2020 Volume 69 Issue 2 Pages 83-91
2020 Volume 69 Issue 2 Pages 83-91
It is well known that lipids form various kinds of self-assembled structures. First, lipid nanoparticles dispersed with hydroxy propyl methyl cellulose acetate succinate (HPMCAS) were introduced. The influence of polymers on the lipid self-assembled structures was evaluated by small and wide angle X-ray scattering (SWAXS). Self-assembled structures containing higher alcohols have attracted much attention in the cosmetic industry. The α-form hydrated crystalline phase (often called α-gel) is one of the hydrated crystalline phases which can be exhibited by surfactants and higher alcohols. As surfactants in this study, an ionic complex or a silicone type were used. This review also reports the lipid membrane fluidity by using fluorescence spectroscopy.
Monoolein (glyceryl 1-oleate) and monolinolein (glyceryl 1-linoleate) are known to form bicontinuous phases in equilibrium with excess water at ambient temperatures1),2),3),4),5),6),7). Lipid-dispersed particles that have bicontinuous phases in their interiors are termed cubosomes. Due to their structural matter, emulsifiers are indispensable to disperse cubosomes stably. Pluronic F127, polyethylene oxide (PEO)- polypropylene oxide (PPO)- polyethylene oxide (PEO) triblock copolymer, have been widely used as emulsifiers of cubosomes. However, they greatly affect the cubosomes’ internal structures8),9),10),11). In addition, Pluronic F127 has been said to irritate the eyes and skin. Monoolein and monolinolein have poor thermal and oxidation stabilities due to their unsaturated bonds. Hence, novel cubic-phase-forming lipids which can be a substitute for monoolein, and emulsifiers which are more biocompatible are both desirable items currently. Boyd et al. have introduced phytantriol-based cubosomes as a novel cubic-phase-forming lipid and have reported that small amounts of impurities significantly alter its lyotropic liquid crystalline phase behavior12),13),14). Hato et al. synthesized various novel lipids and reported that some of them, such as 1-O-(5,9,13,17- tetramethyloctadecanoyl) erythritol (EROCO C22) or 1-O-(5,9,13,17-tetramethyloctadecyl)-β-D-xylopyranoside (β-XP), can form cubic phases15),16),17),18). MacDonald et al. introduced novel cationic phospholipids for lipoplex and transfection studies. They obtained cationic phospholipids by alkylation toward the phosphate group of phosphocholine and reported that 1,2-dioleoyl-sn-glycero-3-hexylphosphocholine forms bicontinuous cubic phases19),20),21),22),23). Polysaccharide polymers have also been assessed as substitute emulsifiers for Pluronic F127 for their ability to prepare cubosomes, though they are not amphiphilic block copolymers. Spicer et al. proposed a pseudoternary phase diagram of a monoolein/hydrophobically modified starch/water system and prepared cubosomes by hydrating starch and monoolein mixtures24). Their hydrophobically-modified system used three times as much starch as monoolein, and the particle size was 600 nm on average, which is too large for injection. Almgren et al. applied hydrophobically-modified ethyl hydroxyethyl cellulose (HMEHEC) to the monoolein based cubic phase25). However, HMEHEC interacted so strongly with monoolein that the monoolein-based cubic phase was easily transformed into lamellar and reversed hexagonal phases. Cellulose products are widely used as thickeners, excipients, disintegrants, or binders in cosmetics, foods, and pharmaceuticals. Hydroxypropyl methyl cellulose acetate succinate (HPMCAS) is a commercially available enteric coating agent and widely used in dry coating or solid dispersion systems26),27),28). Our previous study reported that HPMCAS was a promising emulsifier for stabilizing cubosomes without any modification of their internal structures29).
