2025 Volume 74 Issue 1 Pages 13-23
2025 Volume 74 Issue 1 Pages 13-23
Tangeretin is one of the most abundant polymethoxyflavones in citrus peel and its pharmacological effects are extremely rich. However, due to its poor solubility, bitter taste and poor oral bioavailability, the oral administration of tangeretin is still limited, which seriously limits its application in industrial production. The establishment of encapsulation and delivery systems to improve bioavailability is an effective method. This paper reviewed the research progress of the structure and properties, pharmacological effects and main methods to improve bioavailability of tangeretin, including emulsion delivery, lipid encapsulation, microencapsulation and other delivery and utilization research and application. The article aims to provide theoretical basis for the high-value application of tangeretin in functional food and pharmaceutical industry.
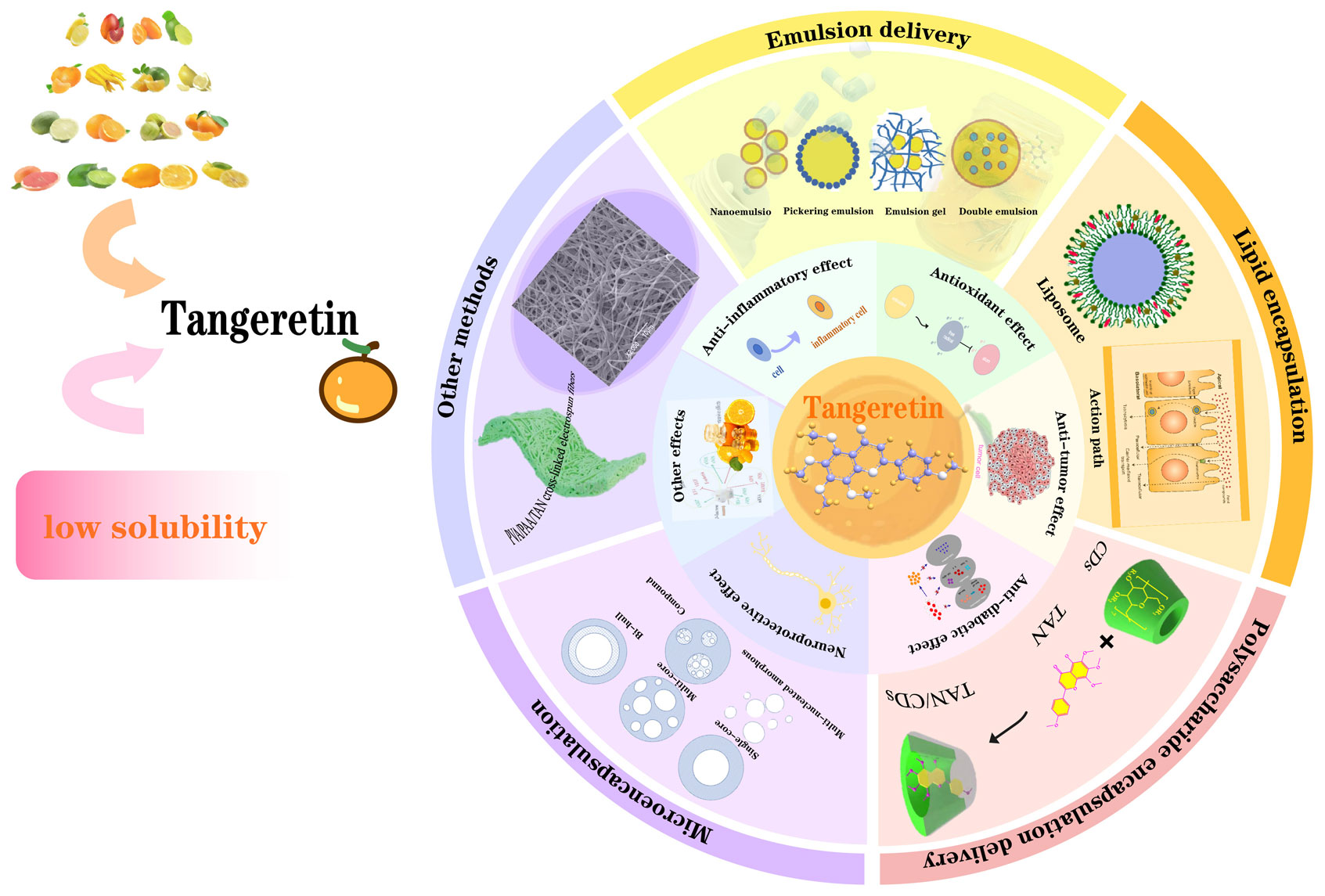
Tangeretin is one of the important polymethoxyflavones (PMFs) in citrus peel. It has a variety of functional properties, including anti-cancer, anti-inflammatory, anti-atherosclerosis, and protection of liver and nerves. However, tangeretin is a highly hydrophobic compound with poor water solubility and bitter taste, which limits its development and application in pharmaceuticals or functional foods1) . Hesperetin is a kind of dihydroflavonoid compound which also exists in citrus peel as tangeretin. At present, through biological modification and chemical modification technology, the development and utilization rate of hesperetin is much higher than that of tangeretin, and its encapsulation technology and solubilization technology have also been mature2) . In contrast, there are relatively few studies on improving the bioavailability of tangeretin. Therefore, seeking effective carriers to improve the stability of tangeretin and promote its biological application has become an urgent need for current research.
