2021 Volume 46 Issue 5 Pages 199-207
2021 Volume 46 Issue 5 Pages 199-207
Cardiovascular complications have been well documented as the downside to conventional cancer chemotherapy. As a notable side effect of cisplatin (CDDP), cardiotoxicity represents a major obstacle to the successful treatment of cancer. It has been reported that Salvianolic acid B (SalB) possesses cardioprotective quality. However, the effect of SalB on cardiac damage caused by conventional cancer chemotherapy remains unclear. In this study, we clarified the protective effect of SalB on cisplatin-induced heart injury. Furthermore, in H9c2 cells, SalB dramatically reduced cisplatin-induced apoptosis and oxidative stress by modulating the nuclear factor erythroid-2-related factor 2 (Nrf2) signaling pathway. In conclusion, SalB had great potential in mitigating cisplatin-induced cardiac injury. Furthermore, more attention should be placed on natural active compounds containing SalB with antioxidant effects for the treatment of cardiomyopathy.
Cardiovascular complications associated with conventional cancer chemotherapy are a global problem (El-Awady et al., 2011; McGowan et al., 2017). Cisplatin, one of the most potent chemotherapeutic agents, has been proved to be effective against a variety of solid tumors, including sarcoma, soft tissue cancer, bone cancer, muscle cancer and vascular cancer, but cisplatin-associated cardiotoxicity, nephrotoxicity and hepatotoxicity are obstacles in clinical application (de Jongh et al., 2003; Ma et al., 2020; Shen et al., 2012; Topal et al., 2018). It has been reported that the cardiotoxicity induced by cisplatin mainly includes ECG changes, arrhythmia, acute myocardial infarction, supraventricular tachycardia, and atrial fibrillation (Dugbartey et al., 2016; El-Awady et al., 2011; Guglin et al., 2009; Ma et al., 2010; Raja et al., 2013). A large number of studies have shown that cisplatin could increase the levels of plasma troponin I (TPI), creatine kinase (CK) and creatine kinase-MB (CK-MB), which might contribute to the damage of myocardial membrane structure (Dugbartey et al., 2016). Eventually, cardiac toxicity can lead to congestive heart failure and sudden cardiac death. Therefore, it is urgent to find an effective strategy for treating cardiotoxicity, and understanding the mechanism of cisplatin-related cardiotoxicity will help to discover novel strategies to prevent cardiovascular complications.
Oxidative stress mediates diverse pathophysiological events in cardiac tissues and contributes to many abnormalities associated with cardiovascular disease (Nabeebaccus et al., 2011). It is now clear that reactive oxygen species (ROS) can be produced by cardiomyocytes with the stimulus of cisplatin, which in turn causes myocardium injury, inflammation, and dysfunction (Dugbartey et al., 2016). In contrast, cells defend against oxidative stress and injury by activating antioxidation signaling proteins, including Nrf2 (Ashabi et al., 2015; Joo et al., 2016). Although the regulatory mechanisms involved in activation of the Nrf2 signaling pathway are not fully understood, there is an emerging association between Nrf2 and natural product.
Salvianolic acid B (SalB) is a kind of polyphenol compound, which is rich in Salvia miltiorrhiza and is the main active component. Emerging studies have shown that SalB was one of the most effective natural antioxidants, which had obvious scavenging effect on oxygen free radicals, and had protective effect on liver injury and fibrosis (Lin et al., 2006; Liu et al., 2002; Yan et al., 2010). In addition, SalB not only improved mitochondrial energy metabolism, but also effectively inhibited hepatocyte apoptosis by regulating apoptosis-related factors in the mitochondrial death pathway (Yan et al., 2010). It has been found that SalB could eliminate oxygen free radicals and reduce lipid peroxidation, so as to improve cardiac function and alleviate myocardial ischemia-reperfusion injury (Li et al., 2019; Wang et al., 2018). However, there is no evidence showing the effect of SalB in cisplatin-induced cardiac injury, and the molecular mechanism is unknown.
In the present study, we investigated the effect of SalB in cisplatin-induced cardiac dysfunction. Our results indicated that cisplatin aggravated oxidative stress response and cell apoptosis in the myocardium, while expectedly, SalB reversed these changes via increasing the expression of Nrf2. Therefore, SalB may be considered to be a protective candidate in cardiovascular complications associated with conventional cancer chemotherapy.
H9c2 cells derived from embryonic BDIX rat heart tissue were purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China) and incubated in DMEM media with 10% fetal bovine serum, 100 U/mL of penicillin, and 100 U/mL of streptomycin at 37°C in a humidified 5% CO2 incubator.
CCK8 assaysH9c2s were seeded into 96-well plates at the density of 3000 cells per well. CCK8 (10 μL) was added into each well for 2 hr at 37°C after incubator with cisplatin or SalB for 24 hr. Optical density was measured at 450 nm using a micro plate spectrophotometer.
Animal experiments8 week-old male C57BL/6 mice, weighing 18-22 g, were obtained from Beijing Vital River Laboratory Animal Technology Co. Ltd. (Beijing, China). In this study, 36 male mice were used. All animal care and experimental procedures were performed in accordance with the directives outlined in the Guide for the Care and Use of Laboratory Animals (US National Institutes of Health). Animal care and experimental protocols were approved by the Ethics Committee of Sanmen People’s Hospital of Zhejiang.
The dosage and treatment time of cisplatin were chosen according to previous studies (Chowdhury et al., 2016; Ibrahim et al., 2019; Xing et al., 2019; Zhao et al., 2018). Experiment 1: 18 male mice were randomly divided into 3 groups: saline group, cisplatin (3 mg/kg/day) group, and cisplatin (10 mg/kg/day) group, with n = 6 in each group. Cisplatin purchased from MedChemExpress (Monmouth, NJ, USA) was dissolved in phosphate buffer and given intraperitoneally for 3 days. The dosage and treatment time of SalB were chosen according to previous published papers (Feng et al., 2015; Liu et al., 2016, 2018; Xiao et al., 2019). Experiment 2: 18 male mice were randomly divided into 3 groups: saline group, cisplatin (10 mg/kg) group, and cisplatin (10 mg/kg/day) +SalB (10 mg/kg/day) group, with n = 6 in each group. SalB was purchased from MedChemExpress and dissolved in 1% Sodium carboxymethyl cellulose. Pretreatment with SalB for 7 days by gavage, cisplatin and SalB were given for 3 days. At 24 hr after final treatment with SalB and cisplatin, transthoracic echocardiography was conducted on the mice to examine their cardiac function, and then all the animals were sacrificed under sodium pentobarbital anesthesia. The body weight and heart weight were recorded and blood samples were collected.
Cardiac functionAfter continuous intraperitoneal injection of cisplatin for 3 days, cardiac function was detected by transthoracic echocardiography (VisualSonics, Toronto, Canada) was performed to measure cardiac function in a non-invasive manner. The LV internal dimension in diastole (LVIDs), LV end-systolic volume (LVESV), LV internal dimension in systole (LVIDd), and LV end-diastolic volume (LVEDV) were assessed from M-mode images. The equations of Fractional shortening (FS) = [(LVIDd - LVIDs)/LVIDd] x100% and Ejection fraction (EF) = LVEDV - LVESV)/LVEDV x100% were respectively used to calculate FS and EF.
Pathological stainingThe hearts were fixed in 4% paraformaldehyde and embedded in paraffin. The sections (5 μm) were stained with hematoxylin and eosin for light microscopy examination by Leica DM2000 microscope.
TdT-mediated dUTP Nick-End Labeling (TUNEL) stainingThe sections (5 μm) were stained with TUNEL staining according to the manufacturer’ s instructions, and images were taken by Leica DM2000 microscope.
Determination of serum LDH and CK-MBThe contents of serum lactate dehydrogenase (LDH) and CK-MB were detected by a commercially available kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s instructions.
Determination of malondialdehyde and superoxide dismutase activityH9c2 cells or cardiac tissues were lysed by RIPA lysis buffer and the protein concentration was measured. Malondialdehyde (MDA) level and the amount of superoxide dismutase (SOD) activity were determined using commercially available kits according to the manufacturer’s instructions (Beyotime Biotech, Nantong, China). The relative amount was normalized to the control group.
Real-time quantitateive PCRThe total RNA was isolated from cardiac tissues (about 20 mg) by Trizol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. SYBR Green was used to detect the mRNA expression levels of Nrf2-regulated genes Heme Oxygenase-1 (HO-1) and NADPH quinineoxidoreductase-1 (NQO-1) by quantitative real-time PCR. The primer sequences are shown in Table 1. The relative amount of each gene was normalized to β-actin.
| Gene | Species | Primers (FW) | Primers (RW) |
|---|---|---|---|
| HO-1 | Mouse | GAGCAGAACCAGCCTGAACT | TTTGAACTTGGTGGGGCTGT |
| NQO-1 | Mouse | AGGGTTCGGTATTACGATCC | AGTACAATCAGGGCTCTTCTCG |
| β-actin | Mouse | CCGTGAAAAGATGACCCAGA | TACGACCAGAGGCATACAG |
| HO-1 | Rat | TCTATCGTGCTCGCATGAAC | CAGCTCCTCAAACAGCTCAA |
| NQO-1 | Rat | ACCTTGCTTTCCATCACCAC | CAAAGGCGAAAACTGAAAGC |
| β-actin | Rat | AAGTCCCTCACCCTCCCAAAAG | AAGCAATGCTGTCACCTTCCC |
The heart tissue (20-40 mg) was lysed with RIPA lysis buffer (adding 1 mL NP-40, 0.88 g NaCl, and 0.25 g sodium deoxycholate into 100 mL 50 mM Tris-HCl, PH7.4) in an ice bath, the protein concentration was measured and the loading amount of each protein was balanced. The protein (100 μg in in vivo experiments or 40 μg in in vitro experiments) was separated by SDS gel, and then transferred to PVDF membrane. After 90 min, the membranes were preincubated with 5% non-fat milk, and then were incubated with specific antibody against BCL2-Associated X (BAX), Nrf2 (Cell Signal Technology, Boston, MA, USA) and α-Tubulin (Proteintech Group, Chicago, IL, USA) overnight. The second antibody conjugated with horseradish peroxidase was incubated for 1 hr and visualized by Tanon-5200Multi using chemiluminescence reagents, super ECL detection reagent purchased from Yeasen Biotech Co., Ltd. (Shanghai, China).
Statistical analysisAll measurement data are shown as mean ± SEM. Difference was obtained by one-way ANOVA analysis followed by multiple comparisons test with Bonferroni correction. It is considered to be significant when P < 0.05.
First, we evaluated the effects of CDDP in H9c2 cells. Incubation with CDDP (0.1, 0.5, 1, 5, 10 μM) for 24 hr or 48 hr reduced H9c2 cell viability in a dose-dependent manner (Fig. 1A-B). In addition, incubation with CDDP (10 μM) for 24 hr resulted in an increased expression of pro-apoptotic Bax (Fig. 1C). These results indicated that CDDP had a damaging effect on H9c2 cells.
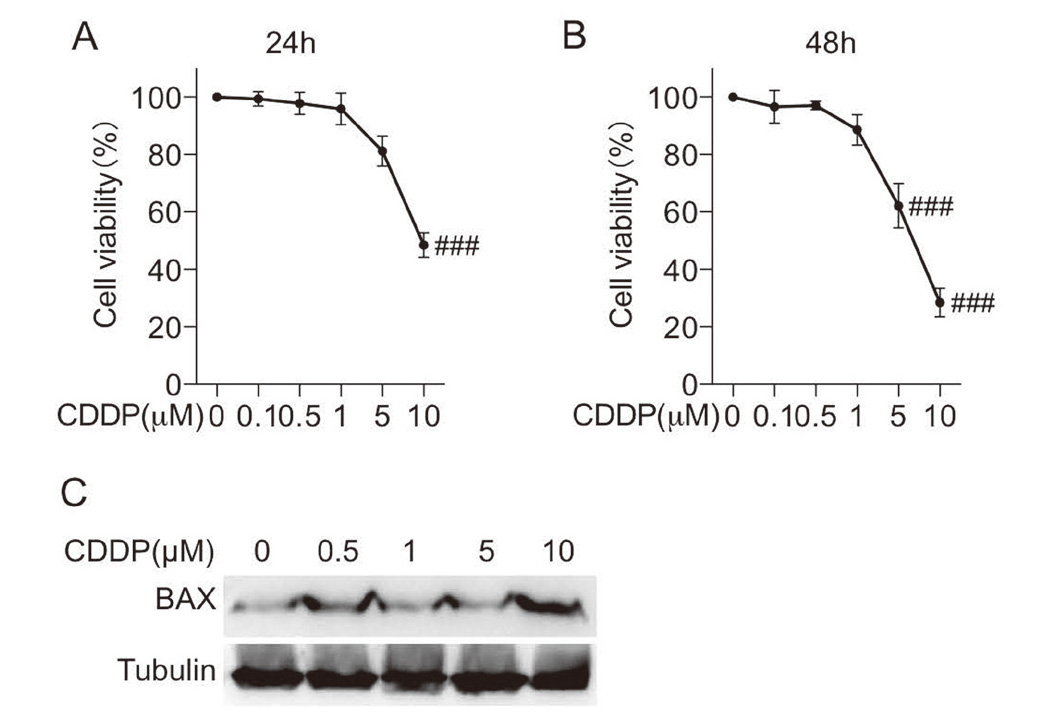
Cisplatin induced cell death in H9c2 cells. A-B. Incubation with cisplatin (0.1, 0.5, 1, 5, or 10 μM) for 24 hr or 48 hr, an then CCK8 assay was performed to detect cell viability in H9c2 cells. C. Incubation with cisplatin (0.1, 0.5, 1, 5, or 10 μM) for 24 hr and then total protein was collected. Western blot analysis was used to detect the expression of BAX. (n = 3 independent experiments; ### P < 0.001, vs. Vehicle group).
H9c2 cells were pretreated with SalB (1, 5, or 10 μM) for 1 hr, and then exposed to CDDP (10 μM) for 24 hr or 48 hr. As shown in Fig. 2A-B, pretreatment with SalB contributed dose-dependently to an increase in the cell viability of CDDP-induced H9c2 cells. Additionally, Fig. 2C shows that the increased expression of Bax induced by CDDP was reversed by pretreatment with SalB, indicating that SalB exerted a protective effect against CDDP-induced cell injury. TUNEL staining showed that cisplatin induced cardiomyocyte apoptosis, but this change was reversed by pretreatment with SalB in H9c2 cells (Fig. 2D-E).
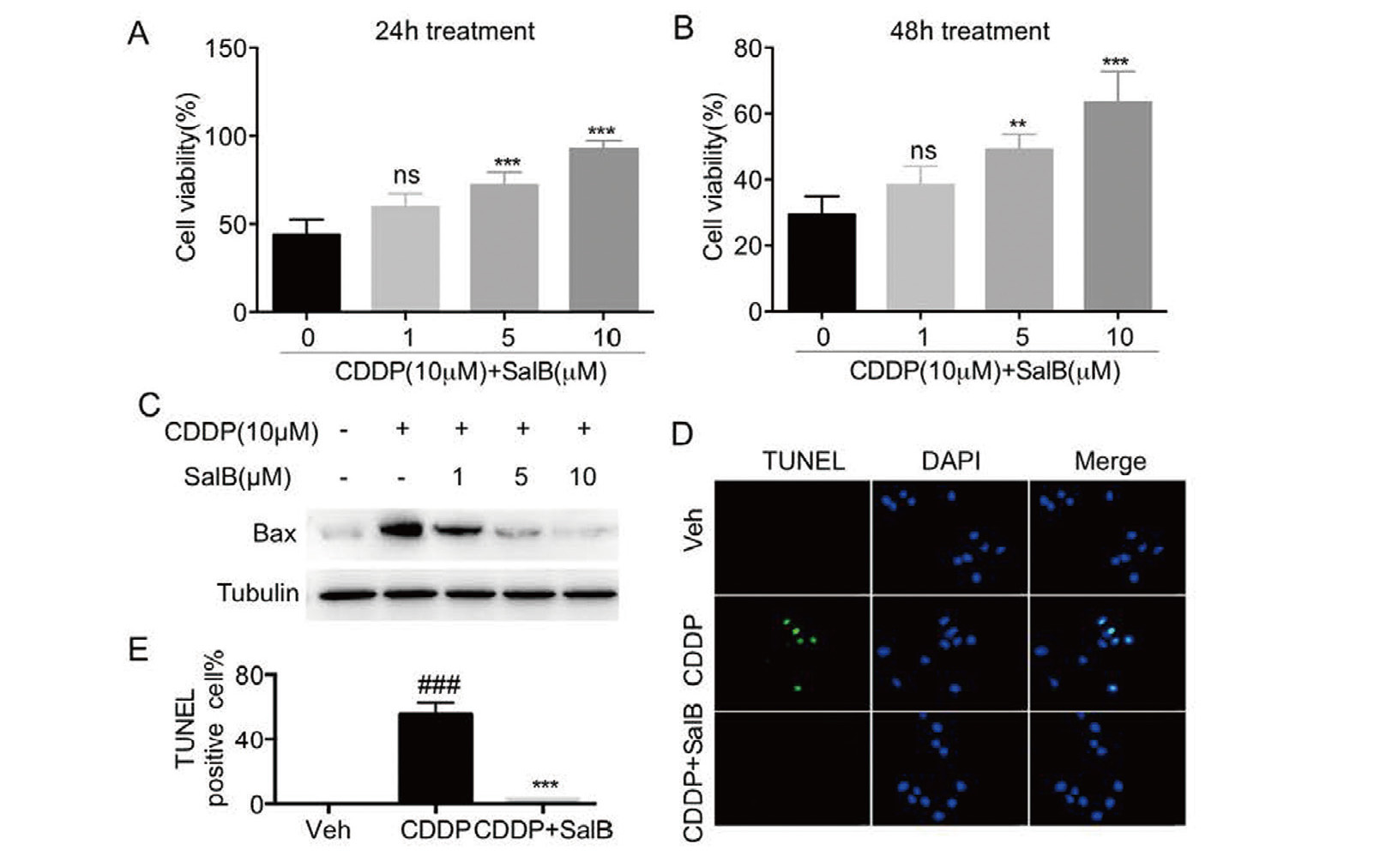
SalB attenuated cisplatin-induced cell death in H9c2 cells. A-B. Pretreatment with SalB (1, 5, or 10 μM) for 1 hr, H9c2 cells were incubated with cisplatin (10 μM) and SalB for 24 hr or 48 hr and then cell viability was measured by CCK8 assay. C. