2012 年 62 巻 4 号 p. 310-319
2012 年 62 巻 4 号 p. 310-319
Heading date in rice (Oryza sativa L.) is a critical agronomic trait with a complex inheritance. To investigate the genetic basis and mechanism of gene interaction in heading date, we conducted genetic analysis on segregation populations derived from crosses among the indica cultivars Bo B, Yuefeng B and Baoxuan 2. A set of dominant complementary genes controlling late heading, designated LH1 and LH2, were detected by molecular marker mapping. Genetic analysis revealed that Baoxuan 2 contains both dominant genes, while Bo B and Yuefeng B each possess either LH1 or LH2. Using larger populations with segregant ratios of 3 : 1, we fine-mapped LH1 to a 63-kb region near the centromere of chromosome 7 flanked by markers RM5436 and RM8034, and LH2 to a 177-kb region on the short arm of chromosome 8 between flanking markers Indel22468-3 and RM25. Some candidate genes were identified through sequencing of Bo B and Yuefeng B in these target regions. Our work provides a solid foundation for further study on gene interaction in heading date and has application in marker-assisted breeding of photosensitive hybrid rice in China.
Heading date (flowering time) is one of the most crucial agronomic traits in rice (Oryza sativa L.) for ecological adaptation to different cultivation areas and cropping seasons, and changes in heading date greatly impact rice yield. Therefore, control of heading date is a key objective in rice breeding. As such, heading date has been comprehensively studied, and hundreds of quantitative trait loci (QTLs) have been reported (http://www.gramene.org/qtl). In recent decades, with the rapid development of molecular marker-based mapping, great progress has been made in fine mapping and cloning of heading date QTLs (Li et al. 1995, Lin et al. 1996, 2003, Lin et al. 1998, Matsubara et al. 2008, Monna et al. 2002, Takahashi et al. 2001, Xiao et al. 1995, Xiong et al. 1999, Yamamoto et al. 2000, Yano et al. 1997). To date, nine QTLs for heading date, Hd1, Hd3a, Hd6, Ehd1, Ehd2, Ehd3, Ghd7, Ghd8 and Hd17 have been cloned using a map-based cloning strategy (Doi et al. 2004, Kojima et al. 2002, Matsubara et al. 2008, 2011, 2012, Takahashi et al. 2001, Xue et al. 2008, Yan et al. 2011, Yano et al. 2000).
Heading date is a complex trait which is affected not only by photoperiod sensitivity but also by basic vegetative growth and temperature sensitivity. Studies on heading date have focused on photoperiod sensitivity, the most important factor influencing flowering. Genetic analysis has been conducted under different day lengths, including long-day (LD), short-day (SD) and natural-field conditions (NF). Photoperiod control of heading date involves a complicated network of genes (Tsuji et al. 2011). Among the identified genes controlling heading date, a number of epistatic interactions have been detected, while the study of complementary genes postponing heading date has not been reported.
In this study, we found that the F1 derived from two early heading indica maintainers, Bo B and Yuefeng B, had a significantly delayed heading date under natural-field conditions. Genetic analysis of the resultant F2 population showed that the late heading phenotype was controlled by a pair of dominant complementary genes derived from each of the two parents. In addition, we discovered that an indica cultivar, Baoxuan 2, extremely late heading in natural-field conditions, possesses both of these dominant complementary genes. By analyzing the segregant populations derived from crosses between Bo B, Yuefeng B and Baoxuan 2, we fine mapped the two complementary genes, analyzed candidate genes in the mapping regions, and developed several usable DNA markers for marker-assisted breeding. These results provide a solid base for molecular cloning of the target genes and prompt further studies of digenic interaction in heading date of rice.
Three rice cultivars were used in this study: Bo B and Yuefeng B, two excellent maintainer lines that are middle-season indica varieties from the middle-lower region of the Yangtze River and the south of China; and Baoxuan 2, a late heading indica variety from southern China that has partial consanguinity with wild rice (Oryza rufipogon Griff). The genetic pedigree of Bo B and Baoxuan 2 (Fig. 1) showed that they share part of the same heredity background. We produced crosses among Bo B, Yuefeng B and Baoxuan 2 and the resulting F1 plants were self-pollinated to obtain F2 populations (ByF2: Bo B × Yuefeng B; BbF2: Bo B × Baoxuan 2; YbF2: Yuefeng B × Baoxuan 2). Recombinant plants selected from the three F2 populations were developed to F3 lines.
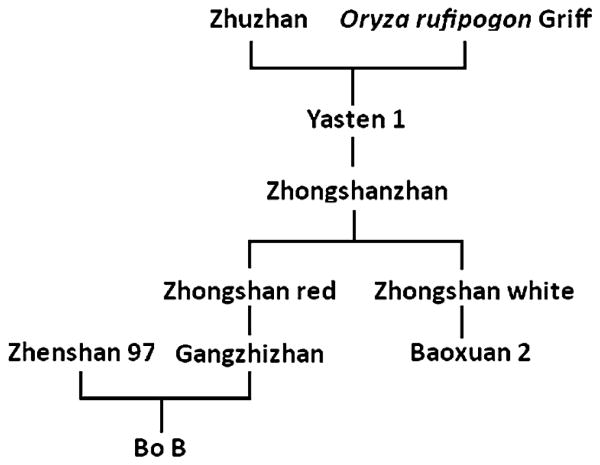
The pedigree of Bo B and Baoxuan 2.
