2019 Volume 44 Issue 9 Pages 611-619
2019 Volume 44 Issue 9 Pages 611-619
The kidney proximal tubule is a target of many renal toxicants, including cadmium (Cd), and also a place of reabsorption of essential metals in glomerular filtrate to systemic circulation. Although the mechanisms of metal transport in the convoluted proximal tubule (S1 and S2 segments) and the straight proximal tubule (S3 segment) may differ, little is known about the segment-specific modes of metal transport. Here, we utilized immortalized cell lines derived from the S1, S2, and S3 segments of mouse kidney proximal tubules, and examined the segment-specific and direction-dependent transport of Cd and manganese (Mn) using a trans-well culture system. The results showed that the uptakes of Cd2+ and Mn2+ from apical sides were the highest in S3 cells, and Cd2+, Mn2+, and Zn2+ mutually inhibited the apical uptake of each metal. As the expression of ZIP8, a zinc transporter having affinities for Cd2+ and Mn2+, was the highest in S3 cells, ZIP8 may contribute largely to the apical uptakes of these metals. The efficient uptake of Mn2+ from apical side of S3 cells may suggest an important role of ZIP8 in proximal tubule in reabsorption of Mn, an essential metal. Our study demonstrated that S1, S2, and S3 cells provide a useful tool for studying the segment-specific and direction-dependent transport of both toxic and essential metals in the kidney’s proximal tubules.
For decades, most studies on the deposition of cadmium (Cd) in the kidney have focused on the roles of metallothionein (MT) (Nordberg et al., 1992; Klaassen et al., 1999, 2009). It has been assumed that the Cd bound to MT (Cd-MT) in blood plasma can be readily filtered through the glomerulus into the lumen of the renal tubule due to its low molecular weight (less than 7,000), and that the Cd-MT in the lumen is taken up by proximal tubule epithelial cells (PTECs), especially at the convoluted proximal tubule (S1 and S2 segments) via endocytosis (Klaassen et al., 1999; Christensen et al., 2009). However, the precise mechanisms of the renal handling of Cd, especially the involvement of non-MT factors, remain unclear. Since the accumulation of Cd, which has a biological half-life of more than 25 years in human kidney (Elinder et al., 1976), is the most important determinant of renal Cd toxicity, better understanding of renal handling of Cd is essential for the elucidation of Cd toxicity and the interaction of Cd with other essential metals.
Recently, several metal transporters have been identified as transporters responsible for cellular uptake of Cd. These include divalent metal transporter 1 (DMT1) (Gunshin et al., 1997), Zrt-/Irt-related protein 8 (ZIP8) and ZIP14 encoded by Slc39a8 and Slc39a14, respectively (Dalton et al., 2005; Girijashanker et al., 2008; Fujishiro et al., 2006, 2009, 2012), and calcium transporter (CaT1) (Min et al., 2008). These metal transporters may be involved in the absorption of non-MT Cd.
In the previous study using immortalized mouse renal cells (PT cells) cultured on a trans-well culture system, we showed that the down-regulation of ZIP8 and ZIP14 by siRNA transfection resulted in a decrease in apical uptake of Cd by about 50%, suggesting that these zinc transporters play important roles in the uptake of Cd from the proximal tubule lumen into PTECs (Fujishiro et al., 2012). In situ hybridization of the mouse kidney showed expressions of ZIP8 and ZIP14 in the straight part (S3 segment) of the proximal tubules (Fujishiro et al., 2012). We also found that a portion of the Cd in the immortalized PT cells was excreted into the media of the apical chamber of the trans-well. Based on these data, we proposed a hypothesis regarding the renal handling of non-MT Cd as follows: small amounts of Cd accumulated in the PTECs in the S1 and S2 segments of the proximal tubule could be released into the lumen, and then incorporated into PTECs in the S3 segment via Zn transporters such as ZIP8 and ZIP14 (Fujishiro et al., 2012). To examine this hypothesis, however, the analysis of apical and basolateral transports of Cd in PTECs derived from each segment of the proximal tubule is required since the original segment of the PT cells used in the previous study (Fujishiro et al., 2012) remains obscure.
We and other groups have demonstrated that ZIP8 and ZIP14 also have affinities for divalent manganese (Mn2+) (He et al., 2006; Fujishiro et al., 2006, 2009, 2011). Recently, human cases of ZIP8 mutations have been reported. In patients with missense mutations in the SLC39A8 gene, blood Mn levels are undetectable or very low. It has been presumed that a marked decrease in Mn-dependent galactosyltransferase activity was associated with the symptoms of glycosylation deficiency found in these patients (Park et al., 2015; Boycott et al., 2015). A clinical study reported enhanced Mn excretion in the urine among these patients, suggesting that renal Mn reabsorption is disordered by ZIP8 mutation (Park et al., 2018). Thus, elucidation of the precise mechanisms of renal handling of Mn is an urgent issue to be solved.