It is well known that there are three types of hydrated crystalline phases for higher alcohols (α, β, and γ-forms)30),31),32),33),34),35),36),37),38),39),40),41),42). The α-form hydrated crystals (often called α-gel, especially in the field of cosmetics) have a white appearance and high viscosity which enables the preparation of cosmetic products with high viscosities, including skincare creams or hair conditioners. Therefore, α-form hydrated crystals are often applied as stabilizers of oil in water (O/W) emulsions and have attracted much attention to the cosmetic industry. The long-periodic structure of the α-form hydrated crystal has a repeating bilayer structure, with a packed (sub-cell) structure of hydrophobic groups forming a hexagonal sub-lattice. Water molecules are solubilized between bilayers. It is also known that triacylglyceride demonstrates a complex polymorphism43),44),45),46). Triacylglycerides are esters derived from glycerols and three fatty acids, and the main constituents of fats in human bodies. Mykhaylyk et al. ascertained that 1,3-distearyol-2-oleoyl-sn-glycerol forms six crystal phases (α2, α1, γ, β1, and β2)43). However, almost no hydration occurrs due to its high hydrophobicity. Therefore, the α-form crystals formed by triacylglycerides are applied as stabilizers of water in oil (W/O) emulsions47). At present, it has been reported that there are various systems which exhibit an α-form hydrated crystalline phase. Fukushima et al. investigated the phase diagram of a cetyl alcohol/stearyl alcohol/polyoxyethylene (15 mol) oleyl ether/water system31),32). The structure of an α-form hydrated crystal of stearyl trimethyl ammonium chloride/1-hexadecanol/water system was proposed by Yamaguchi34),35). Debnath et al. reported molecular dynamics simulations for the behenyl trimetyl ammonium chloride/stearyl alcohol/water system and evaluated the gel to liquid crystalline transitions36). An α-form hydrated crystalline phase of a sodium N-stearoyl N-methyl taurate/behenyl alcohol/water system was introduced by Watanabe37).
It has been reported that mixed cationic-anionic surfactant systems exhibit a significantly low critical micellar concentration (cmc) and unique adsorption behaviors48),49),50). Nakama et al. evaluated the phase diagram of stearyl trimethyl ammonium chloride and a mixed sodium N-lauroyl-N-methyl alanine system and the adsorption of the complex on keratin48),49). Lucassen-Reynders reported the strong synergistic effect on surface pressure for mixed solutions of sodium dodecyl sulfate and dodecyl trimethyl ammonium bromide50). In this review, therefore, the physicochemical properties of the α-form hydrated crystals containing an ionic complex of anionic and cationic surfactants was introduced41). All of these systems contain only hydrocarbon surfactants and there was previously no report indicating that silicone surfactants form α-form hydrated crystalline phases with higher alcohols. Recently, however, it has been reported that a certain type of silicone surfactant can form an α-form hydrated crystal with higher alcohols42).
Various kinds of receptors have been reported to localize in lipid raft domains and the microfluidity of these domains regulates the activation of these receptors51),52),53),54),55),56),57),58). Lipid rafts are the membrane microdomains rich in sphigolipids and cholesterol. They are more ordered and tightly packed than surrounding bilayers, but float freely in the membranes. Szolcsanyi et al. revealed that depletion of different constituents of lipid raft inhibit the gating of the TRPV1 (transient receptor potential vanilloid 1) cation channel by various vanilloid and non-vanilloid agents56). Morenilla-Palao et al. have reported that menthol- and cold-mediated responses of TRPM8 (transient receptor potential melastatine 8), which localizes in lipid raft domains, are potentiated when cholesterol molecules are removed by methyl-β-cyclodextrin from the lipid raft domains51). These results imply the importance of characterizing and controlling the microfluidity of lipid rafts. In the cosmetic industry, it is well known that users generally experience a stronger cooling sensation from conditioners than shampoos, even when the amounts of menthol are the same in both. Based on this empirical rule, the microfluidity of lipid rafts when adding cationic surfactants was evaluated using florescence spectroscopy59).
Figure 1 (a) shows the SWAXS pattern of a non-dispersed liquid crystalline phase formed in a monoolein/water binary system29). The scattering curve showed six peaks ( (110), (111), (200), (211), (220), (300), and/or (221) ) in the characteristic ratio for a cubic structure of the type Pn3m, denoted by Q224 or CD phase2),3),4),6),7),9). For the monoolein/HPMCAS/water ternary systems with 10 and 20 wt% HPMCAS, similar diffraction patterns, corresponding to the Pn3m space group, were observed as shown in Fig. 1 (b) and (c). Since the peak positions of these obtained scattering curves were almost unchanged, it was suggested that HPMCAS hardly affected the self-assembly of monoolein. Nakano et al. reported that self-assembled structures of monoolein were converted from Pn3m to Im3m (also denoted by Q229 or CP) cubic structures by the presence of 5-15 wt% of F12711),60). Unlike F127, HPMCAS has quite low miscibility with monoolein.