In this paper, the structure and properties, pharmacological effects and methods of improving bioavailability of tangeretin were comprehensively described. The paper aims to provide theoretical basis for further research on the high-value application of tangeretin in new functional food and pharmaceutical industry. The main content of the thesis is shown in Fig. 1.
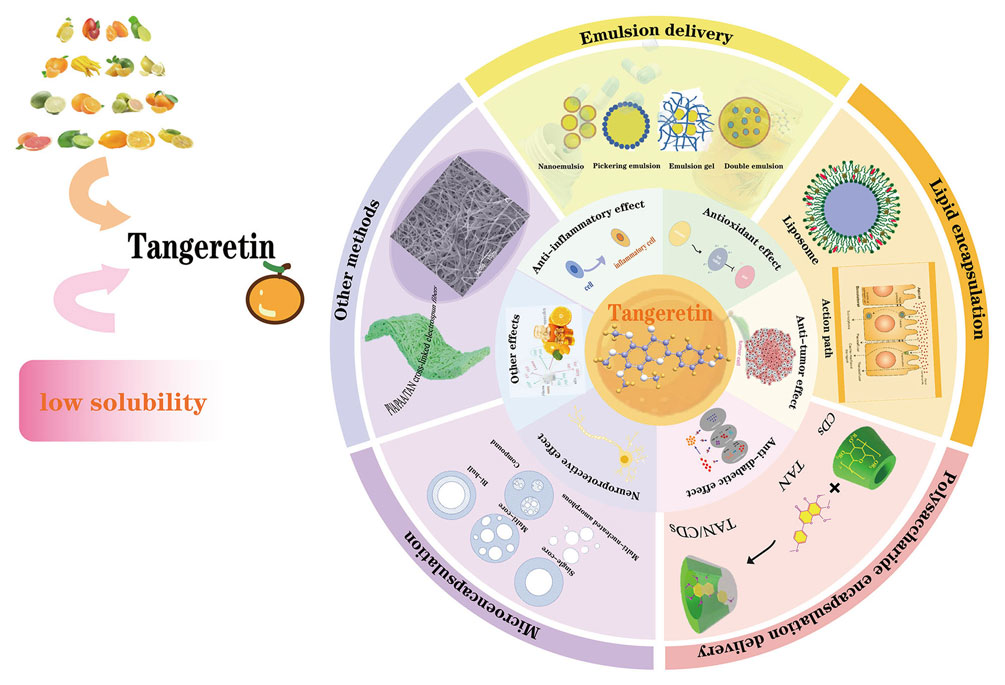
Pharmacological effects of tangeretin and ways to improve bioavailability. The innermost circle is the ball-stick model of tangeretin, the second circle represents the different pharmacological effects of tangeretin and the third cycle represents different methods to improve the bioavailability of tangeretin.
PMFs are a class of flavonoids containing multiple methoxy groups, which are widely found in citrus plants of Rutaceae. Compared with other varieties of citrus, the content of PMFs in the peel of Chinese sweet orange and Chinese citrus is higher3) ,4) . At least 135 kinds of PMFs have been reported in the existing literature, among which the contents of tangeretin (TAN) , nobiletin (NOB) and sinensetin (SIN) are high in citrus fruits5) , and their molecular structures are shown in Fig. 2. However, the current research indicates that tangeretin is almost exclusively present in the peel of citrus fruits, and the content is less6) .

The structural formulas of tangeretin (a) , nobiletin (b) and sinensetin (c) .
Tangeretin, also known as 4’,5,6,7,8-pentamethoxyflavone, is a secondary metabolite of low molecular weight. Tangeretin contains two aromatic rings, a heterocyclic pyran ring and five methoxy groups, one of which has four methoxy groups. The pyran ring is polar, thus endowing the molecule with hydrophilicity. However, all other functional groups extended from the aromatic ring are methyl groups, providing a non-polar surface area for hydrophobic interactions7) .
2.2 Physicochemical properties of tangeretinTangeretin is a light yellow needle-like solid with a melting point of 153-154°C7) , and it is insoluble in water and soluble in organic solvents such as methanol or ethyl acetate. The two most abundant polymethoxyflavones in citrus are TAN and NOB. Tangeretin has five methoxy groups, while nobiletin has six methoxy groups, and the LogP value of tangeretin is 2.78) , while the LogP value of nobiletin is 5.59) , so the water solubility of tangeretin is relatively higher. Tangeretin has the characteristics of low solubility and high crystallinity, which makes its bioavailability low after oral administration, and it is difficult to be compatible with water-soluble food systems, thus greatly limiting its direct application in the field of drugs and functional foods10) .
