Abstract
Enniatins are so-called “emerging mycotoxins” that commonly occur in milligrams per kilogram levels in grains and their derived products, as well as in fish, dried fruits, nuts, spices, cocoa, and coffee. The present study investigated the 28-day repeated oral dose toxicity of enniatin complex in CD1(ICR) mice. Enniatin B, enniatin B1, and enniatin A1 at a ratio of 4:4:1 were administered to male and female mice at doses of 0 (vehicle controls), 0.8, 4, and 20 mg/kg body weight/day. In life parameters did not change during the study period, with the exception of slight reductions in food consumption in male mice administered 4 and 20 mg/kg and in female mice administered 20 mg/kg. Body and organ weights did not change, and no alterations in hematology, blood biochemistry, or histopathology parameters were observed at the end of the administration period. Thus, we determined that the no-observed-adverse-effect level of enniatin complex was 20 mg/kg/day for both sexes under the present experimental conditions.
INTRODUCTION
Fusarium species are common pathogenic microorganisms that contaminate cereal grains, animal feed, and food commodities worldwide (Fraeyman et al., 2017). Enniatins are representative hexadepsipeptidic mycotoxins of this fungal species produced by secondary metabolism (Prosperini et al., 2017). Enniatins are so called “emerging mycotoxins” that commonly occur in milligrams per kilogram levels in grains and their derived products, as well as in fish, dried fruits, nuts, spices, cocoa, and coffee (Ivanova et al., 2017; Prosperini et al., 2017). Moreover, cooking, baking, frying, and roasting do not destabilize the chemical structure of enniatins (de Nijs et al., 2016). Mean chronic exposure to the sum of enniatins has been estimated at 0.42-1.82 µg/kg body weight per day, and the 95th percentile dietary exposure in adults has been estimated to be 0.91-3.28 µg/kg body weight per day (EFSA Panel on Contaminants in the Food Chain, 2014).
Unlike other Fusarium mycotoxins, enniatins in food and feed have not been regulated by authorities until now. Eleven enniatin analogs are known (A, A1, B, B1, B2, B3, B4, D, E, F, and G), and enniatin A, A1, B, and B1 are the most prevalent forms reported as natural contaminants (Santini et al., 2012; Juan et al., 2013; Tittlemier et al., 2013; Yoshinari et al., 2016). Enniatins are known to possess insecticidal, antifungal, antibacterial, and anthelmintic properties (Jestoi, 2008). Moreover, they exert a potent cytotoxic effect in several human and animal cell lines at very low micromolar range (Prosperini et al., 2017). This fact may constitute a great concern for human and animal health. Strong cytotoxicity has been observed in vitro, but the few in vivo studies conducted could not replicate it (Juan et al., 2014; Manyes et al., 2014; Rodríguez-Carrasco et al., 2016; Escrivá et al., 2015). The European Food Safety Authority concluded that acute exposure to enniatins does not indicate concern for human health (EFSA Panel on Contaminants in the Food Chain, 2014). No firm conclusion has been drawn regarding the effects of chronic exposure, and a risk assessment requires toxicity data (EFSA Panel on Contaminants in the Food Chain, 2014).
A 42-day repeated oral dose toxicity study of enniatin B using doses of 0.18, 1.8, and 18 mg/kg/day has been reported in mice (Maranghi et al., 2018), and the authors determined a no-observed-adverse-effect level (NOAEL) of 0.18 mg/kg/day from histomorphometric changes in the thymus, uterus, and spleen in females. However, there were also exposure-unrelated changes in the parameters examined, and a dose-response relationship could not be estimated, requiring general enniatin toxicity studies with appropriate dose intervals.
The present study was performed to obtain a general toxicity profile of enniatins after a 28-day repeated oral exposure in CD1(ICR) mice.
MATERIALS AND METHODS
Chemicals and animals
Enniatin complex (purity: > 95%) was purchased from BioAustralis Fine Chemicals (Smithfield, Australia). According to the constituent analysis by high performance liquid chromatography (HPLC), enniatin complex was consisted of enniatin B, enniatin B1, and enniatin A1, with the ratio of 4:4:1, respectively. The animal experiment was carried out at BoZo Research Center Inc. (Shizuoka, Japan). A total of 40 male and 40 female Crl:CD1(ICR) mice at 5-week of age were purchased from Charles River Laboratories Japan Inc. (Kanagawa, Japan). Animals were maintained in an air-conditioned animal room with a twelve-hour light/dark cycle (room temperature, 23 ± 3ºC; relative humidity, 50 ± 20%). The animals were housed individually in plastic cages with paper bedding and were provided a pelleted basal diet (CRF-1; Oriental Yeast Co., Ltd., Tokyo, Japan) and tap water ad libitum throughout the experimental period.