The plants used in this study were grown under natural field conditions in the Taicang (31°N, 121°E), Jiangsu province, China, from mid-May to November. The mean day-length during the cultivation period was 13.5 h (http://www.weather.com.cn). All materials were planted according to well-established standards: 13.3 cm between plants within each row and 26.4 cm between rows; field management followed normal rice production conditions. For each plant of the mapping populations (F2 and F3), the number of days-to-heading (DTH) required from seeding to heading of the first panicle was scored. For each parental cultivar, 10 plants were scored and mean DTH was calculated.
DNA analysisGenomic DNA from the mapping populations and three parental lines was extracted from the freshly harvested leaves of 60-day-old plants by the cetyltrimethyl ammonium bromide (CTAB) method (Murray and Thompson 1980) with a minor modification. Polymerase chain reaction (PCR) amplification was conducted in 10 μl reaction volumes containing 2 μl (20 ng/μl) template DNA, 5 μl Mix (Takara, Japan), 2 μmol of each primer and 1 μl H2O. Amplification was performed using the following conditions: 5 min at 94°C, followed by 35 cycles (1 min at 94°C, 40 sec at 55°C, 1 min at 72°C), and a final extension at 72°C for 5 min. To detect polymorphisms, PCR products were electrophoresed on 8% polyacrylamide gel in Tris-borate EDTA buffer and the gel was silver stained after completion of the electrophoresis.
Bulked segregant analysis and linkage analysisBulked segregant analysis (BSA) (Michelmore et al. 1991) was performed to determine molecular markers linked to the target genes. Fifteen early-heading and fifteen late heading plants were selected from the F3 segregant progenies to create the early-heading and late heading DNA bulks. The parental DNA and the two bulked samples were used for genotyping. A total of 181 polymorphic simple sequence repeat (SSR) markers, selected from the public database (Rice Genome Research Program 2007), were employed to construct the linkage map. Linkage analysis was performed using MAPMAKER 3.0 (Lander et al. 1987) based on genotypic and phenotypic data from segregant individuals of the F3 population. Genetic distances were calculated by the Kosambi (1943) function.
To further analyze linkage, additional insertion/deletion (InDel) markers were developed by designing primers based on DNA sequence diversity between the indica cultivar ‘93-11’ (http://rise.genomics.org.cn) and the japonica cultivar Nipponbare (http://rgp.dna.affrc.go.jp/). Single-nucleotide polymorphism (SNP) markers were subsequently exploited by sequencing chromosome regions of interest between the two parents. DNA markers used in this study are listed in Table 1.
| Marker | Type | Chr. | Forward primer (5′-3′) | Reverse primer (5′-3′) | Product size |
|---|---|---|---|---|---|
| RM21300 | SSR | 7 | ATAGATTTCGTTCCACCGATCC | GCTAATTATCGAAGCAACCACACC | 226 |
| RM3635 | SSR | 7 | GAGAGACAGTGGAAGGGAAGACG | GTTCCCTCCCTCCTCCTAGTTCG | 90 |
| RM5436 | SSR | 7 | TGAGCTGCACAAGACAGACAAGC | ACCATTTGAACAGGATGGACTGG | 194 |
| RM8034 | SSR | 7 | ATTCCAACGGTCCAGTTCAGAGG | CACGCAGTGTGGTGTGGTAAGC | 281 |
| RM21323 | SSR | 7 | ATTGCGATGTTTGGATGTACCG | AGCAACTCGTCAATGAACATGC | 250 |
| Indel7-1 | InDel | 7 | CCAGGTGAGAGAGAAGACATGACC | CCATCAGTCACACAGATCGACTCC | 280 |
| Indel7-2 | InDel | 7 | TATCAGGTGAAAAAAGGCGG | GGATGAGGGAAAGGTAGGGG | 160 |
| RM22465 | SSR | 8 | CAGAATAGCAAGCGAAGGGAAGG | CTTCTTCTCCCAGTGCACAAACC | 81 |
| RM25 | SSR | 8 | GGAAAGAATGATCTTTTCATG | GAACATTGGTTTTGATGGTAG | 265 |
| RM1111 | SSR | 8 | CCTATACCAAACCGAACCGAACC | CGCTGCCAATATCACCAAAGG | 185 |
| Indel22468-3 | InDel | 8 | TTTGACCTGGCTTGCCCTCT | CACAACCCTCACGCACTATA | 211 |
| Indel22468-4 | InDel | 8 | ATGTCCTTTTGGTCCCTCCT | CTTCCCAGCTTTCCTTGTAA | 216 |
| Snp1-1 | SNP | 8 | ATTGTCCGATGGAGTGGTAG | TGTGCTTTAGATTGAGGTGG | 356 |
| Snp1-2 | SNP | 8 | GATGTTATTGACCGTGTAC | CAAGAGCCAAAGAGTGTGC | 455 |
Full genomes of Bo B and Yuefeng B were sequenced using Illumina/Solexa technology by BGI (Beijing Genome Institute, China). Candidate genes in the target region were also amplified from Bo B and Yuefeng B by PCR with LA-Taq (Takara, Japan). PCR products were purified using a PCR purification kit (Takara, Japan), introduced into the pGEM-T Easy Vector (Promega, USA), and transformed into E. coli strain DH5α. The plasmid was sequenced with an ABI Analyzer. Sequence alignment was performed using DNAssist.