2.3 Absorption and metabolism of tangeretinTangeretin enters the blood mainly through oral and intravenous ways11) . Hung et al.12) collected blood samples from the tail vein of the same animal, obtained plasma samples after centrifugation and found that the maximum concentration (Cmax) of TAN in plasma reached 87±0.33 μg/mL (2.25×10-5 mol/L~2.43×10-5 mol/L) after oral treatment (50 mg/kg b.w.) , the peak concentration appeared at 340±48.99 minutes after administration (Tmax) , and the half-life (t1/2) of the drug was 342.43±71.27 minutes. In contrast, the concentration of TAN in plasma was significantly reduced to 1.11±0.41 μg/mL (1.88×10-6 mol/L~4.89×10-6 mol/L) after intravenous administration (5 mg/kg b.w.) , and reached the peak concentration almost immediately (Tmax=1.11±0.41 min) , while the half-life was shortened to 69.87±15.72 min. Among them, the value of Cmax can reflect the absorption rate and degree of the drug in the body. The preparation with higher Cmax usually means that its bioavailability is higher. Tmax reflects the parameter of the onset speed of the drug. The shorter the peak time is, the faster the drug is absorbed and the faster the onset is. After oral and intravenous administration, the total area under the curve (AUC) was 213.78±80.63 and 78.85±7.39 min μg/mL, respectively. AUC is the most reliable index to evaluate bioavailability. The greater the AUC value, the more the drug is absorbed. The absolute oral bioavailability (F) was 27.11%. The calculation formula is as follows:
 |
The AUCpo and AUCiv correspond to the areas under concentration-time curves after oral and intravenous administration, respectively. Dosepo and Doseiv correspond to the actual doses received via oral and intravenous administration. In addition, only 0.0026% and 7.54% of TAN were found in urine and feces after oral administration, and less than 8% of tangeretin was excreted through urine or feces, indicating that about 92% of tangeretin was absorbed or excreted in the form of its metabolites, mainly demethylated tangeretin and conjugates of glucuronates and sulfates.
Kurowska et al.13) studied the metabolites of PMFs in serum, liver and urine of hamsters by feeding them with diets supplemented with 1% TAN, 0.25% TAN/NOB and 1% TAN/NOB. Quantitative data showed that the tangeretin metabolites in serum solids were mainly composed of glucuronide (91%) . The four main metabolites of liver extract were glucuronide, free glycoside ligand, dihydroxytrimethylflavone and monohydroxytetramethylflavone. The metabolites of tangeretin found in urine were similar to those detected in serum, but the total concentration of metabolites in urine was significantly higher. In the in vivo biotransformation study, Nielsen et al.14) repeatedly fed 100 mg/kg of tangeretin to rats. By HPLC-MS and 1H NMR techniques, the main metabolites found in rat urine and feces were characterized as 4’-demethyltangeretin and 3’,4’-dihydroxy-5,6,7,8-tetramethoxyflavone. Moreover, urine analysis determined that 38% of tangeretin metabolites were excreted in the form of conjugates of glucuronate and sulfate15) . Although tangeretin itself does not contain hydroxyl groups, its metabolites are all demethylation products, which are hydroxylation products to a certain extent. The results showed that the 4’ position seemed to be the main site of demethylation of tangeretin, and the 6 position was the second common demethylation site. In addition, some secondary metabolites were hydroxylated at the 3’ position. Therefore, the glucuronidation or sulfation of tangeretin mainly occurs at the 4’ position, followed by the 6 and 3’ positions14) . Datla et al.16) found that the tissue concentration of tangeretin in liver was the highest, followed by kidney, spleen, lung and heart. Studies have shown that another type of PMFs (NOB) is also mainly accumulated in the liver and kidney after oral administration17) . Overall, these results suggest that PMFs are usually accumulated in the liver and kidney, possibly because they are well-perfused organs18) ,19) .