(a) SWAX pattern from non-dispersed liquid crystals of the monoolein/water binary system at 25°C. (b) and (c) SWAX patterns from non-dispersed liquid crystals of monoolein/HPMCAS/water ternary systems at 25°C. The weight ratio of HPMCAS to monoolein is (b) 1:9 and (c) 1:4.
Figure 2 shows SWAXS patterns from cubosomes of EROCO C22 dispersed with F127 (a) and HPMCAS (b) measured at 25°C. The diffraction pattern indicated that the Im3m space group was coexistent with the Pn3m space group for the EROCO C22/F127 system as shown Fig. 2 (a). Sagalowicz et al. revealed that cubosomes which have a Pn3m space group exist separately from those with the Im3m space group in the same soulution, as seen using cryo-transmission electron scopy61). This result suggests that F127 affects the self-assemblies of EROCO C22 as well as those of monoolein. On the other hand, diffraction peaks derived from the Im3m space group were not observed for the EROCO C22/HPMCAS system as shown Fig. 2 (b). Therefore, HPMCAS seems to simply adsorb on the particle surface.

SWAXS patterns from cubosomes of EROCO C22 dispersed with (a) Pluronic F127 and (b) HPMCAS as emulsifiers at 25°C. The weight ratio of the emulsifier to EROCO C22 is 1:9. Downward arrows nindicate cubic Im3m peaks.
The schematic illustration of cubosomes stabilized by HPMCAS and their internal Pn3m cubic structures are shown in Fig. 3. According to one of our previous studies, the diameter of the cubosomes stabilized by HPMCAS was about 150 nm29). HPMCAS simply adsorbs on the particle surface, resulting in the preservation of the internal Pn3m cubic structure. The particles are negatively charged and stabilized by electrostatic repulsion since HPMCAS has acetate and succinate groups. HPMCAS is a promising stabilizer for cubosomes and can be substituted for F127.
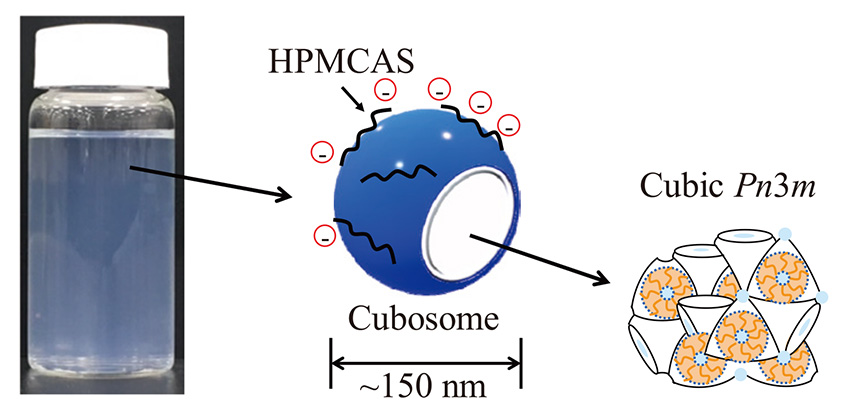
Schematic illustration of cubosomes stabilized by HPMCAS and their internal Pn3m cubic structures.