Experimental design
The test article was prepared by adding an enniatin complex mixture to corn oil supplemented with 6% dimethyl sulfoxide. The stability of the test article was examined by comparing the amount of enniatin B, enniatin B1, and enniatin A1 in 4 mg/mL solution stored one day and 8 days at 4°C after preparation, using HPLC, and no degradation was confirmed with each enniatin analog. After one week of acclimation, the test article was administered to mice by oral gavage (10 males and 10 females/group) at a dose of 0 (vehicle controls), 0.8, 4, and 20 mg/kg body weight/day. The dosage of this study was determined based on the results of the previous 42-day repeated oral dose toxicity study of enniatin B at doses of 0, 0.18, 1.8, or 18 mg/kg/day in mice, with the NOAEL determined to be 1.8 mg/kg/day in males and 0.18 mg/kg/day in females (Maranghi et al., 2018).
During the administration period, animals were observed daily for clinical signs and mortality, and body weight and food consumption were measured. Clinical signs, including any abnormality in the external appearance, nutritional condition, posture, behavior, and excretions, were checked during the administration period (before the administration, immediately and 1-3 hr after the administration) and before unloading animals from the animal room on the necropsy day. Body weight was measured during the administration period on days 1, 4, 7, and thereafter every 7 days, once a week, before unloading animals from the animal room on the necropsy day. Body weight gain throughout the administration day 1 to day 28 was calculated. Food consumption in each animal was measured during the administration period on days 1, 4, 7, and thereafter once a week. One day after completion of the administration period, animals were weighed and euthanized by exsanguination from the abdominal aorta under isoflurane anesthesia without overnight fasting.
Animals were given appropriate environmental enrichment according to the guidelines of the Institutional Animal Care and Use Committee (IACUC). This experiment was conducted with the approval of IACUC and was performed according to each guideline for the animal welfare and to standard operating procedures.
Hematology and blood biochemistry
Before euthanization at the scheduled sacrifice, blood samples were collected from the posterior vena cava to use for hematology and blood biochemistry tests under deep anesthesia. The hematological parameters included the red blood cell count, hemoglobin concentration, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, red blood cell distribution width, reticulocyte count, platelet count, white blood cell count, and differential leukocyte counts. Blood biochemistry parameters were total protein, albumin, albumin/globulin ratio, aspartate aminotransferase, alanine aminotransferase, lactate dehydrogenase, alkaline phosphatase, triglyceride, glucose, blood urea nitrogen, and creatinine.
The dead animal was not tested.
Necropsy and histopathology
At the necropsy of the scheduled sacrifice, all animals were examined carefully the external and all the internal organs. The animal found dead was necropsied as soon as possible. In animals of scheduled sacrifice, gross findings were recorded and abnormal portions were removed along with all organs/tissues. They were fixed with phosphate buffered 10% formalin, with the exception of the testes and epididymides, which were prefixed with Bouin’s solution, and the eyeballs including the optic nerves, which were fixed with phosphate buffered 3.0% glutaraldehyde and 2.5% formalin. Among organs/tissues removed, brain, thymus, heart, lungs, liver, spleen, kidneys, testes, ovaries, epididymides, and uterus were weighed before fixation. One animal found dead was not subjected to organ weight measurement.
In all animals, including an animal which died, the cerebrum, cerebellum, spinal cord (thoracic), sciatic nerves, eyeballs, Harderian glands, pituitary, thyroids including parathyroids, adrenals, thymus, spleen, submandibular lymph node, mesenteric lymph node, heart, aorta (thoracic), trachea, lungs including bronchi, tongue, esophagus, stomach, duodenum, jejunum, ileum including Peyer’s patch, cecum, colon, rectum, submandibular glands, sublingual glands, liver, gall bladder, pancreas, kidneys, urinary bladder, testes, ovaries, epididymides, uterus, prostate, vagina, seminal vesicles including coagulating glands, mammary glands (inguinal; females), sternum, femur skeletal muscles, and skin (inguinal) along with abnormal portions were trimmed, paraffin-embedded, sectioned at 4 µm, stained with hematoxylin and eosin (HE), and assessed histopathologically.