The anti-inflammatory effect is one of the most effective functions of tangeretin, which be used in the treatment of many diseases, such as kidney injury, neuritis, allergic rhinitis, rheumatoid arthritis and so on20) . Sun et al.21) used 4-5-week-old male C57/BL6J mice to explore the potential protective effect of tangeretin on diet-induced metabolic inflammation. The data showed that tangeretin promoted macrophage M2 polarization and reduced inflammation by reprogramming glucose metabolism and promoting lactic acid accumulation. Xu et al.22) used 3-week-old male BALB/c mice as an animal model, and found that tangeretin can reduce the secretion of interleukin-1β by inhibiting the activation of nuclear factor-κB, thereby inhibiting respiratory syncytial virus replication and inhibiting RSV-induced lung inflammation in vivo. Furthermore, the regular consumption of tangeretin at a dose of no more than 10 mg/day can help protect children from RSV infections. Yang et al.23) found that tangeretin had neuroprotective and anti-inflammatory effects on ischemia/reperfusion injury in rats by inhibiting inflammatory response. Research showed that tangeretin attenuated the enhanced inflammatory response by inhibiting pro-inflammatory signaling pathways24) . Lee et al.25) used immortalized mouse BV 2 microglia to demonstrate the anti-inflammatory properties of tangeretin in activated microglia and its potential molecular mechanism. Tangeretin significantly inhibited the production of nitric oxide and pro-inflammatory cytokines in microglia stimulated by lipopolysaccharide (LPS) . Further studies have shown that tangeretin inhibits LPS-induced phosphorylation of mitogen-activated protein kinase and protein kinase B.
3.2 Antioxidant effectTangeretin is a natural antioxidant, which has a very precious value for both medicine and food. Kou et al.26) utilized exhaustive swimming test in mice, and found that tangeretin depended on the activation of Keap1-Nrf2 signaling axis to improve the activity of antioxidant enzymes, reduce oxidative stress and myocardial injury, and protect myocardial morphology and ultrastructure. Tangeretin exerted antioxidant effects by modulating NADPH oxidase and HO-1 expression in LPS-stimulated microglia25) . Wang et al.27) used ICR mice as the research object and in vivo experiments showed that tangeretin could induce and maintain the activity of antioxidant enzymes in mouse liver by inhibiting the ubiquitination degradation of Nrf2, the main regulator of endogenous antioxidant pathway. The gastric concentrations of tangeretin and neohesperidin were 1 mg/kg and 500 mg/kg, respectively, indicating that both tangeretin and neohesperidin could activate antioxidant enzymes in mouse liver, but the effective concentration of tangeretin was lower. In addition, PMFs also have the ability to inhibit hydrogen peroxide-induced oxidative stress. Therefore, the antioxidant function of tangeretin, in addition to the application in the field of pharmaceutical field, is also applied to the food industry, such as food preservative, antioxidant functional food and so on. However, there is few research on its application in food industry20) ,28) .
3.3 Anti-tumor effectTangeretin has been reported to have therapeutic effects on a variety of cancers, including liver cancer, stomach cancer, breast cancer, cervical cancer, bladder cancer, melanoma, etc20) . Dong et al.29) investigated the apoptosis mechanism of tangeretin in human gastric cancer AGS cells and found that tangeretin induced apoptosis of AGS cells mainly through p53-dependent mitochondrial dysfunction and the Fas/FasL-mediated extrinsic pathway. Venkatakarthikeswari et al.30) studied the anti-apoptotic signaling mechanism of tangeretin in oral cancer cells in vitro. Xi et al.31) researched the promoting effect of tangeretin on the apoptosis of human lung cancer cells (H1299 and H1975) induced by tumor necrosis factor-associated apoptosis-inducing ligand (TRAIL) . Tangeretin induced death receptors, enhanced TRAIL-induced apoptosis through upregulation of the ROS-JNK/ERK-CHOP pathways, and provided the idea that tangeretin could be an effective candidate drug in combination with TRAIL to induce apoptosis of cancer cells. Gurunathan et al.32) studied the apoptotic properties of adriamycin enhanced by tangeretin-assisted platinum nanoparticles for the combined treatment of osteosarcoma. Tangeretin exhibited some anti-tumor characteristics in several types of cancer cell lines, such as anti-proliferation, apoptosis, anti-metastasis, regulating the expression of tumor suppressor genes and epigenetic regulation. Therefore, it is promising to become a cytotoxic anticancer compound for optimizing therapeutic methods and searching for new drugs to treat cancer6) .
3.4 Anti-diabetic effectStudies shown that tangeretin has an anti-diabetic effect without negative side effects. Sundaram et al.33) used streptozotocin (STZ) -induced diabetic cardiac complications in experimental rats as an animal model, and showed that tangeretin played a beneficial role in regulating diabetes and its related heart complications through biochemical tests and histological observations. This effect was possibly mediated through inhibition of hepatic gluconeogenic enzymes (glucose-6-phosphatase and fructose-1, 6-bis-phosphatase) and increased the expression of GLUT4 in the cardiac muscle of diabetic rats via enhanced insulin secretion as evidenced by improved histopathology of pancreas. They compared tangeretin with glibenclamide in normalizing disturbed serum and heart lipid levels and found that their effects were comparable. Sun et al.34) evaluated the ability of tangeretin to improve diabetic nephropathy compared with the metformin, a conventional hypoglycemic drug. The results showed that both tangeretin and metformin showed anti-inflammatory properties by improving the increase of inflammatory cytokines TNF-α, IL-6 and IL-Iβ. Tangeretin (25 mg/kg b.w.) could significantly slow down the progression of diabetic kidney in STZ-induced diabetic rats, and was higher than metformin (150 mg/kg b.w.) in increasing the serum insulin level of rats. This may be because tangeretin enhances the ability of surviving pancreatic cells to secrete insulin through its antioxidant capacity. Based on its good anti-sugar effect and minimal toxicity, tangeretin is expected to be a good natural hypoglycemic drug.