Expression analysisRice seedlings of 4-leaf date were transferred to a growth chamber to receive light treatment for 5 d. The treating conditions were set as following: 11 h dark and 13 h light with a temperature of 28°C. For quantitative RT-PCR, we isolated total RNA from the leaves using an RNA extracting kit (TIANGEN, China) and treated with DNase I (Takala, Japan). cDNA was synthesized from 2 μg RNA using PrimeScript RT Master Mix (Takala, Japan). The quantitative analysis of gene expression was performed with SYBR Premix Ex Taq II (Takala, Japan) on an Applied Biosystems 7500 Real-time PCR System. The data were analyzed with the relative quantification method.
Bo B and Yuefeng B are two excellent indica maintainer lines and used as breeding resources in the middle-lower region of the Yangtze River and the south of China. Their average days-to-heading (DTH) at Taicang (31°N, 121°E), Jiangsu province, China, are 68.2 ± 3.2 and 82.6 ± 4.3 (Fig. 2), respectively. Interestingly, the F1 between Bo B and Yuefeng B has an average DTH of 136.2 ± 6.8, a significant delay in growth period. In 2007, the resultant F1 plants were self-pollinated to produce 1791 F2 progenies that formed a large segregant population: 980 late heading individuals (DTH ≥ 115 d) and 811 early heading individuals (DTH < 115 d) (Table 2 and Fig. 2A). The ratio of late heading progenies to early heading progenies was 9 : 7 (χ2 = 0.86, P > 0.05). In 2008, 15 individuals selected randomly from late heading F2 progenies were planted to F3 lines (150–200 plants for each family). Of the 15 F3 lines, two populations showed a consistent late heading phenotype, six populations exhibited a segregant ratio of 3 : 1, and the remaining seven populations exhibited a segregant ratio of 9 : 7 (Table 2). In 2009, we analyzed the DTH of two F2 populations derived from crosses between Baoxuan 2 (DTH = 154.3 ± 6.6) and Bo B and between Baoxuan 2 and Yuefeng B and found that the segregant ratios of late heading to early heading were all 3 : 1 (BbF2: χ2 = 1.98, P > 0.05; YbF2: χ2 = 0.03, P > 0.05) (Table 2 and Fig. 2B, 2C). These results revealed that the late heading phenotype was controlled by a pair of dominant complementary genes, which we named LH1 and LH2. Genetic analysis also indicated that the parents Bo B and Yuefeng B each contain a homozygous dominant gene (LH1 and LH2, respectively), while Baoxuan 2 contains both homozygous dominant genes.
| Pedigree | Popul. | No. of plants | Percentage of late heading plants | χ2 (3 : 1) | χ2 (9 : 7) | ||
|---|---|---|---|---|---|---|---|
| Early heading | Late heading | Total | |||||
| Bo B/Yuefeng B | F2 | 811 | 980 | 1791 | 54.7 | 392.2* | 1.70NS |
| F3-Line 1 | F3 | 47 | 151 | 198 | 76.3 | 0.17 NS | 32.2* |
| F3-Line 2 | F3 | 47 | 139 | 186 | 74.7 | 0.01 NS | 28.5* |
| F3-Line 3 | F3 | 48 | 147 | 195 | 75.4 | 0.02 NS | 29.0* |
| F3-Line 4 | F3 | 41 | 127 | 168 | 75.6 | 0.03 NS | 25.5* |
| F3-Line 5 | F3 | 40 | 119 | 159 | 74.8 | 0.00 NS | 22.3* |
| F3-Line 6 | F3 | 47 | 142 | 189 | 75.1 | 0.00 NS | 27.3* |
| F3-Line 7 | F3 | 66 | 95 | 161 | 59.0 | 37.3* | 0.49NS |
| F3-Line 8 | F3 | 80 | 96 | 176 | 54.5 | 39.2* | 0.21NS |
| F3-Line 9 | F3 | 0 | 188 | 188 | 100.0 | — | — |
| F3-Line 10 | F3 | 83 | 96 | 179 | 53.6 | 43.6* | 0.50NS |
| F3-Line 11 | F3 | 72 | 100 | 172 | 58.1 | 26.1* | 0.25NS |
| F3-Line 12 | F3 | 78 | 109 | 187 | 58.3 | 27.9* | 0.32NS |
| F3-Line 13 | F3 | 0 | 164 | 164 | 100.0 | — | — |
| F3-Line 14 | F3 | 72 | 101 | 173 | 58.4 | 25.5* | 0.31NS |
| F3-Line 15 | F3 | 85 | 108 | 193 | 56.0 | 37.3* | 0.01NS |
| Bo B/Baoxuan 2 | F2 | 1780 | 5631 | 7411 | 75.9 | 1.98NS | 42.6* |
| YuefengB/Baoxuan 2 | F2 | 830 | 2514 | 3344 | 75.1 | 0.03NS | 50.1* |
NS: not significant at the 0.05 level, P > 0.05;
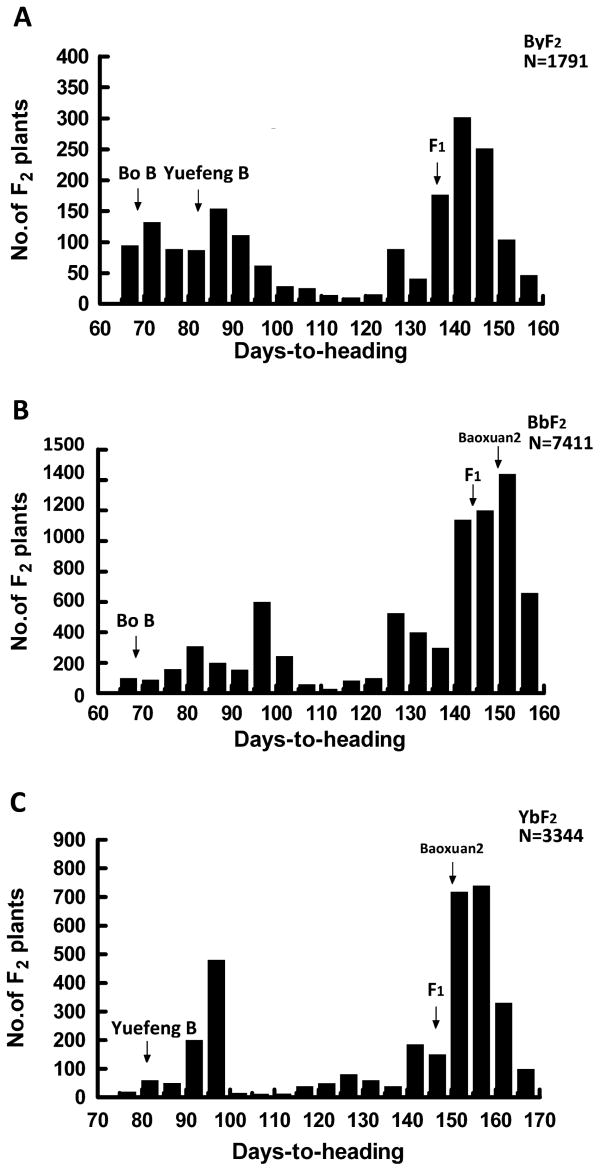
Frequency distribution of days-to-heading in three F2 populations. A. ByF2 (Bo B × Yuefeng B), B. BbF2 (Bo B × Baoxuan 2), C. YbF2 (Yuefeng B × Baoxuan 2). Arrow indicates mean days-to-heading for Bo B, Yuefeng B, Baoxuan 2 and the corresponding F1.
Six F3 lines with segregant ratios of 3 : 1 in growth period were selected as primary populations for mapping the pair of genes controlling the late heading phenotype. In Line 1, fifteen late heading plants and fifteen early heading plants were selected to create late heading and early heading DNA bulks. A total of 597 SSR markers distributed over the 12 chromosomes (McCouch et al. 2002) were used to screen the parents (Bo B and Yuefeng B). Of these, 181 exhibited polymorphism and were applied to the late heading and early heading mixed bulks. Ten SSR markers showed diversity between the two bulked DNA samples and were employed to survey polymorphism in Line 1 individuals. Two out of the ten SSR markers, RM6338 and RM3755 (located on the short arm of chromosome 7), were found to be closely associated with the late heading time among these 198 F3 plants. Linkage analysis with Mapmaker/Exp3.0 software revealed that the genetic distance between RM 6338 and RM3755 was 9.2 cM. This first late heading gene detected was named LH1 (Fig. 3A).

Fine mapping of LH1. A. Location of LH1 on chromosome 7. B. High-resolution linkage map of LH1 using ByF3 population. cM, genetic distance between the SSR markers. C. LH1 was located on a 63-kb region between markers RM8034 and RM5436. The number of recombinants between adjacent markers is indicated below the bar. D. The BAC AP003755 was annotated by TIGR (http://rice.plantbiology.msu.edu/cgi-bin/gbrowse/rice).
Markers RM 6338 and RM3755 were applied to two bulked DNA samples from each of the remaining five F3 lines, and found not to be associated with the late heading time in Lines 3 and 4. These results revealed that the late heading phenotypes in Lines 3 and 4 were controlled by another gene, dubbed LH2. Using the same procedure with Line 3, LH2 was mapped to a 10.2 cM region on the short arm of chromosome 8 flanked by the SSR markers RM3572 and RM6208 (Fig. 4A). Genetic analysis found that the dominant LH1 allele derived from parent Yuefeng B, and LH2 derived from parent Bo B.
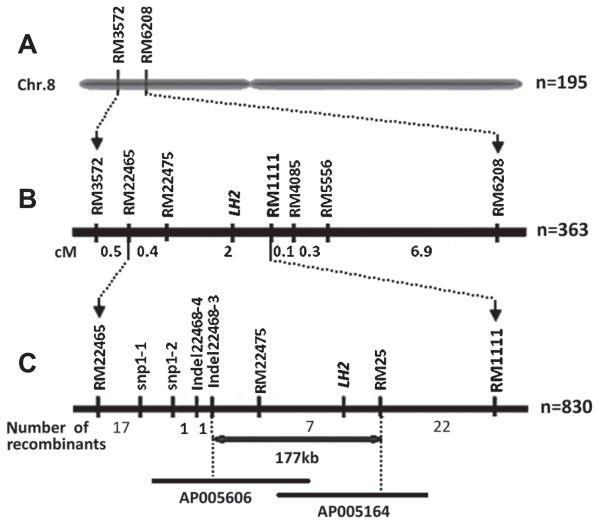
Fine mapping of LH2. A. Location of LH2 on chromosome 8. B. High-resolution linkage map of LH2 using ByF3 population. cM, genetic distance between the SSR markers. C. LH2 was localized to a 177-kb region between markers Indel22468-3 and RM25. The number of recombinants between adjacent markers is shown below the bar.
To fine map LH1 from Yuefeng B, the flanking markers RM 6338 and RM3755 were used to examine 732 individuals from four F3 lines segregated around the LH1 locus. A total of 18 recombinants were found. All recombinants were genotyped using five polymorphic SSR markers within the target interval (Fig. 3B). Recombination analysis revealed that LH1 was located in a region between RM21300 and RM3635, with a map distance of 1.8 cM.
To further refine the position of LH1, an additional large mapping population, BbF2, containing 7411 F2 plants derived from the cross between Baoxuan 2 and Bo B, was developed. Markers RM21300 and RM3635 were used to survey 1780 early heading plants in the BbF2 segregation population. In total, 21 recombinants were identified between these two markers. Two additional Indel markers, Indel7-1 and Indel7-2, were designed based on the publicly available rice genome sequence and the likelihood of detecting diversity between Bo B and Baoxuan 2 was predicted by comparing sequences from Nipponbare (http://rgp.dna.affrc.go.jp/) and the indica cultivar ‘93-11’ (http://rise.genomics.org.cn). A total of seven markers (Table 3) were used to genotype the 21 recombinants. The plants recombinant in the region between Indel7-1 and Indel7-2 are listed in Table 3. The recombinants BBE-42 and BBE-273 restricted LH1 to the region downstream of RM8034, while recombinants BBE-98, BBE-489 and BBE-813 localized the gene to the region upstream of RM5436. Therefore, LH1 was confined to the 63.7-kb region flanked by the SSR markers RM5436 and RM8034 (Fig. 3C).
| Individual | Days-to-heading | Chr. | Markers | ||||||
|---|---|---|---|---|---|---|---|---|---|
| RM 21300 | RM 21323 | Indel 7-1 | RM 8034 | RM 5436 | Indel 7-2 | RM 3635 | |||
| BBE-42 | 90 (EH) | 7 | H | H | H | H | B | B | B |
| BBE-98 | 75 (EH) | 7 | B | B | B | B | H | H | H |
| BBE-273 | 80 (EH) | 7 | H | H | H | H | B | B | B |
| BBE-299 | 85 (EH) | 7 | B | B | B | B | B | H | H |
| BBE-489 | 85 (EH) | 7 | B | B | B | B | H | H | H |
| BBE-552 | 90 (EH) | 7 | H | H | H | B | B | B | B |
| BBE-624 | 80 (EH) | 7 | B | B | B | B | B | H | H |
| BBE-813 | 65 (EH) | 7 | B | B | B | B | H | H | H |
| BBE-1214 | 70 (EH) | 7 | H | H | H | B | B | B | B |
| BBE-1533 | 70 (EH) | 7 | B | B | B | B | B | H | H |
EH, early heading; B, Bo B homozygous genotype; H heterozygous genotype
The same strategy was used to fine map the LH2 gene originating from Bo B. A total of 31 recombinants were found between markers RM3572 and RM6208 among 363 individuals from two F3 lines segregated around the LH2 locus. Five polymorphic SSR markers within the target interval were used to screen the genotypes of the recombinants. Consequently, LH2 was narrowed down to a region between RM1111 and RM22465 with a genetic distance of 2.4 cM (Fig. 4B).
To further locate LH2, another large mapping population, YbF2, containing 3344 F2 plants, was derived from the cross between Baoxuan 2 and Yuefeng B. 830 early heading individuals in the YbF2 segregation population were genotyped using RM1111 and RM22465. In total, 48 recombinants were identified between these two markers. Four additional markers, snp1-1, snp1-2, Indel22468-3 and Indel22468-4 were developed. InDel markers were designed as mentioned above. SNP markers were designed by direct partial sequencing of certain chromosome regions between Baoxuan 2 and Yuefeng B. In total, eight markers were used to genotype the 48 recombinants, including four new, two flanking and two SSR markers within the interval (Table 4). Among these 48 recombinants, two individuals showed that the crossover occurred somewhere downstream of Indel22468-3, while five revealed that the crossover occurred somewhere upstream of RM25 (Table 4 and Fig. 4C) The plants recombinant in the region between snp1-2 and RM25 are listed in Table 4. Therefore, LH2 was mapped between Indel22468-3 and RM25 with a physical distance of 177 kb (Fig. 4C).
| Individual | Days- to-heading | Chr. | Markers | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RM 22465 | Snp 1-1 | Snp 1-2 | Indel 22468-4 | Indel 22468-3 | RM 22475 | RM 25 | RM 1111 | |||
| YBE4-8 | 68 (EH) | 8 | H | H | H | H | Y | Y | Y | Y |
| YBE15-8 | 85 (EH) | 8 | H | H | H | Y | Y | Y | Y | Y |
| YBE30-11 | 90 (EH) | 8 | Y | Y | Y | Y | Y | Y | H | H |
| YBE34-9 | 95 (EH) | 8 | Y | Y | Y | Y | Y | Y | H | H |
| YBE39-3 | 85 (EH) | 8 | H | H | H | H | H | Y | Y | Y |
| YBE45-11 | 70 (EH) | 8 | Y | Y | Y | Y | Y | Y | H | H |
| YBE48-8 | 90 (EH) | 8 | H | H | H | H | H | Y | Y | Y |
| YBE52-2 | 80 (EH) | 8 | Y | Y | Y | Y | Y | Y | H | H |
| YBE55-7 | 80 (EH) | 8 | Y | Y | Y | Y | Y | Y | H | H |
EH, early heading plants; Y, Yuefeng B homozygous genotype; H heterozygous genotype
Fine mapping placed LH1 on chromosome 7 between markers RM5436 and RM8034. Based on the available sequence annotation database (http://www.tigr.org), there were nine predicted genes located in the 63-kb target region (Fig. 3D), five of which had a full-length cDNA and were regarded as candidates for LH1. We sequenced the full genomes of Bo B and Yuefeng B and compared the nucleotide sequences of these five candidate genes. Three (LOC_Os7g15570, LOC_Os07g15600 and LOC_Os7g15640) showed differences between Bo B and Yuefeng B in their coding regions. The second gene identified in this study, LH2, was mapped between markers Indel 22468-3 and RM25 on chromosome 8. Thirty potential genes were identified in the 177-kb target region, fifteen of which had a full-length cDNA and were regarded as candidates for LH2. Polymorphisms between Bo B and Yuefeng B were found in the coding regions of three candidates (LOC_Os08g07660, LOC_Os08g07730 and LOC_Os08g07810) according to the nucleotide sequence alignment results. Potential genes and alignment results are listed in Table 5.
| No. | Chr. | BAC | Gene name | NCBI locus | Full-length cDNA/EST | Gene annotation | SNP | InDel | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Bo B | Yuefeng B | Bo B | Yuefeng B | |||||||
| 1 | 7 | AP003756 | LOC_Os07g15570 | 9054441-9056000 | NM001065855 | pentatricopeptide (PPR) | CCG/ACA/CGG/AGA | CTG/ACG/TGG/AAA | In-TTC | Del-TTC |
| 2 | 7 | AP003756 | LOC_Os07g15590 | 9063927-9066617 | CT857039 | expressed protein | – | – | – | – |
| 3 | 7 | AP003756 | LOC_Os07g15600 | 9068117-9071382 | AK067947 | PRLI-interacting factor G | CTG | GTG | – | – |
| 4 | 7 | AP003756 | LOC_Os07g15610 | 9079693-9080221 | AK288525 | hypothetical protein | – | – | – | – |
| 5 | 7 | AP003756 | LOC_Os07g15640 | 9104013-9109947 | NM001065857 | selenium-binding protein-like protein, CRR4 | ATT/CGG | ATC/AGG | – | – |
| 1 | 8 | AP005606 | LOC_Os08g07490 | 4205886-4206807 | AK240638 | expressed protein | – | – | – | – |
| 2 | 8 | AP005606 | LOC_Os08g07500 | 4210446-4211917 | AK100902 | DUF617 domain containing protein | – | – | – | – |
| 3 | 8 | AP005606 | LOC_Os08g07540 | 4236013-4241588 | AK101266 | hemimethylated DNA binding domain containing protein | – | – | – | – |
| 4 | 8 | AP005606 | LOC_Os08g07550 | 4241938-4242886 | AK111274 | hAT dimerisation domain-containing protein | – | – | – | – |
| 5 | 8 | AP005606 | LOC_Os08g07600 | 4267286-4267978 | AK111113 | expressed protein | – | – | – | – |
| 6 | 8 | AP005164 | LOC_Os08g07660 | 4289421-4290286 | AK241700 | expressed protein | – | – | In-TG | Del-TG |
| 7 | 8 | AP005164 | LOC_Os08g07690 | 4299556-4300473 | AK062724 | expressed protein | – | – | – | – |
| 8 | 8 | AP005164 | LOC_Os08g07700 | 4302905-4303699 | AK241762 | AP2 domain containing protein | – | – | – | – |
| 9 | 8 | AP005164 | LOC_Os08g07720 | 4315163-4317641 | AK108243 | transferase family protein | – | – | – | – |
| 10 | 8 | AP005164 | LOC_Os08g07730 | 4323890-4328771 | NM001067641 | transferase family protein | GCG | GTG | In-GCG | Del-GCG |
| 11 | 8 | AP005164 | LOC_Os08g07740 | 4330976-4335183 | AB693204 | Hd5 gene for heading date 5 | – | – | – | – |
| 12 | 8 | AP005164 | LOC_Os08g07760 | 4343174-4349505 | AK103038 | BRASSINOSTEROID INSENSITIVE 1- associated receptor kinase1 precursor | – | – | – | – |
| 13 | 8 | AP005164 | LOC_Os08g07774 | 4351810-4360461 | AK071471 | disease resistance protein RPM1 | – | – | – | – |
| 14 | 8 | AP005164 | LOC_Os08g07790 | 4362358-4364522 | AK288674 | CRS2-associated factor 2, mitochondrial precursor | – | – | – | – |
| 15 | 8 | AP005164 | LOC_Os08g07810 | 4370025-4371933 | AK73494 | strictosidine synthase | GCG | GAG | – | – |
To investigate the relationship of LH1 and LH2 to genes associated with the circadian clock and those known to be involved in photoperiod flowering, we compared the expression patterns of six additional genes in Bo B, Yuefeng B and ByF1 population under long day conditions. These six genes, including OsGI, OsLhy and OsPRR, were associated with the circadian clock, and Hd1, Ehd1 and Hd3a, are known to have key roles in photoperiod-controlled heading (Doi et al. 2004, Kojima et al. 2002, Yano et al. 2000). The results showed that the expression level of these genes are slightly different between Bo B and Yuefeng B. However, the expression patterns in ByF1 population appeared markedly vary. The expression of the clock-associated genes and Hd1 is promoted, in contrast, the expression of Ehd1 and Hd3a is suppressed in ByF1 population (Fig. 5). According to the photoperiod flowering pathway (Tsuji et al. 2011), we speculate that the epistasis effect of genes LH1 and LH2 acts on the clock-related genes, which are upstream of Hd1, Ehd1 and Hd3a in the photoperiod flowering pathway.
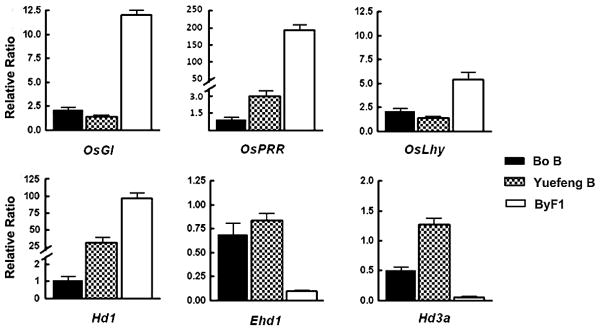
The expression of rice flowering genes in Bo B, Yuefeng B and ByF1. qRT-PCR was performed with total RNA from leaves of 30-d-old plants under LD conditions. Samples were collected at the initiation of the light phase (ZT = 0). These experiments were repeated at least three times.
Heading date has been extensively studied as one of the essential agronomic traits in rice. A total of 734 QTLs related to heading date have been reported to date (http://www.gramene.org/qtl). Epistatic interactions among these have also been observed (Gu and Foley 2007, Kojima et al. 2002, Komiya et al. 2009, Lin et al. 2003, Uwatoko et al. 2008, Yano et al. 2000, Yu et al. 2002) but have not lead to major phenotypic variation in heading date. In this study, we detected two new interacting genes, LH1 and LH2. A single dominant gene did not cause variation in heading date but the two dominant genes together, regardless of homozygous or heterozygous types for each, significantly delayed heading date. This digenic interaction to produce a new phenotype is typical of the complementary gene model. This phenomenon was first discovered in pea flower colors by Bateson and Punnett (Bateson et al. 1906), and has also been reported in rice: Thakur and Roy (1975) found that the uneven kernel trait is controlled by two dominant complementary genes, Una and Unb, and clustered habit is controlled by Cla and Clb. Recently, many other dominant-complementary genes have been identified in rice: e.g. Rhz2 and Rhz3 controlling rhizomatousness (Thakur and Hille Ris Lambers 1989), and Stva and Stvb controlling RSV (resistance to stripe virus) (Maeda et al. 2004). However, dominant complementary genes controlling heading date have never been reported. Here, the genes LH1 and LH2 identified in this study provide an example for further study of gene interactions and networks in the rice flowering pathway.
With the completion of the rice genome sequence project and advances in molecular marker-based mapping, many heading-date-related genes have been identified. Key heading date genes located on chromosome 7 are as follows (Fig. 6): Hd2, tightly linked with the RFLP marker C728 (Yano et al. 2000), Hd4, located between RFLP markers R46 and C39 (Lin et al. 2003), and Ghd7, located between SSR marker RM5436 and RFLP marker C39 (Xue et al. 2008). In the present study, we fine mapped LH1 to a 63-kb region flanked by two SSR markers, RM5436 and RM8034, upstream of Ghd7 and near the centromeric region of chromosome 7. Genetic prediction analysis was conducted using the JCVI database (http://rice.plantbiology.msu.edu) and nine potential genes were identified, of which five had full-length cDNA (Table 5). We sequenced the full genome of Bo B and Yuefeng B and compared their nucleotide sequences. Alignment results showed that three of these genes, LOC_ Os7g15570, LOC_Os07g15600 and LOC_ Os7g15640 have differences in their coding regions between Bo B and Yuefeng B. In LOC_Os7g15570, which encodes a pentatricopeptide repeat (PPR)-like protein, we found four amino acid changes and one serine deletion. LOC_Os07g15600 and LOC_Os7g15640, which are known to encode a putative PRLI-interacting factor G and a selenium-binding type protein, respectively, also showed several single-nucleotide substitutions between the two cultivars. Further study will be required to pinpoint the candidate gene for LH1.
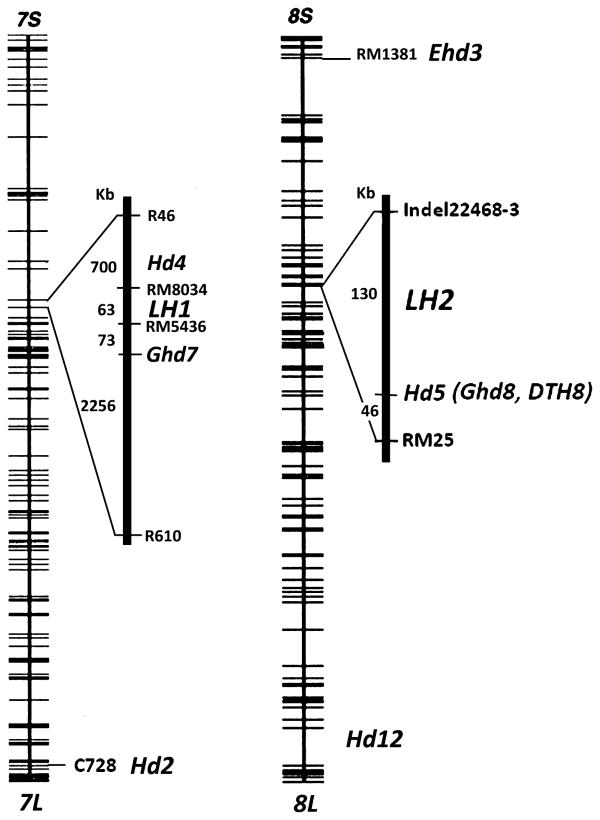
Linkage maps of chromosomes 7 and chromosomes 8 showing the locations of LH1 and LH2. Genes in the parentheses on chromosome 8 means Hd5, Ghd8 and DTH8 are the same genes.
We also found a second gene, LH2, which was located on chromosome 8 and subsequently limited to a 177-kb region through analysis of recombination events. In previous studies, there have been other reports of heading date genes on chromosome 8 (Fig. 6) (Lin et al. 2003, Matsubara et al. 2011, Nonoue et al. 2008, Zhang et al. 2006, Zhou et al. 2001). One such gene, Ghd8, also named Hd5 (Lin et al. 2003) and DTH8 (Wei et al. 2010), is located in the interval of LH2. It is known to encode the OsHAP3 subunit of a CCAAT-box binding protein (HAP complex). To investigate whether Ghd8 was the candidate gene, we sequenced and compared the nucleotide sequences of Ghd8 in Bo B and Yuefeng B, yet no differences were found. We also sequenced and compared the other 14 candidates for LH2, three of which, LOC_Os08g07660, LOC_Os08g07730 and LOC_Os08g07810, showed polymorphisms in their coding regions between Bo B and Yuefeng B. These genes are known to encode a strictosidine synthase, a transferase family protein and an unknown protein, respectively. Our preliminary results have excluded Ghd8 as a prospective gene but further study is required to confirm this. Previous studies have not shown that genes on chromosomes 7 and 8 have a complementary action to postpone heading date as we have reported here. Thus, we assume that LH1 and LH2 might be two novel genes. However, cloning and functional complementarily tests need to be conducted to further identify LH1 and LH2 and clarify the molecular mechanism that regulates heading date in rice and other plants.
Photoperiod control of heading date involves a complicated network of genes. We investigated the relationship of LH1 and LH2 to genes associated with the circadian clock and those known to be involved in photoperiod flowering. Expression analysis showed that the epistasis effects of LH1 and LH2 act on the clock-related genes, which are upstream of Hd1, Ehd1 and Hd3a in the photoperiod flowering pathway. Gene cloning and transgenic test are needed to integrate the LH1 and LH2 genes into the gene network of flowering time in rice.
Heading date is a complex trait that is mainly affected by photoperiod sensitivity, basic vegetative growth and temperature sensitivity, among which photoperiod sensitivity is the most important factor influencing flowering. Most of the heading date related genes are sensitive to the photoperiod. In this study, we investigated the photoperiod sensitivity of Bo B, Yuefeng B, Baoxuan 2 and ByF1 population under natural-field conditions. The DTH of Bo B and Yuefeng B vary only slightly between the Taicang (31°N, 121°E, Jiangsu Province, China, NLD: 13.5-h light on average) and Sanya (18°N, 109°E, Hainan Province, China, NSD: 11.5-h light on average), while the DTH of Baoxuan 2 and ByF1 population increased more than 60 days under NLD conditions than NSD conditions. The results indicated that the epistasis of LH1 and LH2 increases the photoperiod sensitivity of rice.
Growth from sowing to heading is a complex period in rice comprising a vegetative growth phase and a reproductive phase. The vegetative growth duration is composed of a basic vegetative growth phase and a photoperiod-sensitive phase that is controlled by many genes and environmental factors such as temperature and day length. Many studies have shown that advanced heading date shortens the vegetative growth period of rice and results in a reduction of grain yield, while delayed heading provides the crop with sufficient vegetative growth to lead to better grain yield and/or biomass (e.g. Peng et al. 1999). In the rice-planted areas of southern China, super hybrid rice has justifiably popularized planting only one crop per year. To make the most of light energy from the sun and improve rice yield, appropriately delaying heading date is a key selection criterion during rice breeding and production. As a prospective gene resource for late heading, LH1 and LH2, a set of dominant complementary genes, could effectively promote the breeding of new hybrid rice combinations to achieve high yield, high quality and cost-saving potential.
This work was supported in part by the National Natural Science Foundation of China (30900881) and the Key Programme of Guangdong Province, China (2009A020102003) and the earmarked fund for Modern Agro-industry Technology Research System (CARS-01-10).