Statistical analysis
Numerical data, i.e., body weight, body weight gain, food intake, hematology test, blood biochemistry test, and organ weight, were analyzed between the vehicle controls and each enniatin-administered group, using SAS Release 9.1.3 (SAS Institute Inc., Cary, NC, USA). Data were analyzed using Bartlett’s test for homogeneity of variance. If the variance was homogenous, numerical data were assessed using Dunnett’s test. For heterogeneous data, Steel’s test was applied.
With regard to categorical data, such as the incidence and severity of histopathological findings, incidence of finding was compared using Fisher’s exact probability test and severity of change was compared using Mann-Whitney’s U-test using Excel Statistics 2010 software package (Social Survey Research Information Co. Ltd., Tokyo, Japan).
RESULTS
Clinical signs, body weight and food consumption
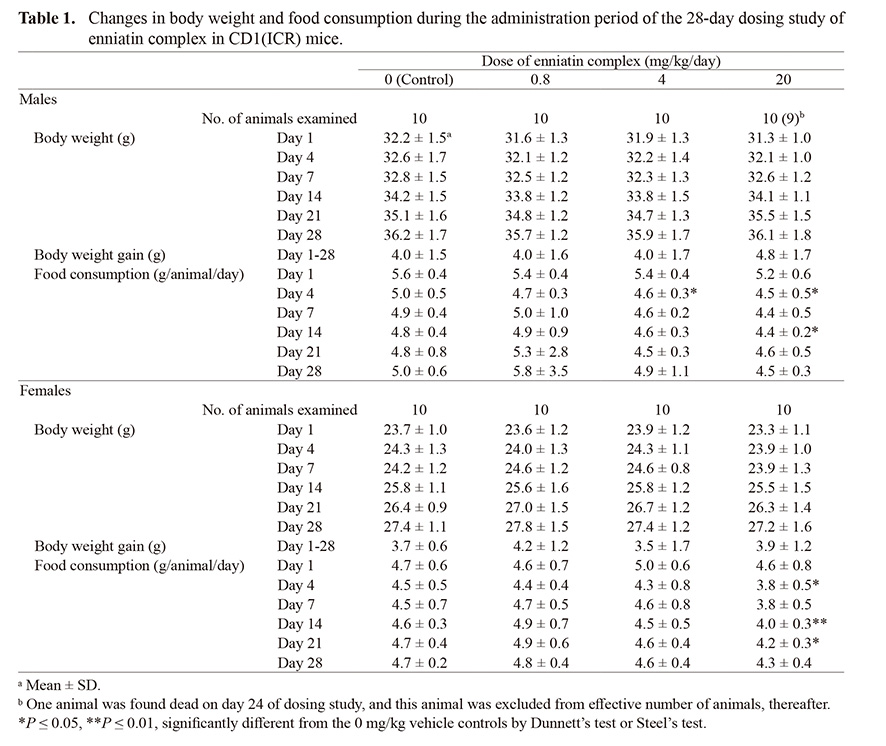
On administration day 22 and 23, one male in the 20 mg/kg group exhibited decreased locomotor activity and looked pale. Then the animal was found dead on administration day 24. Other animals in all groups showed no abnormal clinical signs. Body weight and body weight gain during the administration period were not statistically different between the 0 mg/kg vehicle controls and any of the enniatin groups (Table 1). Food consumption was significantly decreased on administration day 4 in the 4 and 20 mg/kg groups and on administration day 14 in the 20 mg/kg group, as compared with the 0 mg/kg vehicle controls in males (Table 1). In females, food consumption was significantly decreased on administration day 4, 14 and 21 in the 20 mg/kg group, as compared with the 0 mg/kg vehicle controls.
Table 1. Changes in body weight and food consumption during the administration period of the 28-day dosing study of enniatin complex in CD1(ICR) mice.
 Hematology and blood biochemistry
Hematology and blood biochemistry
There were no parameters to show statistically significant difference in the hematology and blood biochemistry in any of the enniatin-administered groups as compared with the 0 mg/kg vehicle controls (Tables 2 and 3).
Table 2. Hematological data at the end of repeated administration of enniatin complex for 28 days in CD1(ICR) mice.

Table 3. Blood biochemistry data at the end of repeated administration of enniatin complex for 28 days in CD1(ICR) mice.