3.5 Neuroprotective effectTangeretin has the potential to prevent neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease by regulating a variety of signaling pathways, including stress-activated protein kinase, phosphoinositide 3-kinase, mitogen-activated protein kinase, Nrf2, extracellular signal-regulated kinase, CRE dependent transcription and so on, thus inhibiting oxidative and inflammatory pathways11) . Bao et al.35) showed that TAN supplementation could prevent cognitive deficits in APP/PS1 mice. Notably, TAN supplementation alleviated synaptic dysfunction and integrity. In addition, TAN supplementation reduced β-amyloid accumulation and β-secretase activity. These findings suggest that dietary supplementation of TAN can be considered as a preventive therapy for patients with Alzheimer’s disease. In oral acute toxicology experiments, high concentrations of TAN exposure had no effect on cell viability. Long-term application of high-dose tangeretin to mice did not show any decrease in neurons and no significant change in body weight. These results indicate that TAN is a safe dietary supplement without obvious side effects. Takano et al.36) used C57 BL/6 mice (male, 8-12 weeks old) to establish a mouse endoplasmic reticulum stress model by a single intraperitoneal injection of urethromycin (1 mg/kg) . Studies have shown that tangeretin has a strong protective effect on endoplasmic reticulum stress in vitro and in vivo. The enhancement of endoplasmic reticulum stress can lead to cell death in various pathophysiological states. Preadministration of tangeretin in mice enhanced expression of glucose-regulated protein (GRP) 78 in the substantia nigra pars compacta and protected dopaminergic neurons against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, a neurotoxin that induces both oxidative and ER stress. Alla et al.37) used adult male Wistar rats (200-220 g) for research and found that tangeretin had neuroprotective, cognitive and memory enhancement effects on global cerebral ischemia. Tangeretin treatment significantly improved cognition and memory by enhancing acetylcholine levels through the amelioration of acetylcholinesterase activity in bilateral common carotid artery occlusion (BCCAO) rats. In addition, it showed that tangeretin exhibited neuroprotective effects by reducing oxidative stress, inflammation and apoptosis in BCCAO rats. Based on the various effects of tangeretin on the nervous system and excellent physicochemical and pharmacokinetic characteristics11) , it has great development prospects in the field of neural adjuvant therapy and can also be used as a health food to prevent neurodegenerative diseases.
3.6 Other effectsIt has shown that tangeretin has other pharmacological effects, such as anti-thrombosis, lowering blood pressure and regulating metabolism. Tangeretin has a certain effect on platelet function, signal transduction and hemostasis, and inhibits human platelet activation induced by agonists such as collagen in a concentration-dependent manner, which can reduce the risk of thrombotic diseases38) . Li et al.39) found that orange peel oil rich in PMFs reduced systolic and diastolic blood pressure in ω-nitroarginine-induced hypertensive rats. Liu et al.40) showed that tangeretin had great potential in the treatment of exercise-induced asthma (EIA) , and was expected to provide a new way for EIA intervention in athletes. Kou et al.41) found that tangeretin enhanced mitochondrial biogenesis via activating the AMPK-PGC1-α pathway, resulting in the improvement of exercise performance, and tangeretin may be a potentially novel mitochondria regulator in foods. In addition, tangeretin has anti-obesity biological activity and can improve fat metabolism.
Although tangeretin has various pharmacological effects, the poor water solubility and low bioavailability of tangeretin seriously hinder its further development and utilization. Therefore, how to improve the bioavailability of tangeretin has become the focus of current research. The characteristics of oils directly affect the bioavailability of tangeretin in four aspects. Firstly, oil can dissolve many fat-soluble substances, so choosing appropriate oil as the carrier or solvent of tangeretin can significantly improve the solubility and dissolution rate of tangeretin in vivo, so as to improve its bioavailability. Secondly, the viscosity of the oils helps to protect the tangeretin from the destruction of the external environment (such as gastric acid, enzyme, etc.) , improve its stability in the gastrointestinal tract and increase its bioavailability. Thirdly, by forming an emulsion of tangeretin and oil, its surface area can be increased, and the dispersion and absorption of the drug in the gastrointestinal tract can be promoted, thereby improving its bioavailability. Fourthly, chemically stable oil, as a carrier of tangeretin can inhibit its degradation during storage and transportation and maintain its biological activity.
The following is a classification of current methods for improving the bioavailability of tangeretin.