Electrolytes often affect the stability of O/W emulsions. Konno developed an O/W emulsion system stabilized by an α-from hydrated crystal composed of sodium N-stearoyl N-methyl taurate and also investigated the influence of electrolytes on the system’s stability62). This system had excellent salt tolerance. However, it had poor viscosity stability and viscosities decrease over time in a non-electrolyte condition. Figure 4 shows the effect of distearyl dimethyl ammonium chloride (DSAC) on the viscosity changes. Detailed formulae for viscosity measurements have previously been reported41). All the samples for this measurement contained 0.20 wt% of sodium N-stearoyl N-methyl taurate and 1.40 wt% of higher alcohol to form the α-from hydrated crystalline phase. A clear concentration dependence of DSAC was observed. As shown in Fig. 4, the viscosity of the sample without DSAC decreased over time from 3,560 to 2,950 mPa·s (open circles). It kept decreasing over 1 year and became lower than 2,000 mPa·s (data not shown). In contrast, the viscosity stability of the sample containing 0.05 wt% DSAC was largely improved and its viscosity decreased only a little from 4,080 to 3,950 mPa·s (open squares). It remained higher than 3,500 mPa·s after 1 year (data not shown). Figure 5 shows the cryo-SEM images of O/W emulsion samples. Basically, the formulae for the cryo-SEM observation were the same as those for the viscosity measurement. Humectants were removed from the formulae for the cryo-SEM observation since they prevent samples from freezing. Cryo-SEM images of samples without DSAC after 1 week and 2 months are shown in Fig. 5 (A) and (B), respectively. Stratified vesicle structures with 3 or 4 layers around the emulsion particles were clearly observed as shown in Fig. 5 (A)62). After 2 months, the number of stratified vesicle structures seemed to increase as shown in Fig. 5 (B). This increase in the number of stratified vesicle structures was assumed to cause the decrease in viscosity. To the contrary, such stratified vesicle structures completely disappeared and, in their place, plate-like structures were observed in the system containing 0.05 wt% DSAC as shown in Fig. 5 (C) and (D). Even after 2 months, no structural changes were observed (Fig. 5 (D) ). The structural transition from vesicle to plate-like structures was assumed to result from the addition of DSAC, and this transition improved the viscosity stability.

Time course of the viscosity of O/W emulsion samples stabilized by α-from hydrated crystals composed of 0.20 wt% of sodium N-stearoyl N-methyl taurate. Plots represent viscosities of samples containing 0 wt% (circles), 0.02 wt% (triangles), 0.05 wt% (squares), 0.07 wt% (diamonds), and 1.00 wt% (crosses) DSAC.

Cryo-SEM images of O/W emulsion samples stabilized by α-from hydrated crystals composed of 0.20 wt% of sodium N-stearoyl N-methyl taurate. The magnifications of the images were 500 and cryo-SEM observation was performed between −70 and −90°C. Sample without DSAC (A) after 1 week and (B) after 2 months. Sample containing 0.05 wt% DSAC (C) after 1 week and (D) after 2 months. The arrows in the images (C) and (D) represent plate-like structures.
This study has reported for the first time that a certain silicone surfactant can also form an α-from hydrated crystal with higher alcohols42), and we designed and synthesized a novel silicone surfactant, 3-(10-carboxydecyl-1,1,1,3,5,5,5-heptamethyl trisiloxane (CDTS). CDTS was neutralized with triethanol amine (TEA) and the molar ratio of CDTS to TEA was 5:6. Figure 6 shows the pseudo ternary phase diagram of the CDTS-TEA/behenyl alcohol (BEA)/polyoxyethylene (5 mol) glyceryl monostearate (GMS-5) /water system at 25°C. Unlike other α-from hydrated crystal systems previously reported30),31),32),33),34),35),36),37),39),41),62), an α-from hydrated crystal cannot be formed in the CDTS-TEA/BEA/water system since the miscibility of CDTS to higher alcohols was too poor to form a eutectic complex. This study found that GMS-5 had excellent miscibility to CDTS as well as higher alcohols and that single α-form hydrated crystalline phase was obtainable at an ambient temperature by adding GMS-5 molecules to CDTS-TEA/BEA/water systems. Figure 7 (a) shows the SWAXS pattern of the α-form hydrated crystalline phase at 25°C in the CDTS-TEA/BEA/GMS-5=2/4/3 system (represented as a star symbol in Fig. 6). A sharp peak at 15.1 nm−1 was clearly observed, which indicated the existence of a hexagonal sub-lattice of the α-form hydrated crystal. Periodic peaks at low q region (0.2<q<2.0 nm−1) indicated a repeated bilayer structure. The interplanar distance was calculated from the peak positions at the low q region to be 24.2 nm. From the DSC thermogram shown in Fig. 7 (b), it turned out that there were three phase transitions in this system (γ-form to β-form, β-form to α-form, and α-form to liquid crystal). The melting points of each phase transition were 8, 20, 63°C.