 Gross findings and organ weights at necropsy
Gross findings and organ weights at necropsy
The dead male animal in the 20 mg/kg group showed pallor skin, fading of various organs, one dark red lesion in the subcutis of the left axilla (25 × 20 mm), and dark red discoloration of the left forelimb. At the scheduled necropsy, one female animal in the 0 mg/kg vehicle controls showed a focal dark discoloration at the liver surface. Other animals in all groups did not show any gross findings.
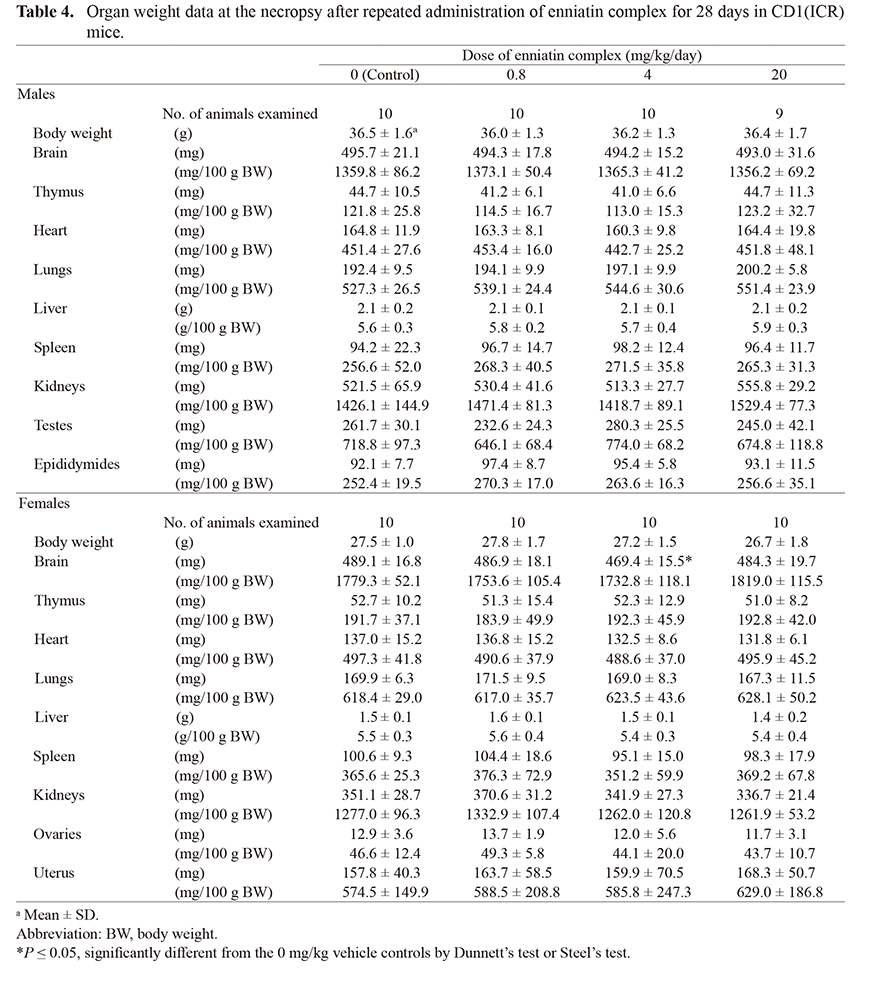
With regard to organ weight, absolute brain weight of the 4 mg/kg group was lower than that of the 0 mg/kg vehicle controls in females. Other organs/tissues did not show any changes in the absolute or relative weight (Table 4).
Table 4. Organ weight data at the necropsy after repeated administration of enniatin complex for 28 days in CD1(ICR) mice.
 Histopathological analysis
Histopathological analysis
In animals of scheduled sacrifice, histopathological lesions were observed in the glandular stomach, kidneys, liver, and ovaries; however, there was no statistically significant difference in the incidence of any lesion developed between the 0 mg/kg vehicle controls and any of the enniatin complex-administered groups (Table 5). The found dead male animal in the 20 mg/kg group showed subcutaneous hemorrhage, in accordance with grossly appeared lesions.
Table 5. Histopathological data after repeated administration of enniatin complex for 28 days in CD1(ICR) mice.