4.1 Emulsion deliveryEmulsion delivery systems, such as emulsions and nanoemulsions, are highly efficient mediums for encapsulating, protecting and delivering lipophilic bioactive ingredients for food, beverage, dietary supplements and pharmaceutical applications42) . These systems inhibit bioactivity degradation during storage and increase bioavailability after ingestion, thereby enhancing its overall bioactivity43) . The following is a summary of the studies on the enhancement of tangeretin bioavailability by emulsion delivery system from functional food and medical pharmaceutical aspects.
4.1.1 Functional foodYu et al.44) encapsulated sinensetin, tangeretin and nobiletin in pickering emulsion stabilized by zeolysin/amylopectin complex colloidal particles (ZPPs) , and studied their protective effects and in vitro digestion. The results showed that ZPPs-pickering emulsion loaded with PMFs had a strong gel-like network structure and improved the free radical scavenging ability of PMFs. During the digestion process, the bioaccessibility of sinensetin, tangeretin and nobiletin reached 7.96%, 7.03%, and 5.56%, respectively. Bioaccessibility here refers to the extent to which food, drugs or other substances are released in the digestive tract and their availability in the organism. Its usage is similar to bioavailability. Among them, the lower bioavailability of nobiletin may be due to the fact that it has one more methoxy group than the other two, and the structural formulas of the three are shown in Fig. 2.
Hu et al.45) studied the effects of adding hydroxypropyl methyl cellulose (HPMC) , cinnamaldehyde (CA) and gum arabic (GA) biopolymers on the bioavailability of tangeretin in whey protein emulsions in vivo and in vitro. The results of in vivo pharmacokinetics in rats showed that in the presence of HPMC, the concentration of tangeretin in plasma increased from 4 times to 20 times after encapsulation. HPMC also prolonged the release of tangeretin to 22 hours. The results of in vitro digestion showed that the addition of CA, GA and HPMC to the stable whey protein concentrate slightly slowed down the rate of lipid digestion. The three additives increased the bioavailability of tangeretin encapsulated in whey protein emulsions from about 36% to 90%, 56%, and 58%, respectively. Compared with cinnamaldehyde and gum acacia, adding hydroxypropyl methyl cellulose to emulsion is the most effective way to improve the bioavailability of tangeretin.
Ting et al.46) used different in vitro and in vivo models to elucidate mechanically the effects of emulsification on oral bioavailability of tangeretin. The results showed that the digested rate of emulsified tangeretin was faster than that of unprocessed tangeretin oil suspension and its oral bioavailability was 2.3 times higher than that of unprocessed tangeretin oil suspension. Yang et al.47) successfully prepared PMFs loaded emulsions using four different citrus oils (citrus oil, orange peel oil, sweet orange oil and bergamot oil) as raw materials and analyzed physicochemical properties and encapsulation efficiency. The results showed that the loading capacity and encapsulation efficiency of the emulsion with bergamot oil as the carrier were the highest. Based on a diet that consumes 2,000 kilocalories per day48) , the effective dose of PMFs is about 50-250 mg per day. The concentration of PMFs in most citrus peel molasses is less than 100 μg/mL, that is, to achieve the minimum effective dose, at least 500 mL of citrus peel molasses is required49) . However, with the loading level of PMFs emulsion, the minimum effective dose can be easily achieved by consuming preparations as low as 1.52 g50) . The excellent loading capacity of the emulsion system makes tangeretin more bioaccessible.
These results are helpful to design tangeretin into a more complete emulsion delivery system, which can be used to encapsulate functional foods and deliver PMFs into beverages and other nutritional supplements, and significantly improve the stability and bioavailability of PMFs.
4.1.2 Medical pharmaceuticalsTing et al.50) emulsified tangeretin and 5-demethyl tangeretin with lecithin based viscoelastic emulsion, and investigated the effectiveness of lecithin based viscoelastic emulsion system on PMFs encapsulation. The results showed that the emulsion system had excellent storage stability and significantly reduced the relative viability of liver cancer and colon cancer cells. They also administered different doses of tangeretin to mice using an optimized lecithin-based viscoelastic emulsion (LE) system and medium-chain triglyceride (MCT) suspension as carriers, and the results showed that the LE system increased the administration efficacy of tangeretin and significantly improved its system availability and potential anti-cancer efficacy51) . Wan et al.10) successfully prepared susaturated self-emulsifying nanoemulsion of tangeretin using soybean protein isolate, which significantly enhanced the oral absorption of water-insoluble drugs. The release behavior of the nano-emulsion was effectively enhanced under the simulated conditions of gastrointestinal tract, and the efficient encapsulation and protection of tangeretin was realized, thus improving the bioavailability of tangeretin. Tan et al.52) prepared a polyethioether-polyester prodrug micelle triggered by active oxygen by self-assembly method. The micelle had a hydrophilic shell of polyethioether and a hydrophobic core of tangeretin, with a loading efficiency of 63.15%. It had good biocompatibility and effectively enhanced the antioxidant activity of tangeretin. Biocompatibility refers to the reaction of living tissues to inactive substances, and generally refers to the compatibility between materials and hosts. After the biomaterial is implanted into the human body, it will have an impact and effect on the specific biological tissue environment, and the biological tissue will also have an impact and effect on the biomaterial. The cycle of the two continues until the balance is reached or the implant is removed53) . Under the stimulation of 1.0 mmol/L H2O2, the prodrug micelle showed excellent drug release efficiency. In addition, the emulsion delivery system improved the water solubility of tangeretin, gastric retention time, intestinal uptake and metabolic stability to improve its drug efficacy. Relevant studies shown that tangeretin emulsion delivery system significantly inhibited the occurrence of colitis-related tumors54) .