To study the segment-specific transports of metals, cultured PTECs derived from each segment of proximal tubule may serve as an excellent tool. In earlier studies, Dr. Endou and coworkers have established immortalized cells from the S1, S2, and S3 segments of the proximal tubules of mouse kidneys (Takeda et al., 1995, 1998; Hosoyamada et al., 1996), but characterization of these cells have not been fully performed. Recently, using microarray analyses, we found that these immortalized PTECs maintain the segment-specific gene expression patterns similar to those observed under in vivo conditions as far as several marker proteins and metal transporters are concerned (Fujishiro and Himeno, 2019). Thus, in the present study, we examined the usability of these immortalized PTECs, hereafter called S1, S2, and S3 cells, in the studies of metal transports. We first confirmed the segment-specific expression of some marker proteins and metal transporters at the protein levels. Since PTECs maintain polarity in vivo, we also examined whether these cells can be used for examining the direction-dependent transports of Cd and Mn using a trans-well culture system. Here we show that the apical uptake of both Cd2+ and Mn2+ were the highest in S3 cells, where ZIP8 is highly expressed. These cells will provide a useful tool for in vitro analyses of segment-specific and direction-dependent transports of toxic and essential metals and the segment-specificity of renal toxicants.
[109Cd]-CdCl2 was purchased from RIKEN (Wako, Saitama, Japan). [54Mn]-MnCl2 was purchased from PerkinElmer (Boston, MA, USA). ThinCert™ Cell Culture Inserts (24-well, pore size 1.0 μm) were purchased from Greiner Bio-One GmbH (Kresmunster, Austria). Mouse anti E-cadherin polyclonal antibody was purchased from Becton Dickinson (Franklin Lakes, NJ, USA), Alexa 488 anti-rabbit IgG antibody was purchased from Invitrogen (Carlsbad, CA, USA). Rabbit anti-Nramp2 (DMT1) polyclonal antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit anti-ZIP8 polyclonal antibody was purchased from Proteintech (Chicago, IL, USA). Rabbit anti-NET34 (ZIP14) polyclonal antibody and mouse anti-Glutamine Synthetase (GS) monoclonal antibody were purchased from EMD Millipore (Darmstadt, Germany). Rabbit anti-β-actin polyclonal antibody, rabbit anti-4F2hc/CD98 polyclonal antibody, and anti-rabbit IgG HRP-linked antibody were purchased from Cell Signaling Technology (Danvers, MA, USA). Rabbit anti-Oct1 polyclonal antibody was purchased from Abcam (Cambridge, MA, USA). Rabbit anti-SLC38A3/SNAT3 polyclonal antibody was purchased from LS Bio LifeSpan BioScience, Inc. (Seattle, WA, USA).
Cell cultureS1, S2 and S3 cells were kindly provided by Prof. Nobuhiko Anzai (Chiba University, Japan). These cells were cultured in Dulbecco’s modified Eagle’s medium/Ham’s Nutrient Mixture F12 supplemented with 5% fetal bovine serum (FBS), 1 μg/mL insulin, 10 ng/mL epidermal growth factor, 10 μg/mL transferrin, penicillin and streptomycin under 5% CO2 at 37°C.
S1, S2, and S3 cells (1 × 105 cells) were transferred onto the membrane of ThinCert™ Cell Culture Inserts placed in the chamber of a 24-well culture plate filled with medium. The structural integrity of the monolayer of these cells was confirmed by immunohistochemical staining of E-cadherin. The experiments on metal transport were carried out 3 days after the transfer of the cells onto the membrane of the culture insert, when the values of transepithelial electrical resistance (TEER) across the cells showed sufficiently high values (Supplemental Fig. 1), indicative of tight junction formation (Supplemental Fig. 2).
Measurement of metal concentrations in cellsMeasurement of Cd and Mn concentrations in cells was performed using tracers of 109Cd and 54Mn, respectively, as described previously (Fujishiro et al., 2011). All experiments for metal analyses were performed on the third day after the cells were transferred to ThinCert™ Cell Culture Inserts, when the formation of tight junctions was confirmed.
For the measurement of the initial uptake rates of Cd2+ and Mn2+ from the apical or basolateral side of S1, S2, and S3 cells, 0.1 μM [109Cd]-labeled CdCl2 or 0.1 μM [54Mn]-labeled MnCl2 was added to the medium of the upper or lower chambers of the trans-well culture insert. S1, S2, and S3 cells were pre-incubated with FBS-free media for 30 min, and then exposed to 0.1 μM metals for 30 and 60 min in FBS-free media. The cells were washed three times with 0.3 mL ice-cold medium containing FBS followed by a washing with PBS containing 0.05% EDTA, and then harvested with 0.4 mL RIPA buffer (50 mM Tris, pH8, 150 mM NaCl, 1% NP-40, 0.5% deoxycholate, 0.1% SDS) and transferred to a test tube.
For the measurement of the excretion of Cd and Mn, S1, S2, and S3 cells cultured on ThinCert™ Cell Culture Inserts were treated with FBS-free media containing 0.1 μM [109Cd]-labeled CdCl2 or 0.1 μM [54Mn]-labeled MnCl2 for 2 hr, washed with 0.3 mL of medium containing FBS followed by a washing with PBS containing 0.05% EDTA, and then incubated further for 30 and 60 min in metal-free media. The media of the upper chambers (apical side) and lower chambers (basolateral side) of the cell culture insert were collected.
The radioactivities of 109Cd or 54Mn were measured using an auto-well gamma counter (Wizard2; PerkinElmer Japan, Kanagawa). All experiments were carried out three times and the average values were used.