DISCUSSION
In the present study, a repeated oral dose toxicity study of enniatin complex in mice revealed decreased food consumption in male mice administered 4 and 20 mg/kg and in female mice administered 20 mg/kg. However, no clinical changes or alterations in body weight were observed during the experiment, and no alterations in hematology, blood biochemistry, or histopathology parameters were observed at the end of the administration period. On day 24, one male animal in the 20 mg/kg group deteriorated and died. Histopathological analysis revealed bleeding from the forelimb which may have been the cause of death. Because other animals did not deteriorate, we surmise that the cause of death was accidental injury and not related to enniatin exposure. Histopathological findings were not dose-related and were identical to spontaneous lesions commonly observed in rodents. These results suggest that a 28-day exposure to enniatin complex at doses that suppress food consumption does not induce toxicity in mice.
A previous 42-day repeated oral dose toxicity study of enniatin B in CD1 mice, an identical strain to that used in the present study, estimated the lowest-observed-adverse-effect level (LOAEL) as 1.8 mg/kg/day from histomorphometric changes in the thymus, uterus, and spleen in females (Maranghi et al., 2018). Males exhibited vacuolization of enterocytes in the duodenum and higher levels of reactive oxygen species and lower production of glutathione in the brain following exposure to 18 mg/kg/day. These findings suggest that enniatin B is highly toxic to mice, but the histomorphometric changes in females were unrelated to organ weights. Moreover, the duodenal epithelial vacuolization was observed in all groups, including vehicle controls, in females, so the toxicological relevance of the vacuolization is unclear. The production of reactive oxygen species in the brain was not accompanied by weight and histopathological changes. Multiple dose-unrelated alterations were observed and a dose-response relationship could not be estimated because of the wide intervals between doses. In the present study, the highest dose of enniatin B exceeded the LOAEL estimated in the 42-day study (Maranghi et al., 2018), and we did not observe changes suggestive of toxicity.
It is possible that enniatin toxicity varies by analog and by animal species or strain. However, other than the apparent in vivo toxicity of enniatin B in CD1 mice reported by Maranghi et al. (2018), only three studies have examined the in vivo toxicity of any enniatin analog. Two studies investigated the effects of a 28-day oral exposure to enniatin A in Wistar rats; one observed T cell subpopulation changes in peripheral blood (Juan et al., 2014), and the other reported no apparent effect on the duodenal tract (Manyes et al., 2014). In both studies, enniatin A levels in serum or the liver increased. The third in vivo study examined the effects of intraperitoneal injections of enniatin B on two consecutive days in CB-17 scid/scid mice, with no acute toxicity observed (Rodríguez-Carrasco et al., 2016). In that study, phase I metabolites were detected in the liver and colon, suggestive of biotransformation in these organs. Similarly, a single oral administration of enniatin B1 induced hepatic biotransformation in pigs (Ivanova et al., 2017). However, a single oral administration of a mixture of enniatins A, A1, B, and B1 did not elevate serum or urine levels of any of the constituents in Wistar rats, although enniatins were detected in feces and their concentrations peaked 6 hr after administration (Escrivá et al., 2015). Enniatin B and enniatin B1 have been reported to be poorly absorbed after oral administration in broiler chickens, with absolute oral bioavailability of 0.05 and 0.11, respectively (Fraeyman et al., 2016). While enniatins are biotransformed in the liver, these findings suggest that at least enniatin B and B1 are poorly absorbable after oral administration. Most of the enniatin complex we used is composed of the B and B1 analogs, and thus poor absorption explains why we did not observe toxicity even after 28 days of exposure.
In conclusion, enniatin complex did not exert toxicity at doses up to 20 mg/kg/day following a 28-day oral administration in mice of both sexes, although slight reductions in food consumption were observed. Thus, the NOAEL of this enniatin complex is estimated at 20 mg/kg/day under the present experimental conditions. Because tolerable daily intake levels have not been established for enniatins, 90-day exposure studies are necessary.
ACKNOWLEDGMENTS
This work was supported by Health and Labour Sciences Research Grants (Research on Food Safety) from the Ministry of Health, Labour and Welfare of Japan (Grant No. 19KA1004), and by a Research Fund from Institute of Global Innovation Research, Tokyo University of Agriculture and Technology. We thank Edanz Group (https://en-author-services.edanz.com/ac) for editing a draft of this manuscript.
Conflict of interest
The authors declare that there is no conflict of interest.
REFERENCES
- de Nijs, M., van den Top, H., de Stoppelaar, J., Lopez, P. and Mol, H. (2016): Fate of enniatins and deoxynivalenol during pasta cooking. Food Chem., 213, 763-767.
