/plant and 636 mg/plant in HV-FS and HV-S respectively, 39% and 32% of Ndfsf amount taken up until 12 WAT. Ndfhv uptake occurred mainly by the first 4 weeks after transplant and more than 60% of Ndfsf was continuously taken up until 12 WAT.
3) Competition of Ndfhv and Fast-release fertilizerThe rate of N uptake derived from HV to total N uptake in tomato plants (%Ndfhv) in HV-S at 2 and 4 WAT was 43.2% and 43.1%, higher than in HV-FS, 33.8% and 35.8% (Fig. 1). The value of %Ndfhv decreased gradually in both HV-FS and HV-S after 4 WAT and was 20.5–21.5% at 12 WAT, reflecting the lack of difference between HV-FS and HV-S. Kumar et al. (2005) also pointed out a similar tendency in tomato production in the mid-Atlantic USA.

Effects of N fertilizer treatments on the change in rate of plant N uptake derived from HV to total N uptake. Plots of HV were fertilized with 240 kg N·ha−1, and HV was applied (1008 mg N/pot). FS contained 20% fast-release fertilizer + 80% slow-release fertilizer, and S contained 100% slow-release fertilizer. Means followed by asterisk are significantly different among treatments in the investigated weeks at 5% by t-test test. NS: not significant.
Tomato plants take up N mineralized from HV, soil, and fertilizer without distinction. In this experiment, the amount of Ndfhv taken up until 4 WAT was more than 430 mg/plant in HV-FS and HV-S, accounting for 80–90 kg N/ha. It was assumed that the HV application was sufficient for tomato N demands in the early stage. In addition, chemical fertilizer released enough inorganic N to tomato plants because the NH4+ and NO3− concentrations were 4.5–8.2 mg/100 g soil and 4.8–12.1 mg/100 g soil, respectively, until 4 WAT (Table 6). Competition in N uptake was supposed to have occurred between Ndfsf and Ndfhv, and it can be inferred that Fast masked the effect of HV.
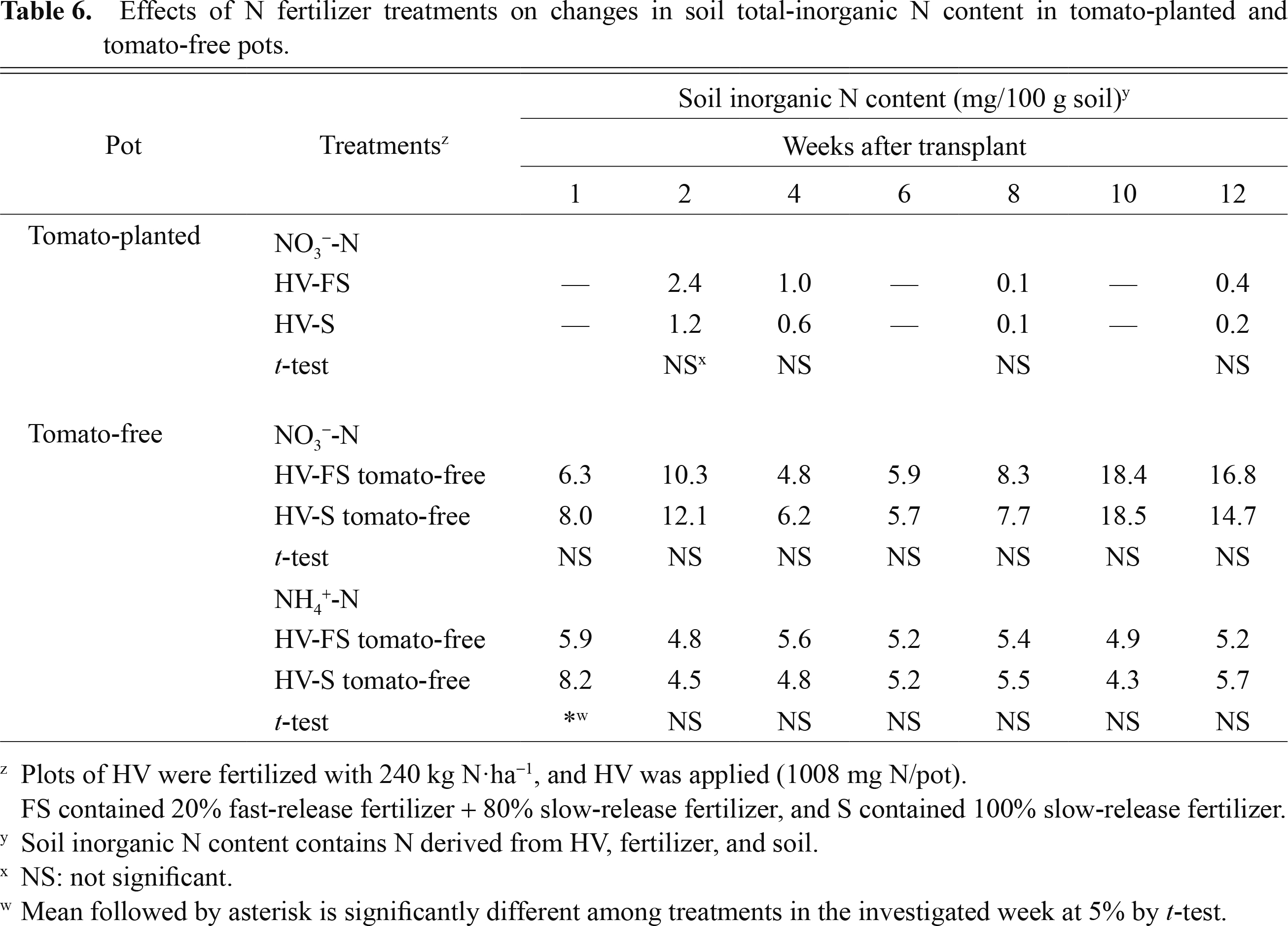
Effects of N fertilizer treatments on changes in soil total-inorganic N content in tomato-planted and tomato-free pots.