Immunoblot analysisProteins extracted from cells were separated by 10% SDS polyacrylamide gel electrophoresis and electrophoretically transferred to a polyvinylidene fluoride membrane. The transblot was preincubated with 5% nonfat dry skim milk in Tris-buffered saline (TBS, pH 7.4), and then incubated overnight with the antibody against each protein. The membrane was washed with TBS/0.05% Tween 20 and then incubated with anti-rabbit IgG HRP-linked antibody (1:3000). After the membrane was washed with TBS/0.05% Tween 20, immunoreactive bands were detected using enhanced chemiluminescence reagents.
Statistical analysisStatistically significant differences in the competition experiment were determined by one-way ANOVA followed by Williams multiple comparisons. Statistically significant differences in the uptake and efflux experiments were determined by two-way ANOVA followed by Turkey multiple comparisons.
We first compared the protein levels among S1, S2, and S3 cells of several marker proteins known to be expressed in kidney proximal tubules in vivo in a segment-specific manner. The results of Western blot analysis (Fig. 1A) showed that the protein level of organic cation transporter 1 (Oct1), which is expressed highly in the S1 segment and absent in the S3 segment (Karbach et al., 2000), was detected highly and faintly in S1 and S2 cells, respectively, but was undetectable in S3 cells. The protein levels of CD98 (4F2hc, subunit of heteromeric amino acid transporter), which is used for identifying the early proximal tubule in immunostaining (Rossier et al., 1999), was higher in S1 and S2 cells than in S3 cells. The protein levels of Na+-dependent glutamine transporter (SNAT3) and glutamine synthetase (GS) were the highest in S3 cells, in accordance with immunostaining data showing high expression of SNAT3 and GS proteins in the S3 segment of the mouse kidney proximal tubule (Moret et al., 2007; Cristofori et al., 2007). The differences in the protein levels shown here were basically similar to the differences in the mRNA levels of these genes observed in the microarray analysis performed in S1, S2, and S3 cells (Fujishiro and Himeno, 2019). These results suggest that the immortalized S1, S2, and S3 cells retained the fundamental properties of their original segments, as far as the expressions of these marker proteins are concerned.
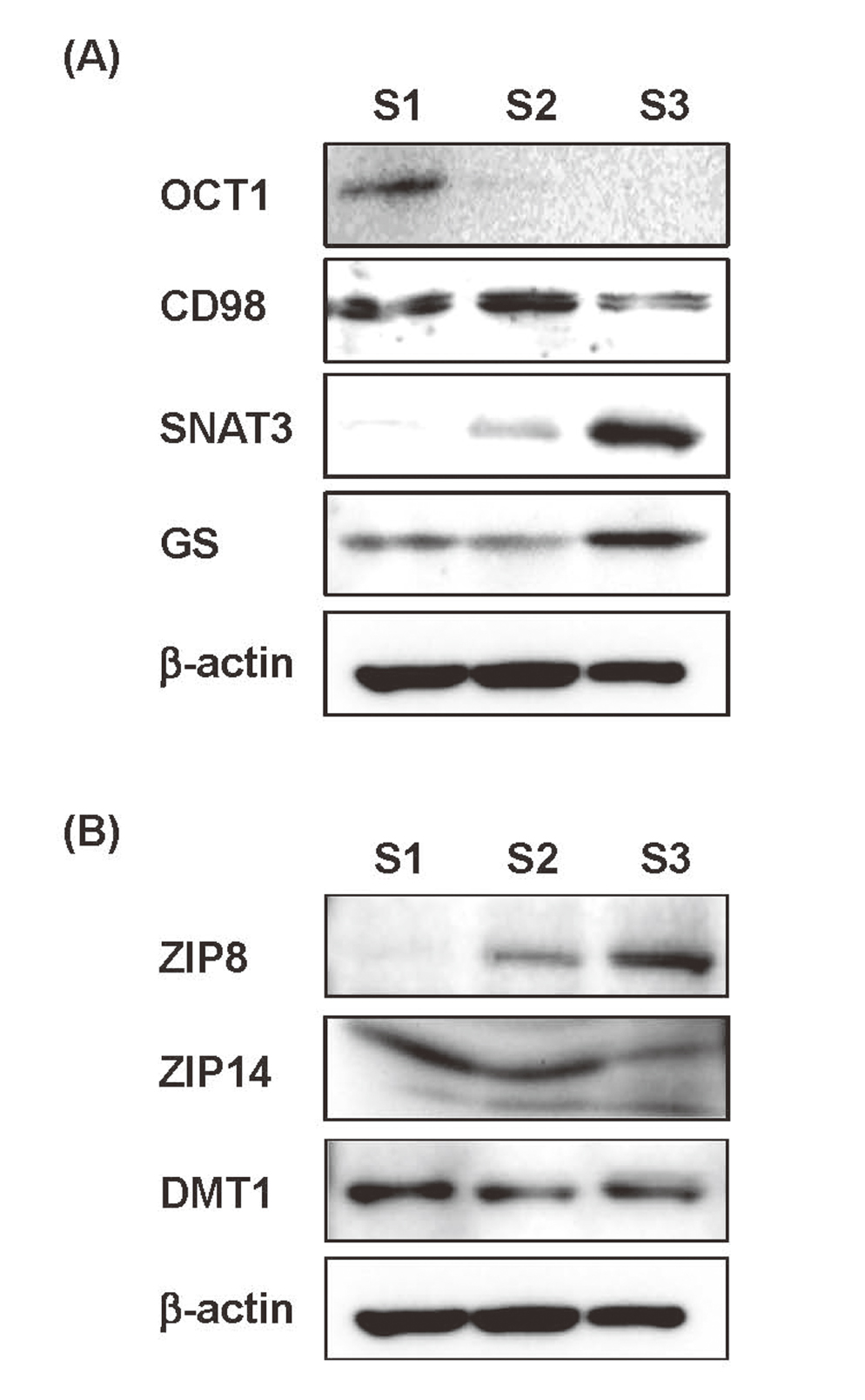
Western blot analyses of segment-specific marker proteins and metal transporters. Whole-cell lysates of S1, S2, and S3 cells were subjected to Western blot analyses. β-actin was used as a loading control. (A) The protein levels of markers for each segment such as OCT1 for the S1 segment, CD98 for the S1 and S2 segments, and SNAT3 and GS for the S3 segment showed similar expression patterns as those observed in in vivo studies. (B) The protein level of ZIP8 was the highest in S3 cells, while those of ZIP14 and DMT1 were roughly similar among S1, S2, and S3 cells.