- EFSA Panel on Contaminants in the Food Chain (CONTAM). (2014): Scientific opinion on the risks to human and animal health related to the presence of beauvericin and enniatins in food and feed. EFSA J., 12, 3802.
- Escrivá, L., Font, G. and Manyes, L. (2015): Quantitation of enniatins in biological samples of Wistar rats after oral administration by LC-MS/MS. Toxicol. Mech. Methods, 25, 552-558.
- Fraeyman, S., Croubels, S., Devreese, M. and Antonissen, G. (2017): Emerging Fusarium and Alternaria Mycotoxins: Occurrence, Toxicity and Toxicokinetics. Toxins (Basel), 9, 228.
- Fraeyman, S., Devreese, M., Antonissen, G., De Baere, S., Rychlik, M. and Croubels, S. (2016): Comparative Oral Bioavailability, Toxicokinetics, and Biotransformation of Enniatin B1 and Enniatin B in Broiler Chickens. J. Agric. Food Chem., 64, 7259-7264.
- Ivanova, L., Uhlig, S., Devreese, M., Croubels, S. and Fæste, C.K. (2017): Biotransformation of the mycotoxin enniatin B1 in pigs: A comparative in vitro and in vivo approach. Food Chem. Toxicol., 105, 506-517.
- Jestoi, M. (2008): Emerging fusarium-mycotoxins fusaproliferin, beauvericin, enniatins, and moniliformin: a review. Crit. Rev. Food Sci. Nutr., 48, 21-49.
- Juan, C., Mañes, J., Raiola, A. and Ritieni, A. (2013): Evaluation of beauvericin and enniatins in Italian cereal products and multicereal food by liquid chromatography coupled to triple quadrupole mass spectrometry. Food Chem., 140, 755-762.
- Juan, C., Manyes, L., Font, G. and Juan-García, A. (2014): Evaluation of immunologic effect of Enniatin A and quantitative determination in feces, urine and serum on treated Wistar rats. Toxicon, 87, 45-53.
- Manyes, L., Escrivá, L., Serrano, A.B., Rodríguez-Carrasco, Y., Tolosa, J., Meca, G. and Font, G. (2014): A preliminary study in Wistar rats with enniatin A contaminated feed. Toxicol. Mech. Methods, 24, 179-190.
- Maranghi, F., Tassinari, R., Narciso, L., Tait, S., Rocca, C.L., Felice, G.D., Butteroni, C., Corinti, S., Barletta, B., Cordelli, E., Pacchierotti, F., Eleuteri, P., Villani, P., Hegarat, L.L., Fessard, V. and Reale, O. (2018): In vivo toxicity and genotoxicity of beauvericin and enniatins. Combined approach to study in vivo toxicity and genotoxicity of mycotoxins beauvericin (BEA) and enniatin B (ENNB). EFSA Supporting Publications, 15, 1406E.
- Prosperini, A., Berrada, H., Ruiz, M.J., Caloni, F., Coccini, T., Spicer, L.J., Perego, M.C. and Lafranconi, A. (2017): A Review of the Mycotoxin Enniatin B. Front. Public Health, 5, 304.
- Rodríguez-Carrasco, Y., Heilos, D., Richter, L., Süssmuth, R.D., Heffeter, P., Sulyok, M., Kenner, L., Berger, W. and Dornetshuber-Fleiss, R. (2016): Mouse tissue distribution and persistence of the food-born fusariotoxins Enniatin B and Beauvericin. Toxicol. Lett., 247, 35-44.
- Santini, A., Meca, G., Uhlig, S. and Ritieni, A. (2012): Fusaproliferin, beauvericin and enniatins: occurrence in food—review. World Mycotoxin J., 5, 71-81.
- Tittlemier, S.A., Roscoe, M., Trelka, R., Gaba, D., Chan, J.M., Patrick, S.K., Sulyok, M., Krska, R., McKendry, T. and Gräfenhan, T. (2013): Fusarium damage in small cereal grains from Western Canada. 2. Occurrence of fusarium toxins and their source organisms in durum wheat harvested in 2010. J. Agric. Food Chem., 61, 5438-5448.
- Yoshinari, T., Suzuki, Y., Sugita-Konishi, Y., Ohnishi, T. and Terajima, J. (2016): Occurrence of beauvericin and enniatins in wheat flour and corn grits on the Japanese market, and their co-contamination with type B trichothecene mycotoxins. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess., 33, 1620-1626.





