2019 Volume 68 Issue 6 Pages 525-539
2019 Volume 68 Issue 6 Pages 525-539
The combination of polymers and surfactants is an important means to create various functions in recent detergents and personal care products. In particular, detergents mixing oppositely charged anionic surfactants and cationic polymers induce coacervation by the dilution of the washing and rinsing process, and the complexes effectively adsorb onto surfaces and can change their characteristics. The driving force of the coacervation is electrostatic interaction between the anionic groups of the surfactant and the cationic groups of the polymer. Normally, the coacervation is controlled by selecting the molecular structure or the amount of polymer and surfactant. In shampoo and body wash compositions, we studied the complex precipitation (CP) regions and the morphology and rheological properties of precipitated complexes by focusing on the number of ionic groups in the anionic surfactants and cationic polymers, the mixed electrolyte and the ionic strength as a whole. This clarified the factors related to complex functions. For coacervation in shampoo based on alkyl ethoxylate sulfate (AES), the degree of cationization of the cationic cellulose (CC) and coexisting electrolyte greatly contributed to these functions. In a combination of moderately cationically charged CC and AES mixed amphoteric surfactant, the precipitated complexes became a loose mesh-like morphology, which was also formed when the charge shielding effect was enhanced by adding electrolyte. The precipitated complexes with a looser mesh-like morphology gave a smooth texture to the hair surface during rinsing.
On the other hand, for coacervation in body wash based on fatty acid salt, the complexes were effectively precipitated in a combination with a synthetic polymer, poly diallyldimethylammonium chloride (PDADMAC), which has a higher cationic charge than CC. The precipitated complexes had high adsorbability onto skin and contributed to a moisturizing effect by lowering transepidermal water loss (TEWL).
In this review, we introduce the controllable factors of coacervation in shampoo and body wash systems by focusing on the relationship between dilution processes and precipitation behavior.
The combination of surfactants and polymers is a remarkably important way to create product functions in the field of personal care, like cosmetics, pharmaceuticals and foods, because it can take a variety of complex states which differ greatly in appearance and viscosity. In particular, attempts to mix anionic surfactants as detergent and oppositely charged cationic polymers have been applied to shampoo since the late 1970’s1),2),3). When the oppositely charged surfactants and polymers coexist in solution, the complexes take three different dissolved states by electric equivalent ratio (S/P), in which S is the number of surfactant ions and P is the number of ionized groups of the polymer (Fig. 1)4),5),6). The first state (Stage 1) is S/P <1 at a surfactant concentration below cmc, where surfactant ions cooperatively bind to the polar groups of the polymer. The mixed solution is a single phase (1φ) and the complexes still have the polymer’s cationic charge. The onset of the second state (Stage 2) is led by electric neutralization, S/P=1, and the complexes become insoluble and precipitate around the electric neutralization line. This stage is called the coacervation region or complex precipitation (CP) region. As the surfactant concentration increases at S/P>1 (Stage 3), micelles are gradually formed on the precipitated complexes and the complexes are finally re-dissolved and coexist with free micelles in Stage 3. Current conditioning shampoos containing cationic polymers are mixed solutions in Stage 3. Shampoo has a mechanism in which the complexes coacervate by the dilution of the shampoo composition in the washing and rinsing process from Stage 3 to Stage 2.