Next, the expression levels of ZIP8, ZIP14 and DMT1, metal transporters having affinities for both Cd2+ and Mn2+ in S1, S2, and S3 cells were examined by Western blot analysis (Fig. 1B). We found that the protein level of ZIP8 was the highest in S3 cells, in accordance with the previous in situ hybridization study showing the highest expression of ZIP8 in the S3 segment of mouse kidney proximal tubule (Fujishiro et al., 2012). In contrast, the protein levels of ZIP14 and DMT1 were approximately similar among S1, S2, and S3 cells, in accordance with a reported in vivo observation (Fujishiro et al., 2012). The results of the microarray analysis in the previous study also showed the higher mRNA levels of ZIP8, but not ZIP14 or DMT1, in S3 cells than in S1 or S2 cells (Fujishiro and Himeno, 2019). These data suggest that the S1, S2, and S3 cells may be useful at least for studying segment-specific transport of Cd and Mn by metal transporters, although the transport systems for other metals and renal toxicants should be further examined in a future study.
Next, we checked whether S1, S2, and S3 cells can be cultured on the membrane of the trans-well for studying direction-dependent transports of the metals. As shown in Supplemental Fig. 1, S1, S2, and S3 cells showed TEER values higher than 150 Ω, which is known to be enough for the formation of tight junctions, and the high TEER values were maintained from day 2 to day 10 or over (Nakagawa and Niwa, 2014). Especially, S1 and S2 cells showed high levels of TEER, which is also confirmed by the E-cadherin staining in these cells (Supplemental Fig. 2). Relatively lower TEER values in S3 cells than S1 and S2 cells may reflect the lower mRNA levels of E-cadherin and occludin in S3 cells than in S1 or S2 cells detected in the microarray analysis (Fujishiro and Himeno, 2019). However, we confirmed no leakage of the solutes added in the apical or basal chambers of the trans-well when S3 cells were cultured on the membrane of the cell insert under confluent conditions. Thus, we proceeded to examine the metal transports using S1, S2 and S3 cells cultured on the trans-well.
Uptake rates of Cd2+ and Mn2+ in trans-well cultured S1, S2, and S3 cellsUsing S1, S2, and S3 cells cultured on the trans-well, we determined the initial uptake rates of Cd2+ and Mn2+ from the apical and basolateral sides of cells. When Cd2+ was added to the medium of the apical chamber of the trans-well, the highest uptake of Cd2+ was detected in S3 cells (Fig. 2A). Similarly, the uptake rate of Mn2+ from the apical side of the cells was the highest in S3 cells. On the contrary, the uptake rates of Cd2+ and Mn2+ from the basolateral side of cells showed no substantial difference among S1, S2, and S3 cells (Fig. 2B). These results suggest that the metal-transporting system on the apical side of S3 cells may have the highest ability for the incorporation of Cd2+ and Mn2+.