These studies are to improve the stability and water solubility of tangeretin by optimizing the composition, formula and properties of the emulsion, thus increasing its bioavailability, which provides a strong support for the application of tangeretin in the medical and pharmaceutical field.
4.2 Lipid encapsulationSolid lipid nanoparticles and nanostructured lipid carriers are similar to oil-in-water emulsions, which have completely or partially cured lipid cores43) . Studies shown that the use of lipid-based nanocarriers improved the water solubility, bioaccessibility, bioavailability and anti-degradation stability of lipophilic core materials55) . Chen et al.56) studied the effect of dietary lipids on the gastrointestinal fate of tangeretin-supported zein nanoparticles and found that the bioavailability of tangeretin increased with the increase of fat content, and the bioavailability of hydrophobic bioactive agents could be promoted by using a mixed colloidal system composed of lipid nanoparticles and protein nanoparticles. Zheng et al.57) constructed liposomal encapsulated citrus peel PMFs extract (mainly composed of nobiletin and tangeretin) , which improved the solubility and anti-lipase activity of citrus peel PMFs.
Therefore, the bioavailability and various activities of tangeretin can be improved by the encapsulation of tangeretin with lipids, which provides important theoretical basis and practical guidance for its application in the field of medicine.
4.3 Polysaccharide encapsulation deliveryPolysaccharides, as a ubiquitous biomolecule, have shown great potential in improving the availability of bioactive substances. The different functional groups in polysaccharides endow them with high customizability, which enables the preparation of various biological nanostructures with unique functions. In addition, polysaccharides also have biocompatible and biodegradable properties58) , which provide security for their application in the food and pharmaceutical fields.
4.3.1 Dextrin encapsulated tangeretinCyclodextrin (CD) is a series of cyclic oligosaccharides produced by amylose under the action of cyclodextrin glucosyltransferase produced by Bacillus. Elhennawy et al.59) studied the pharmacokinetics of tangeretin after intravenous and oral administration. The results showed that tangeretin plasma coated with methylated β-cyclodextrin was at least twice as exposed (mean bioavailability was 6.02%) as unprocessed tangeretin suspension (mean bioavailability was<3.05%) . Gu et al.60) constructed three new host-guest inclusion compounds with different types of CDs (β-cyclodextrin (β-CD) , 2-hydroxypropyl-β-cyclodextrin (HP-β-CD) and 1,3,6-β-cyclodextrin (TM-β-CD) ) and TAN, and the host-guest inclusion ratio of TAN and CDs were all 1:1. The results showed that TANCDs inclusion compounds had good water solubility and were significantly more stable than TAN itself. In the simulated human gastric juice (pH=1.5) environment, the stability of TAN-β-CD, TAN-HP-β-CD and TAN-TM-β-CD inclusion complexes was increased by 10.45%, 17.11% and 15.18%, respectively. Russo et al.61) collected the high-purity nobiletin and tangeretin, and evaluated the ability of 2-hydroxypropyl-β-cyclodextrin to enhance the water solubility of two PMFs by compatibility study. The results showed that the water solubility of nobiletin and tangeretin was increased by four times and ten times, respectively, thereby improving their bioavailability. Kaempferia parviflora (KP) contains several methoxyflavones, including 5,7-dimethoxyflavone (DMF) , 5,7,4’-trimethoxyflavone (TMF) and 3,5,7,3’,4’-pentamethoxyflavone (PMF) . Studies have shown that the oral bioavailability of KP-HP-β-CD complex is 21.63 times, 34.20 times and 22.90 times that of KP (PMF, TMF and DMF) , respectively62) . The above substances have similar structures to tangeretin, only the number and position of methoxy groups are different, but this method had different degrees of improvement in their bioavailability.