Schematic representation of the three dissolved states of oppositely charged surfactant/polymer complex in solution. Stage 1 and stage 3 are clear one phase solution. Stage 2 is turbid by insoluble coacervated complex. Images taken from the reference 6.
Goddard et al.7) proposed this change in the dissolved state of complexes as a dilution-deposition system. It has also been reported that the precipitated complexes possess functions such as the increase of bubbles8),9),10), reduction of frictional force11),12), and adsorption promotion of dispersed colloids like silicone emulsion on hair12),13),14). The precipitated complexes greatly influence the feeling during rinsing and the finish after drying, which are directly recognized by consumers13),15),16).
For the 30+ years that cationic polymers have been used in shampoos, complex coacervation with various combinations of oppositely charged surfactants and polymers has been studied. In the early stage of the studies, this focused on the cooperative binding behavior between surfactant ions and charged groups of the polymer in Stage 117),18) and phase diagrams showing the coacervation region19). Thereafter, the effect on coacervation of conditions such as the charges of the surfactants and polymers related to complex precipitation behavior20),21),22), the ionic strength with coexisting electrolytes23),24), the molecular weight, the concentration and the main chain flexibility of the polymer25), the other components added in the bulk such as solvents and the temperature and shear26),27),28),29) were also evaluated using various methods. Furthermore, the dissolved state of the complexes and the adsorption state at the gas-liquid and the solid-liquid interfaces6),29),30) have also been studied. However, in these fundamental research fields, sodium dodecyl sulfate (SDS) was often used as an anionic surfactant. Coacervation in shampoo whose main surfactant is AES is considerably different from that of SDS. In addition, although hair texture after rinsing with precipitated complexes depends on the type of surfactant and polymer in the conditioning shampoo, the mechanism of coacervation has not been sufficiently understood.
Previously, we studied the CP regions and morphology and rheological characteristics of precipitated complexes in model shampoos of AES and CC by focusing on the charge densities, the charge per unit volume, with AES and CC and the coexisting electrolyte concentration. Thereby we clarified the relationship between coacervation and the functions of the precipitated complexes in conditioning shampoo17),32),33),34). Recently, we succeeded in utilizing coacervation in body wash based on fatty acid salt as an anionic surfactant and improved the skin moisturizing effect by absorbing many moisturizing polymers. In this review, we present our results and views related to the control factors of coacervation and the functions of precipitated complexes in shampoo and body wash.
AES is widely used as an anionic surfactant for shampoo. In addition, nonionic surfactants, amphoteric surfactants, amino acid surfactants and others are mixed in to alleviate stimulation and improve the properties of the foam. Mixing other surfactants reduces the anionic charge of AES in the composition and affects the coacervation region. On the other hand, cationic polymers used for shampoo are cationized cellulose (Polyquartanium-10), cationized guar gum12),35), and synthetic polymers such as a homopolymer of DADMAC (PDADMAC, Polyquartanium-6), copolymers with DADMAC and acrylamide (Polyquartanium-7)36), and the like. A number of novel synthetic cationic polymers have been developed in order to expand application functions37). However, the mechanism of coacervation in the dilution process has not been comprehensively understood because there are an infinite number of surfactant and polymer combinations.
When the application of CC in shampoo began, surfactant-polymer concentration phase diagrams of SDS and CC were reported by Goddard et al.4) and those of AES and CC by Yamaguchi et al.38) (Fig. 2). In the phase diagrams of both SDS and AES, the complex precipitation region exists around the charge neutralization line. The lower part is Stage 1 in which surfactant monomers are bound to CC and the upper part is Stage 3 of the dissolved complexes in which surfactant micelles are bound to CC. Both stages are a single phase (1φ) region. The charge neutralization line of AES is located at a somewhat higher surfactant concentration than that of SDS because the molecular weight of AES is slightly larger than SDS. The composition of the dilution process of a model shampoo containing 15% surfactant and 1% polymer is shown with arrows in the figures. Generally, it is thought that shampoo is diluted from 5 times to 10 times, which correspond with washing process and a start of rinsing one. It is preferred that the complexes precipitated around 5 to 10 times dilutions in shampoo. In the phase diagrams of both AES and SDS, the arrows show that the model shampoo is diluted without reaching the complex precipitation region in Fig. 2. In these cases, it can easily be predicted that a surface modification function cannot be obtained by the precipitated complexes. In order to reach the complex precipitation region with a diluted composition of the model shampoo solution, it is necessary to shift the charge neutralization line and/or the CP region toward the higher surfactant concentration in which the diluted composition exists. There are a few ways to achieve this. One is to reduce the anionic charge of the surfactant by mixing amphoteric and/or nonionic surfactants. The other is to enhance the coacervation by adding electrolyte which affects salting-out and charge shielding.