Thus, if HV is applied sufficiently, we can reduce the Fast in order to avoid the competition between HV and Fast, and more efficiently obtain the nutritional effect of HV. Blanco-Canqui et al. (2012) reported that high N application masked the benefits of cover crop-derived soil N, and appeared to limit the cover crop effects on crop yields. In their examination using a summer cover crop, sunn hemp (Crotalaria juncea L.), this increased the yield of subsequently cultivated sorghum by 1.18 to 1.54 times at 0 kg N·ha−1 fertilizer, whereas it had no effect on sorghum yield at 66 kg N·ha−1 fertilizer application. It was suggested that Ndfhv was utilized more effectively by reducing the N fertilizer.
In tomato-free pots, the content of NH4+-N and NO3−-N in soil was more than 4.3 mg/100 g soil and 4.8 mg/100 g soil until 12 WAT, respectively (Table 6). On the other hand, NO3−-N content was quite low throughout the growth period in tomato-planted pots. Tomatoes are known to take up N continuously throughout the growth period (Ito et al., 1990). The present data indicate that inorganic N was released and accumulated in soil by chemical fertilizer and accumulated N was taken up by tomato plants. It was necessary for healthy tomato growth to ensure sufficient N in the late growth period even if HV is applied during cultivation. The uptake amount of Ndfhv by tomato plants reached 513 mg/plant and 538 mg/plant in HV-FS and HV-S, respectively, at 12 WAT. Nitrogen use efficiency (NUE) derived from HV was 50.9% (513/1008) and 53.4% (538/1008) in HV-FS and HV-S, respectively. It is understood that HV is an important source of nitrogen supply at all times.
In conclusion, the authors recognized that applied HV acted as a fast-release fertilizer, as pointed out by other reports (Acosta et al., 2011; Kumar et al., 2005; Seo et al., 2006), and a high amount of chemical fertilizer tended to suppress the uptake of Ndfhv in tomato cultivation. Ndfhv was mainly taken up by tomato plants until 4 WAT, whereas N uptake by tomato plant continued until the late stage of growth. These results can be applied to establish a suitable combination of HV and chemical fertilizer for tomato production. Further research will be needed in the greenhouse in practice.
The authors wish to deeply thank Mr. Hideki Nakano, a technician at the Field Science Center for Northern Biosphere, Hokkaido University, for his valuable technical support with tomato cultivation. We also wish to thank Ms. Aiko Agui, a technician at the Graduate School of Environmental Earth Science, Hokkaido University, for her pivotal technical support with 15N analysis.