4.3.2 Other polysaccharides encapsulated tangeretinLi et al.63) comprehensively characterized the obtained composite nanoparticles with different cationic short linear dextran (CSLG) concentrations and encapsulated tangeretin as a carrier. The results showed that at simulated gastrointestinal fluid conditions, high methoxy pectin-CSLG nanoparticles exhibited higher encapsulation efficiency and slower release rate than tangeretin. Hu et al.64) successfully prepared starch-based nanocarriers by combining ultrasonic treatment and recrystallization methods, which effectively solved the problems of poor water solubility and weak stability of flavonoids such as tangeretin. Wang et al.65) used debranched starch with different degree of polymerization (DP) as a carrier to encapsulate tangeretin. The results showed that the debranched waxy corn starch/tangeretin inclusion body could protect tangeretin from degradation during storage, and the debranched starch with shorter DP could be used to encapsulate and protect crystalline hydrophobic bioactivators.
The solubility, stability and bioavailability of tangeretin can be improved by a stable polysaccharide-encapsulated delivery system, thereby improving its bioavailability and efficacy. Therefore, the polysaccharide encapsulation delivery technology can be further explored and optimized to better meet the application requirements of tangeretin in the fields of food and medicine.
4.4 MicroencapsulationMicroencapsulation is a process of encapsulating bioactive components in coating to form microcapsule, which can improve the stability of the active components66) . This method is used in the field of food additives and drugs. Sun et al.1) encapsulated different concentrations of tangeretin in citrus pectin/sodium alginate matrix with bergamot oil as carrier by spray drying. The obtained microcapsule powder showed good physical and structural properties with high retention rate and encapsulation efficiency. Hu et al.67) encapsulated tangeretin in hydroxypropyl methyl cellulose (HPMC) modified whey protein concentrate microcapsules, in which Ca2+ cross-linking was used to induce gel formation. Ca2+ cross-linking and HPMC modification improved the encapsulation efficiency of tangeretin microcapsules. HPMC greatly improved the bioavailability of tangeretin and the bioavailability increased from about 28% to 80%. Sun et al.68) developed cinnamaldehyde (CA) modified whey protein (WPC) stabilized microcapsules (WPC/CA microcapsules) to encapsulate nobiletin. The presence of CA maintained or increased the bioavailability of nobiletin in microcapsules (range 82%-94%) . The structure of nobiletin is similar to that of tangeretin, which suggests that microencapsulation is a feasible and effective method to improve the bioaccessibility of PMFs.
Therefore, tangeretin microcapsules can be applied to the food and pharmaceutical industries, such as dairy products, processed foods and nutritional health products based on the characteristics of the encapsulated ingredients and consumer demand.
4.5 Other methodsIn addition, there are other methods to improve the bioavailability of tangeretin. For example, Zhan et al.69) prepared water-resistant polyvinyl alcohol (PVA) /polyacrylic acid (PAA) electrospun fibers encapsulating tangeretin by emulsion electrospinning method. The in vitro release test proved that PVA/PAA/tangeretin cross-linked electrospun fibers have long-lasting release characteristics and lower burst release rate than pure tangeretin emulsion, which can be used as a model technology in the field of water-insoluble drug delivery and sustained release. The Langmuir-Blodgett membrane technology is a method to improve the bioavailability of natural active ingredients. This method is very promising, but its contribution to the current construction of mixed membrane system by tangeretin quite limited.
The emulsion delivery system, lipid encapsulation, and microencapsulation all involve the properties of oils. For the emulsion system, different carrier oils have different effects on the loading capacity and encapsulation efficiency of the emulsion system. Solid lipid nanoparticles and nanostructured lipid carriers are similar to oil-in-water emulsions. Liposomes can greatly improve the water solubility, bioavailability and stability of lipophilic tangeretin. It can be said that improving the bioavailability of tangeretin in previous studies was closely related to the characteristics of oils.
The paper mainly discussed the pharmacological effects of tangeretin and the research progress of improving its bioavailability. The pharmacological effects and mechanisms of tangeretin, including anti-inflammatory, antioxidant, anti-tumor, anti-diabetic and neuroprotective effects were discussed, which clearly shows that the molecule has the potential to become a drug for the treatment of various diseases, but the efficacy of the molecule is currently not fully utilized. In view of the poor water solubility and low bioavailability of tangeretin, scholars have studied the improvement of bioavailability and achieved certain results. In this paper, their research methods were summarized, including emulsion delivery, lipid encapsulation, polysaccharide encapsulation delivery and microencapsulation of tangeretin. However, related research is still in its infancy, and further research and optimization are needed. For example, it is necessary to further study the interaction and mechanism between tangeretin and other components, as well as the impact on the efficacy of tangeretin. At the same time, it is necessary to further explore the safety and effectiveness of the mixed system in practical applications. The mixed molecular membrane of flavonoids and stearic acid is a promising new drug agent technology. In the future, the mixed molecular membrane of tangeretin and stearic acid can be further studied, which is expected to provide a new solution for improving the bioavailability of tangeretin.
This work was financial supported by College Students’ Scientific Research and Entrepreneurship Action Plan (X018) .
There are no conflicts of interest to declare.