Figure 3 shows the surfactant-CC concentration phase diagram with AES mixed lauroyl amidopropyl betaine (LPB) as an amphoteric surfactant. In comparison with the AES-CC concentration phase diagram in Fig. 2b, the charge neutralization line is shifted and the CP region is expanded toward a higher surfactant concentration. The diluted composition could reach the upper end of the CP region; that is, a blue turbid solution in which the complexes are slightly coacervated and dispersed.
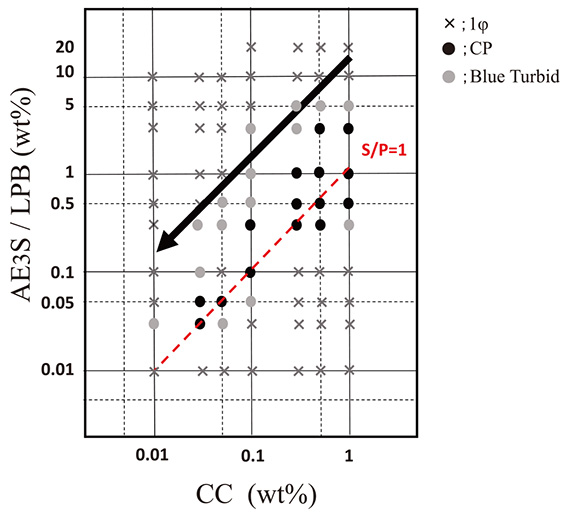
Surfactant-polymer concentration phase diagrams in the combination with AES/LPB and CC α=0.4, in which crosses show the 1φ region, black circles the CP region, and the gray circles the blue turbid solution. The dotted line shows charge neutralization line, the gray zone the complex precipitation region, and the arrow the diluted composition of model shampoo solution.
On the other hand, Fig. 4a is an electrolyte-SDS concentration phase diagram in which CC is fixed at 0.1%. As ionic strength increases with the electrolyte concentration, the CP region expands toward a higher surfactant concentration. When this expansion is superimposed on the SDS-CC concentration phase diagram in Fig. 4b, the diluted composition of the model shampoo with SDS and CC reaches the CP region and the complexes precipitate in the dilution process. Even in the model shampoo with CC and AES, it is important to enlarge the CP region using electrolyte in order to cause coacervation by dilution (Fig. 5). It would be important to adjust both the anionic charge of the surfactant and the ionic strength for effective coacervation of CC in the dilution process. In this case, the electrolyte contributed to obtaining more complexes.

Effect of electrolyte concentration on the CP region in the phase diagram with SDS and CC α=0.4. (a) Electrolyte-SDS concentration partial phase diagram at 0.1% polymer, in which 1φ is a single phase region, CP is a complex precipitation region and PS is phase separation region. (b) The expansion of the CP region, which is shown with gray circles, by adding electrolyte (Na2SO4).

The expansion of CP region by mixing 0.3% electrolyte in the AE3S and CC concentration phase diagram (our data), in which crosses show the 1φ region, black circles the CP region, and the gray circles the blue turbid solution. The dotted line shows charge neutralization line, and the arrow the diluted composition of model shampoo solution.
Miyake et al.16) investigated the effect of the anionic charge of AES, changed by mixing LPB, the degree of cationization of CC and the coexistent electrolyte concentration on coacervation induced in a model shampoo containing 15% surfactant, 1% polymer, and 3% coexistent electrolyte. Figure 6 shows the electrolyte-AES partial phase diagram at constant CC concentration. The dot in the phase diagram of 1% CC represents the model shampoo composition before dilution; in that of 0.1% CC, its 10-times diluted composition. The arrow corresponds to the dilution process. When the degree of cationization of CC decreases, the CP region shifts to a lower electrolyte concentration and lower surfactant concentration. The 1φ region, where the complexes are re-dissolved by charge shielding, appears in the high electrolyte concentration. As a result, in the case of CC with α=0.4 and 0.2, the diluted composition reached the CP region in the dilution process, but in the case of CC with α=0.1, the diluted composition passed through the 1φ region and the complexes did not precipitate. The degree of cationization of CC also had a significant influence on shifting the CP region.