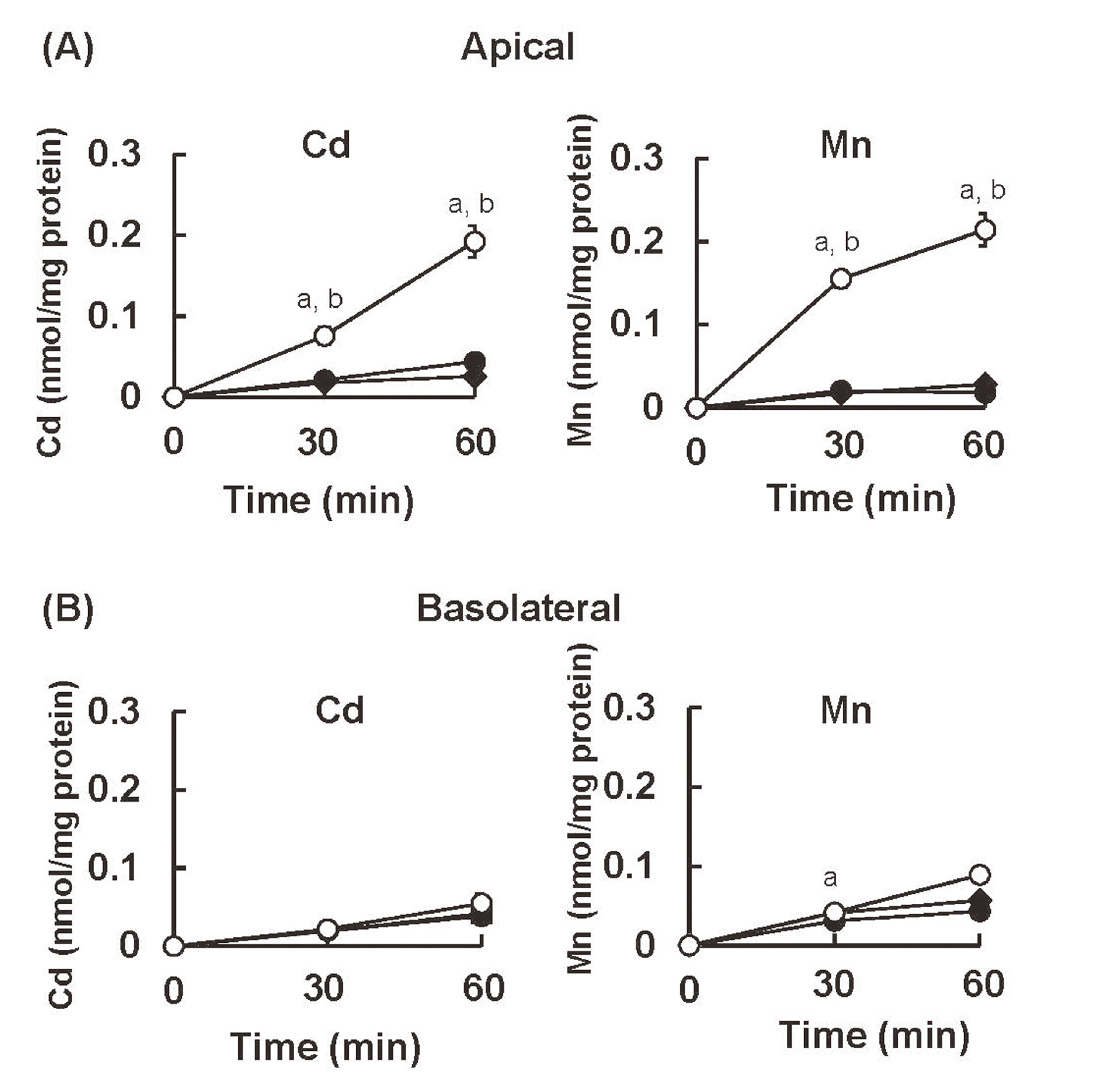
The uptake rates of Cd2+ and Mn2+ into S1, S2, and S3 cells from the apical and basolateral sides of cells. (A) S1, S2, and S3 cells were cultured on the trans-well. Radiolabeled CdCl2 (0.1 μM) and MnCl2 (0.1 μM) were added to the medium in the upper chamber (A) or the lower chamber (B) of the trans-well. Cellular concentrations of Cd and Mn were determined at the time points indicated by their radioactivities. Values are means ± SD. Closed squares, S1 cells; closed circles; S2 cells; open circles, S3 cells. Statistically significant differences were determined by two-way ANOVA followed by Turkey multiple comparison. a, significant difference between S3 vs S1 (p < 0.01); b, significant difference between S3 vs S2 (p < 0.01).
We next examined whether the uptakes of Cd2+ and Mn2+ from the apical side of S3 cells could be inhibited by each other. As shown in Fig. 3A, simultaneous addition of Mn2+ or Zn2+ with Cd2+ in the apical media dose-dependently inhibited the uptake of Cd2+ in S3 cells. Similarly, the addition of Cd2+ or Zn2+ inhibited the apical uptake of Mn2+ (Fig. 3B). The competitive inhibition of metal uptake shown here strongly suggests that the transport of Cd2+, Mn2+, and Zn2+ at the apical membrane of S3 cells is mediated by ZIP8, ZIP14 or DMT1, which all have affinities for Cd2+, Mn2+, and Zn2+. Among these metal transporters, ZIP14 and DMT1 showed similar levels of expression among S1, S2, and S3 cells (Fig. 1B), suggesting that the high rates of Cd2+ and Mn2+ uptake in S3 cells could not be explained by the expressions of ZIP14 or DMT1. In addition, the stronger inhibition of Mn uptake by Cd than Zn (Fig. 3B) suggests that this competition may be mediated by ZIP8, which has higher affinities for Mn and Cd than Zn (Girijashanker et al., 2008). Thus, the high expression of ZIP8 in S3 cells (Fig. 2B) may be the primary cause for the efficient incorporations of Cd2+ and Mn2+ from the apical side of S3 cells, although additional involvement of ZIP14 and DMT1 could not be completely excluded.