Pseudo ternary phase diagram of CDTA-TEA/BEA/GMS-5/water system at 25°C. Total surfactant concentrations were set to 20 wt%. Compositions represented on the ternary phase diagram were based on mole fractions. The phases shown are the lamellar liquid crystalline (Lα), α-form hydrated crystalline (α-form), β-form hydrated crystalline (β-form), and γ-form hydrated crystalline (γ-form) phases. The star symbol represents the CDTA-TEA/BEA/GMS-5=2/4/3 system.

(a) SWAXS pattern for CDTS-TEA/BEA/GMS-5/water system at 25°C. (b) DSC thermogram for CDTS-TEA/BEA/GMS-5/water system. The CDTS-TEA/BEA/GMS-5 molar ratio is 2:4:3.
Our previous study evaluated the membrane microfluidity of liposomes containing raft domains by fluorescence spectrometry59). Liposomes consisting of egg-yolk L-α-phosphatidylcholine (eggPC), egg-yolk sphingomyelin (eggSM), and cholesterol were used as models of cell membranes. The eggPC/eggSM/cholesterol molar ratio of liposomes was 7:6:6. Accoding to de Almeida et al., liquid-ordered (lipid raft) and liquid-disordered phases are thought to coexist under this molar ratio63),64). The microfluidity of the membranes was characterized via fluorescence-generalized polarization of 2-dimethylamino-6-lauroylnaphtalene (Laurdan)65),66),67),68),69). The emission spectra of Laurdan were recorded with excitation at 350 nm at 32°C. Methanol solutions of each surfactant (10 mg/mL) were added to the liposome (100 μM of total lipids, 2 mL). Figure 8 (a) shows the changes in the emission spectra of Laurdan in liposomes upon the addition of dilauryl dimethyl ammonium chloride (DLAC). With the addition of DLAC, the emission maxima of the spectra red-shifted from 440 to 490 nm and a clear isosbestic point was observed. Laurdan displays emission maxima at 440 and 490 nm when the lipid membranes are in liquid ordered and liquid disordered phases, respectively65),66),67),68),69). Hence, this result suggested that DLAC modified the membrane microfluidity of liposomes. The shift of the emission maximum of Laurdan can be quantified via the generalized polarization (GP) value, which is calculated from the emission spectra as follows: GP= (I440−I490) / (I440+I490), where I440 and I490 are the intensities of the emissions at 440 and 490 nm, respectively. According to Sanchez et al., the GP values in the liquid crystalline phase are between −0.3 and 0.3, whereas those in the gel phase are typically between 0.5 and 0.666). Figure 8 (b) shows the changes in the GP values of Laurdan in liposomes as a function of the higher surfactant concentration. When DLAC molecules were added to the liposome solution, the GP values largely decreased. The minimum GP value in the presence of DLAC was approximately −0.3, indicating that the raft regions are considered to entirely convert to the liquid disordered phase. The obtained results indicated a possibility that one could control the membrane fluidity of liposomes as well as living cells.

(a) Changes in the emission spectra of Laurdan in eggPC/eggSM/cholesterl liposomes at 32°C upon the addition of DLAC. (b) Changes in GP values of Laurdan in eggPC/eggSM/cholesterl liposomes as a function of DLAC concentration.
This review focused on lipid self assemblies and lipid membrane fluidity. In order to control self-assembled structures, miscibility or hydrophilic-lipophilic balance must first be considered. Understanding and controlling membrane fluidity is important for living cells as well as liposomes. There is a much higher number of self assemblies in addition to the ones mentioned in this review. Hence, further study is still required to fully understand their mechanisms and their physicochemical properties.
The author is indebted to Prof. Dr. Handa and Prof. Dr. Nakano for their guidance and support. Also, the author would like to thank CytoPathfinder and Dow Corning Toray for providing the materials necessary for this study.