Electrolyte-CC concentration partial phase diagrams at constant polymer concentration. The back them include 1% CC, and the front them 0.1% CC. In these figures, dots show the compositions with model shampoo and 10-times diluted solution. The arrows show dilution process.
When the mixing ratio of AES and LPB and the degree of cationization of CC were changed, hair texture when rinsing with the model shampoos changed as shown in Fig. 7. In the combination of CC α=0.4 and AES (both having the highest charges among the evaluated combinations), hard gel-like complexes were precipitated and the hair texture became sticky during rinsing. When both charges decreased moderately, soft gel-like complexes were precipitated and the hair texture became smooth. These rheological property changes in texture suggest that the morphology of the precipitated complexes was determined by the charge balance of both surfactant and polymer.

Texture of precipitated complexes (upper side) and hair texture during rinsing in the combination of AES/LPB and CC.
Figure 8 is the SEM images observed after the precipitated complexes were dried at a supercritical condition of carbon dioxide, and it shows the morphology of the precipitated complexes in the combination of AES/LPB mixed surfactant and CC16). In the case of the highest charges with AES and CC α=0.4, the morphology of the precipitated complexes was like film with polymers densely packed. As the charges of both the surfactant and polymer decreased, that morphology became a looser structure like mesh. The amount of precipitated complexes having looser structure increased, compared with that having densely packed structure. On the other hand, in the combination of the lowest charges (AES/LPB=5/5 and CC α=0.2), the morphology of precipitated complexes changed to small particles and the amount of precipitated complexes decreased.

Morphology of complexes precipitated from a 10-times diluted model shampoo in the combination of AES/LPB and CC.
These morphologies are formed in the dilution process. The changes in the dissolved state of the complexes can be followed by the relative light scattering intensity, ΔIcomplex, which is the scattering intensity of the solution containing the surfactant and electrolyte (Imicelle) subtracted from the one containing the surfactant, polymer, and electrolyte (Iwhole) at the same concentration (Fig. 9). This scattering intensity results from the polymer chain density in the dissolved complexes compared with the one containing the polymer and electrolyte16),32). The ΔIcomplex increases in the dilution process before the complexes precipitate (Fig. 10). In the model shampoos in which the AES/LPB ratio and the degree of cationization of CC were changed, the ΔIcomplex of a more cationized CC α=0.4 increased significantly in the dilution process compared with that of the less cationized CC α=0.2. In each CC, the ΔIcomplex just before complex precipitation decreased with the decrease in anionic charge by mixing LPB with the AES. In comparison to the SEM images in Fig. 8, this suggests that the dissolved complexes with a high ΔIcomplex just before precipitation formed the densely packed morphology and those with a low ΔIcomplex formed the looser mesh-like morphology. Therefore, the mechanism to determine the morphology of the precipitated complexes is shown as follows (Fig. 11) : In the model shampoo containing electrolyte, the dissolved complexes are influenced by a certain degree of charge shielding. The dilution breaks down the charge balance of the dissolved complexes because it releases the charge shielded ions and loses the bound micelles on the polymer. At that time, when the charges of both the surfactant and polymer are high, the polymer chains are packed densely by recombination at multiple points. On the other hand, when their charges are low, the polymer chains form a looser mesh-like structure because the recombination point is reduced.

Observation of dissolved complexes by relative light scattering of polymer conformation, ΔIcomplex.

Change in relative light scattering of polymer conformation, ΔIcomplex, during the dilution process of the model shampoo with AES/LPB and CC.

Morphology formation of complexes precipitated in dilution process.
Furthermore, when the electrolyte concentration of the model shampoo with AES and CC was increased, the morphology of the precipitated complexes changed from a densely packed state to a looser mesh-like state. This result suggests that the coacervation in this system could be controlled by the charge shielding effect, in addition to the number of oppositely charged ions in the surfactant and polymer.
2.4 Effect of polymer main chain on coacervationIn addition to the widely-used CC, various synthetic cationic polymers can be used in shampoo, but their hair textures during rinsing differ greatly from that of CC34). The synthetic polymer has a larger number of cationic groups, a hydrophobic main chain, and higher flexibility compared with CC. Such polymers tend to undergo a counter ion condensation and have a very compact dissolved state. In reality, CC with rigid sugar chains dissolves in rod shapes at a large size, but cationized dextran (CD) with flexible sugar chains and synthetic copolymers with vinyl chains, for example poly(2-hydroxyethyl methacrylate-co-ethyldimethylammonium methacrylate) (HEMA-EDMAMA), dissolve in coil shapes at smaller sizes than CC (Table 1). In the model shampoo containing these flexible cationic polymers, the ΔIcomplex was higher than that of the CC before dilution and increased sharply by dilution (Fig. 12). This suggests that the complexes, in which the polymer chains were densely packed, precipitated by abrupt condensation among the complexes because the flexible polymer chains shrank and dissolved at a high polymer chain density16). In other words, the flexibility of the polymer chains also contributes to the morphology formation of complexes and greatly affects the rheological characteristics of precipitated complexes.

Change in relative light scattering of polymer conformation, ΔIcomplex, during the dilution process of the model shampoo with AES/LPB and flexible polymers.
The most desirable function in body wash, which is a skin cleanser, is to prevent issues such as dry skin, poor texture, irritation and so on. Consumers tend to desire body wash with a moisturizing effect. There are many moisturizers used in cosmetics: polyols such as glycerin, oils such as jojoba oil, various polymers such as collagen and natural products such as amino acids and plant extracts. However, applying these moisturizers to body wash does not currently achieve a sufficient moisturizing effect. The main reason is that the moisturizers are rinsed off and cannot be absorbed and remain on the skin. Accordingly, using coacervation as in shampoo is expected to effectively adsorb the moisturizer to the skin. However, coacervation techniques in body wash have not made much progress. In Japan, fatty acid salt (C12K) is preferred as an anionic surfactant in body wash due to advantages such as creamy foaming and a clean feeling after rinsing. So far, the application of coacervation in body wash has not made much progress because the coacervation in the combination of fatty acid salt and cationic polymers has not been studied in depth compared with the combination of SDS or AES and cationic polymers.
3.2 Coacervation and complex functions with fatty acid salt and cationic polymersThe ideal coacervation in body wash is to precipitate a large number of complexes at a lower dilution ratio from foaming to washing in order to adsorb the complexes to a large area of the whole body.
Therefore, first of all, we evaluated the complex precipitation behavior of fatty acid salt in a model body wash containing 20% surfactant, 1% polymer, and 10% propylene glycol compared with AES (Table 2). The numerical values in Table 2 represent the dilution ratio at which the complexes began to precipitate. The smaller value means that the complexes can be precipitated without increasing the dilution ratio. In order to cause coacervation by dilution in a model body wash containing CC α=0.4, both AES and C12K need to be mixed with large amounts of amphoteric surfactant, lauroyldimethylbetaine (LDB), and the complexes of C12K do not precipitate unless it is considerably diluted compared with those of AES (3). Next, the conditions of cationic polymer that cause favorable coacervation in a mixed surfactant of C12K and LDB were evaluated in a model body wash solution containing CCs with different degrees of cationization and synthetic DADMAC type polymers whose molecular structure differs greatly from CC (Table 3). We found that complex precipitation started from a low dilution ratio in the combination of C12K and PDADMAC without LDB, and that complex precipitation with a copolymer of DADMAC and acrylamide was not observed unless LDB was mixed, similar to CC. Thus, in the model body wash solution with the combination of C12K and PDADMAC, enough precipitated complexes can be expected to adsorb to the skin to obtain a moisturizing effect. Figure 13 shows the adsorbed amount of cationic polymers evaluated using three-dimensional cultured skin. The model body wash solution was diluted to 5 times, treated on the cultured skin for 2 minutes, and then rinsed with water to quantify the polymer adsorbed on the cultured skin. From the cultured skin washed with a model body wash, the polymer was extracted with methanol and an anion exchange resin was added to remove the C12K, then following filtration, solvent removal, and redissolution in water, the cationic polymer was quantified by size exclusion chromatography. With Body Wash A containing the copolymer of DADMAC and acrylamide, the complexes were not precipitated at the dilution in the washing process (10 times dilution), so the polymer was hardly adsorbed. In contrast, with Body Wash B containing the PDADMAC, the complexes were precipitated and polymer absorption was observed.



The amount of cationic polymer adsorbed onto 3D-cultured skin during washing with model body wash. Body Wash B precipitates complexes during washing while Body Wash A does not.
In the former case, the water content of the cultured skin, higher after washing, decreased to the level before washing in about 25 minutes. On the other hand, the cultured skin treated with Body Wash B maintained a high moisture content, confirming that polymer adsorption as complexes is effective for moisture retention. Furthermore, with Body Wash B, we observed a moisturizing feeling, equal to lotion applied to the skin, at 5 minutes after the washed skin was dried with a towel.
3.3 Coacervation region with fatty acid salt and cationic polymersWhat kind of control factors are involved in the coacervation of fatty acid salt and cationic polymers in the model body wash solution? What is the difference from the combination of AES and CC in shampoo? To comprehensively understand this, we compared the surfactant-polymer concentration phase diagrams. Figure 14 shows the C12K-CC concentration phase diagram. Compared with the AES-CC concentration phase diagram in Fig. 3, the charge neutralization line of C12K is positioned at a somewhat lower surfactant concentration than that of AES, but is nearly equivalent to the charge neutralization line of SDS, which has a similar molecular weight. Furthermore, in the C12K-CC concentration phase diagram, the boundary of the CP region, at which the complexes started to re-dissolve, was at a higher surfactant concentration and the CP region was more expanded than that of AES. However, both arrows representing the dilution composition of the model body wash solution were located at a high surfactant concentration compared with the charge neutralization line and the CP region. This shows that the model body wash solution of C12K is diluted without precipitating the complexes.

C12K-CC concentration phase diagram, in which crosses show the 1φ region, black circles the CP region, and gray circles the turbid blue solution. The dotted line is the charge neutralization line and the arrow the diluted composition of model shampoo solution.
Next, in the case of the C12K-PDADMAC concentration phase diagram (Fig. 15a), the charge neutralization line was pushed up to a high surfactant concentration due to the high cationic charge and the complexes precipitated within the wide polymer concentration at a higher C12K concentration. The composition of the model body wash solution was located in the 1φ region, and the 2-times diluted solution reached the CP region. Therefore, the complexes were precipitated with a wide range of diluted compositions. On the other hand, in the phase diagram with C12K and a copolymer of DADMAC and acrylamide as a polymer (Fig. 15b), the cationic charge of the copolymer decreased compared with PDADMAC, shifting the charge neutralization line to a low surfactant concentration. In this case, the CP region was separated from the diluted composition and no complexes were precipitated in the dilution process. These phase diagrams support the complex precipitation by dilution in model body wash A and B in Fig. 13.

C12K-synthetic polymer concentration phase with (a) PDADMAC and (b) copolymer of DADMAC and acrylamide, in which crosses show the 1φ region, black circles the CP region, and gray circles the turbid blue solution. The dotted line is the charge neutralization line and the arrow the diluted composition of model shampoo solution.
How would the polar groups of the surfactant affect the coacervation of CC and PDADMAC? Firstly, by comparing Fig. 2 and Fig. 14, the contribution of the polar groups to the coacervation of CC combined with C12K, SDS, and AES can be inferred. Since SDS and C12K have a similar molecular weight, the charge neutralization line is located at similarly low surfactant concentrations, but that of AES is shifted to a slightly higher concentration because AES has a slightly larger molecular weight and the anionic charge decreases in the solution. Compared to the CP region of C12K and SDS, the boundary between the CP region and the 1φ region with C12K, where precipitated complexes are re-dissolved, is located at a higher surfactant concentration than with SDS. Since the sulfate group is strongly dissociative, charge repulsion between the micelles bound onto CC is generated when the complexes re-dissolve. This likely accelerates the re-dissolution of precipitated complexes with SDS and CC, narrowing the CP region. In addition, the CP region with AES and CC tended to be wider than that of SDS. Their charge repulsion between micelles at the re-dissolution of precipitated complexes is likely weakened for AES due to a decrease of charge density by EO chain and for C12K due to the lower dissociation property of carbonyl groups. Those factors would affect the expansion of their CP regions. On the other hand, comparing Fig. 15a and Fig. 16 reveals the influence of polar groups in the coacervation of PDADMAC combined with C12K, SDS, and AES. Since PDADMAC has a large cationic charge, the charge neutralization line is located at a high surfactant concentration compared with CC. The complex precipitation region is observed in the vicinity of the charge neutralization line. However, apart from the charge neutralization line, the CP regions tend to be expanded at a high polymer concentration and high surfactant concentration. The CP region appearing in these regions suggests that precipitation of complexes with PDADMAC was promoted by shrinking complexes, as the ionic strength there is increased with the large charge of polymer or surfactant. In the case of PDADMAC and SDS and/or AES, the composition of the model body wash is in the CP region, but in the case of PDADMAC and C12K, it is in the 1φ solution re-dissolved complexes. This suggests that the boundary of re-dissolution with the PDADMAC and C12K precipitation region shifts toward a low surfactant concentration. The influence of surfactant polar groups in the ionic strength is likely reduced by the lower dissociation than sulfate and the salting out effect to the complexes is weakened. Furthermore, the contribution to complex re-dissolution by the EO chain of AES was not observed at high surfactant concentration under high ionic strength. In this result, it is unclear whether the EO chain of AES has an influence in the shrinkage of complexes.

Surfactant-PDADMAC concentration phase diagram with (a) SDS and (b) AE3S, in which crosses show the 1φ region, black circles the CP region, and gray circles the turbid blue solution. The dotted line is the charge neutralization line and the arrow the diluted composition of model shampoo solution.
Detergents combining an anionic surfactant and cationic polymer, such as shampoo and body wash, cause coacervation in the process from washing to rinsing. Thereby active ingredients can be adsorbed to a high degree, enhancing product functions. As representative examples using this mechanism, this review introduced hair texture during rinsing with shampoo and moisturization with body wash. The basic principle of coacervation is charge neutralization between oppositely charged anionic surfactants and cationic polymers. But coacervation cannot be utilized unless the diluted composition of the product reaches the CP region. The charge of the composition is determined by selecting the anionic surfactants and polymers, thereby shifting the charge neutralization line and the CP region significantly.
Moreover, the positional relationship between the CP region and the diluted composition determines the realization of coacervation in the product dilution process. The contribution of the charge to the neutralization line seems to be larger in the polymer. If the charge of the polymer is low (CC) and the charge neutralization line is located at a low surfactant concentration rather than the dilution composition, the CP region can be expanded to a high surfactant concentration, in which the dilution composition is located, by adding electrolyte. When the charge brought in by the polymer itself is large (PDADMAC), the charge neutralization line is pushed up to a high surfactant concentration, so coacervation with C12K was realized at a lower dilution ratio. However, with SDS and AES, the composition before dilution also precipitated the complexes. Thus, in addition to its own polymer and surfactant charges, the dissociation property of polar groups and the coexisting electrolyte are control factors for coacervation. In addition, the function of the precipitated complex depends not only on the quantity of precipitated complexes but also on their morphology. That morphology is determined by crosslink and aggregation between polymer chains through micelles in the dilution process. In addition to the charge balance of both surfactants and polymers, the flexibility of the main chain of the polymer has a great impact on the coacervation. The charge control of surfactants to coacervate is also carried out by mixing nonionic surfactants and amphoteric surfactants. We found that not only the charge and ionic strength but also the dissociation property of the anionic polar group itself had an influence on the coacervation under high ionic strength. In order to control the coacervation, it is necessary to determine the charge neutralization line and the CP region according to the phase diagram and construct a methodology by which the CP region corresponds to the diluted composition of the product. For that purpose, it is important to control the anion/cation electrostatic interaction via the charge density and the ionic strength by selecting the polymer and mixing other surfactants and electrolytes.
Nowadays, in the field of personal care such as cosmetics, the options for surfactants are expanding, including for example amino acid and sugar-based surfactants. Therefore, their coacervation control factors must also be clarified. Furthermore, in order to enhance functions and add value, it is necessary to develop coacervation technology as a technique for effectively adsorbing carriers such as micelles and emulsions including active ingredients. We would be delighted if our findings could be a help in such considerations.