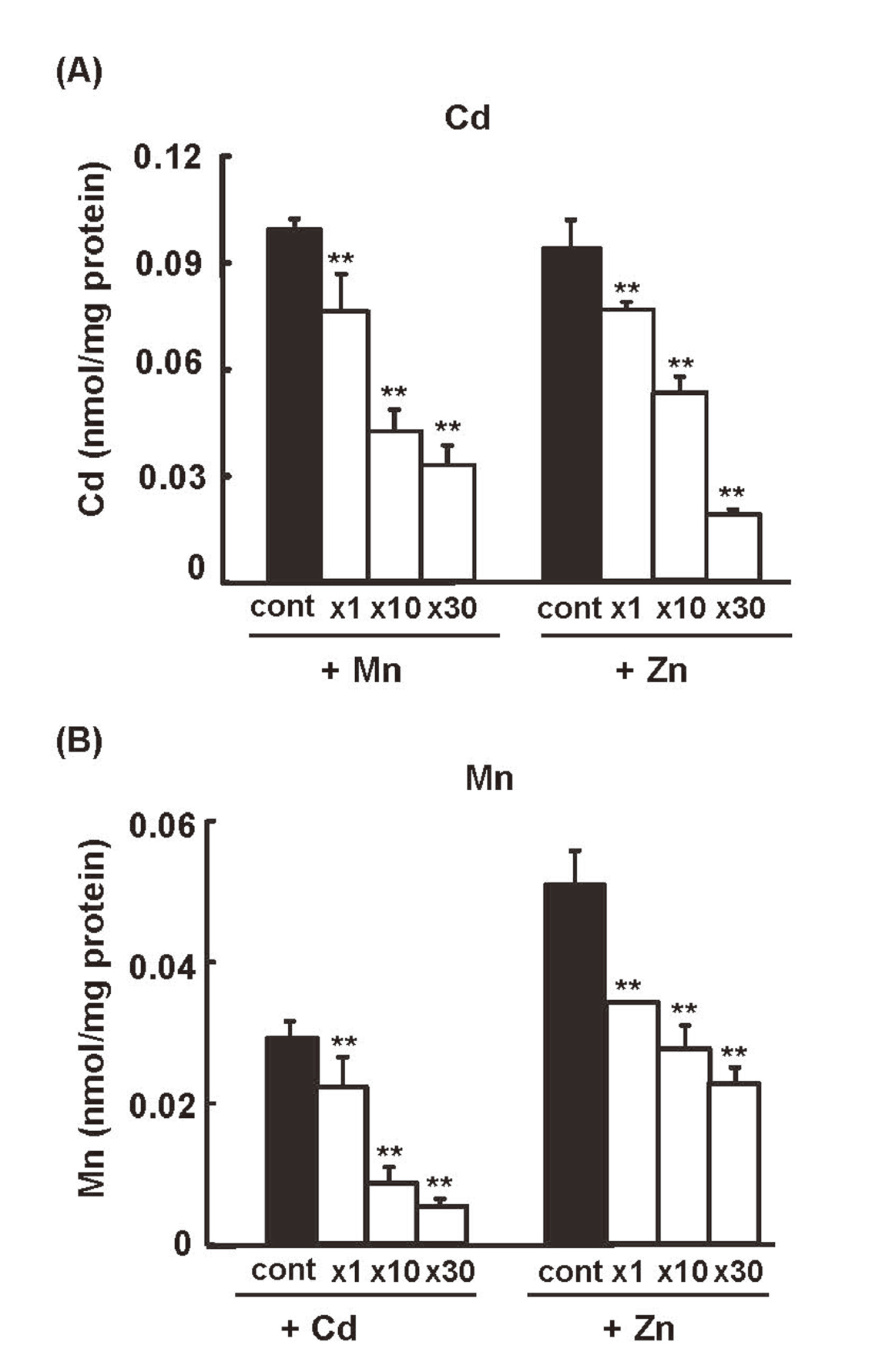
Competitive inhibition of the apical uptake of Cd2+ and Mn2+ in S3 cells. S3 cells cultured in the trans-well were exposed to radiolabeled CdCl2 (0.1 μM) from the apical side in the presence or absence of the indicated molar ratios of MnCl2 or ZnCl2 (A), or exposed to radiolabeled MnCl2 (0.1 μM) from the apical side in the presence or absence of the indicated molar ratios of CdCl2 or ZnCl2 (B). Cellular concentrations of Cd and Mn were determined 1 hr after the addition of the metals by their radioactivities. Significantly different from control “no competitor”, statistically significant differences were determined by one-way ANOVA followed by Williams multiple comparisons. *p < 0.05, ** p < 0.01.
The trans-well cell culture system can also be used for the measurement of metal excretion into the apical and basolateral chambers separately. After S1, S2, and S3 cells were incubated with CdCl2 or MnCl2, which was added to the medium of the apical chamber for 2 hr, the medium was changed to a Cd- or Mn-free one, and then the amounts of Cd and Mn excreted to the apical and basolateral chambers during 30 and 60 min were determined.
In contrast to the uptake rates of Cd2+ and Mn2+, the excretion efficiencies of the two metals did not show clear segment-specific differences (Fig. 4). It was evident, however, that higher amounts of Cd and Mn were excreted to the apical media than to the basolateral media from S1, S2, and S3 cells. At 30 min, more than 10-fold Cd and 4- to 10-fold Mn, respectively, was excreted to the apical media compared to the basolateral media. The excretion efficiencies of Mn were higher (1.5 to 2.5 times on the apical side and 5 times on the basolateral side at 30 min) than those of Cd.
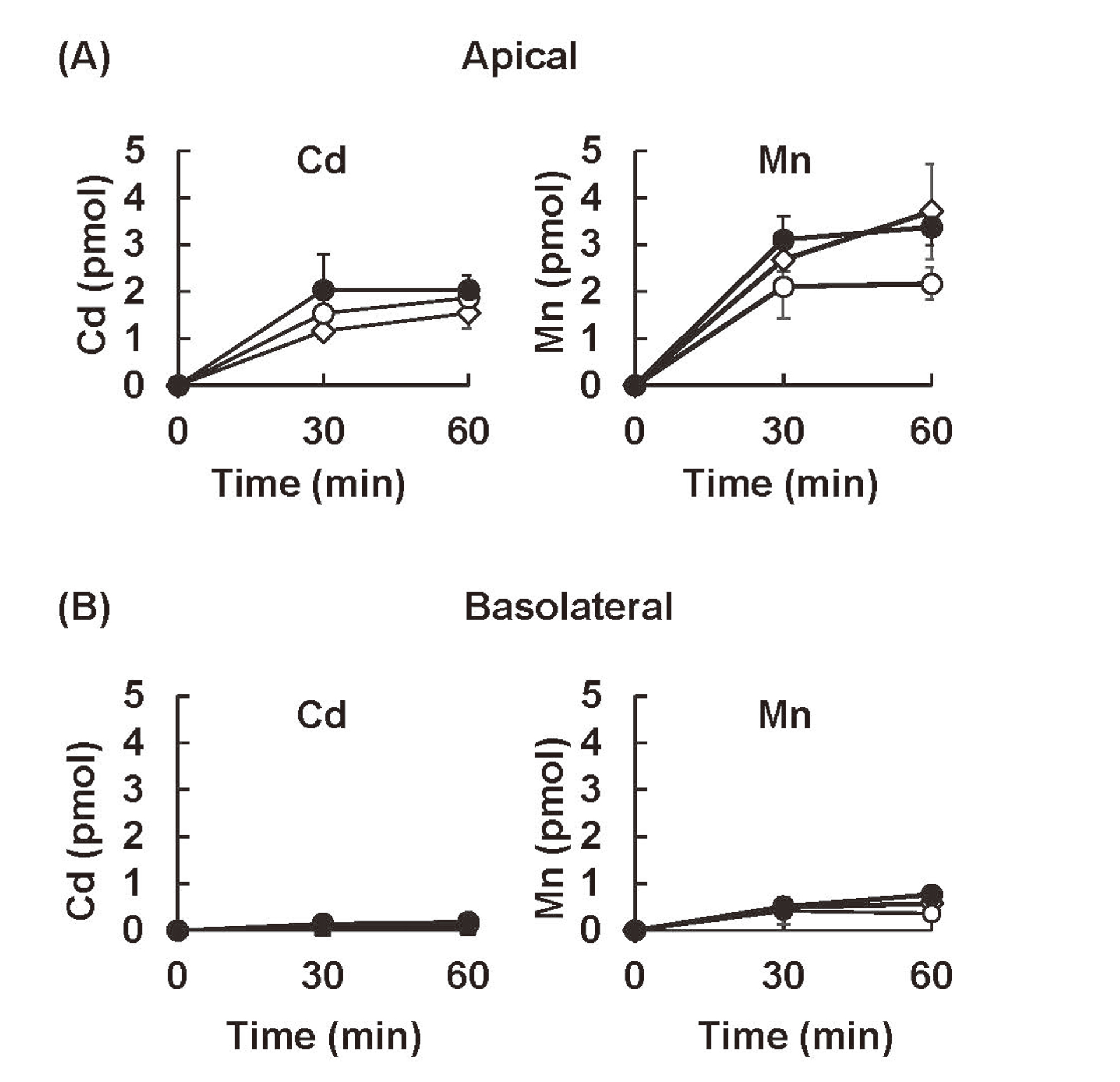
The excretion of Cd and Mn from the apical and basolateral sides of S1, S2, and S3 cells. The cells cultured on the trans-well were exposed to radiolabeled CdCl2 (0.1 μM) or MnCl2 (0.1 μM) from the apical side for 2 hr, washed with serum-containing media, and then incubated further with metal-free media. The concentrations of Cd and Mn excreted into the media in the apical (A) and basolateral (B) chambers were determined by their radioactivities 30 and 60 min after the media were changed. Statistically significant differences were determined by two-way ANOVA followed by Turkey multiple comparison. No significant differences were detected between each cell line by the multiple comparisons.
These data obtained in cultured cells suggest the possibility that some of the Cd and Mn is excreted to the proximal tubule lumen from the PTECs in the kidney. Then, some of the Cd and Mn excreted into the lumen could be reabsorbed into the PTECs in the S3 segment since the S3 cells used in this study showed the highest uptake efficiencies of Cd2+ and Mn2+ from the apical side (Fig. 2).
In a previous study using trans-well cultured PT cells, we presented the hypothesis that small amounts of Cd and Mn are excreted into the lumen of the renal proximal tubule via the apical side of PTECs and then reabsorbed into the PTECs in the lower part of proximal tubule, probably the S3 segment (Fujishiro et al., 2012). At that time, however, segment-specific PTECs were not used. To obtain further evidence for proving this hypothesis, we compared the segment-specific and direction-dependent transports of Cd and Mn using immortalized S1, S2, and S3 segment-specific cell lines. Characterization of these cells for the expression levels of the marker proteins, known to be expressed in a segment-specific manner, and those of the metal transporters involved in Cd and Mn (Fig. 1) demonstrated that these cells are useful at least in studying the segment-specific transports of Cd and Mn. We also confirmed the usability of these cells for examining the direction-dependent transports of metals using a trans-well culture system.
Our results showed that when the metals were added to the media of the apical chamber of the trans-well, the highest uptake of Cd2+ as well as Mn2+ was observed in S3 cells (Fig. 2). Since the expression levels of ZIP14 and DMT1 were roughly similar among S1, S2, and S3 cells (Fig. 1B), the highly efficient and mutually inhibitable uptakes of Cd2+ and Mn2+ in S3 cells (Fig. 3) might be closely connected to the higher expression of ZIP8 in S3 cells (Fig. 1B). The Cd and Mn released from the PTECs via the apical membrane (Fig. 4) may provide, at least partly, a source of Cd2+ and Mn2+ for the uptake by the PTECs in the S3 segment via metal transporters, including ZIP8. However, further studies including the determination of the chemical forms of Cd or Mn excreted from these cells in a short term are required to further characterize the dynamic transports of metals between each segment of proximal tubule.
It has been well known that uric acid in the kidney undergoes intra-proximal tubule handling between the lumen and PTECs characterized by four- or seven-compartment models (Gutman et al., 1959; Diamond and Paolino, 1973). A similar model has also been proposed for renal handling of the copper (Cu) bound to MT based on in vivo observation of differential expression of MT protein in the S1 segment and MT mRNA in the S3 segment of the rat kidney proximal tubule (Okabe et al., 1996). It seems likely that there exists a dynamic intra-proximal-tubule mobilization of Cd similar to those of uric acid and Cu, but its efficiency is very low because the cellular Cd is tightly bound to MT.
Contrary to the case of Cd, intra-proximal tubule mobilization of Mn appears to play a more important role since there is no interference by MT, which does not bind Mn, in renal Mn handling. Moreover, the excretion efficiency of Mn into the basolateral side in S3 cells was shown to be about five times higher than that of Cd (Fig. 4). These results suggest that efficient uptake of Mn in the S3 segment by metal transporters, especially by ZIP8, may serve as a recovery system allowing the Mn in the proximal tubule lumen to return to systemic circulation.
Recent findings of human cases of ZIP8 mutations causing serious clinical symptoms have highlighted the crucial role of ZIP8-dependent renal Mn reabsorption (Park et al., 2015; Boycott et al., 2015). In Germany and Egypt, rare cases of mutations in the SLC39A8 gene encoding human ZIP8 have been identified as the cause of symptoms similar to those observed in congenital glycosylation deficiency (Park et al., 2015; Boycott et al., 2015). Since blood Mn levels in these patients were undetectable or very low, it has been postulated that the resulting dysfunction of galactosyltransferase, the enzymatic activity of which is completely dependent on Mn, led to defects in the glycosylation of a variety of important proteins (Park et al., 2015; Boycott et al., 2015). The treatment of these patients with a high dose of galactose as well as Mn was effective in alleviating their symptoms (Riley et al., 2017; Park et al., 2018).
Undetectable levels of Mn in the blood of such patients may be caused by the loss of Mn from the body via the enhanced excretion of Mn. A clinical study showed that the urinary excretion of Mn in the ZIP8-mutated patients during Mn therapy was much higher than that in normal people (Park et al., 2018). This clinical observation may be strongly associated with our observation of the efficient uptake of Mn2+ via ZIP8 from the apical side of S3 cells. Further studies on the precise mechanisms of ZIP8-mediated renal Mn reabsorption are warranted to elucidate the mechanisms of ZIP8 mutation-related diseases and the physiological roles of renal Mn reabsorption in the maintenance of whole-body Mn homeostasis.
In the present study, we demonstrated that S1, S2, and S3 cells provided an excellent tool for studying the segment-specific and direction-dependent transports of Cd and Mn. Further characterization of these cells, i.e., the elucidation of the expression levels of the transporters and detoxification systems for other renal toxicants, are required for proving the usefulness of these cells as an in vitro test tool for studying the transports and toxicities of various types of renal toxicants. S1, S2, and S3 cells may also provide an excellent tool for elucidation of the mechanisms by which the homeostasis of an essential element is regulated in the kidney via the transfer of the element from the lumen into PTECs and then either to systemic circulation or back to the lumen.
This work was partly supported by JSPS KAKENHI Grant Number C-18K06646 and the Study of the Health Effects of Heavy Metals organized by the Ministry of the Environment, Japan.
Conflict of interestThe authors declare that there is no conflict of interest.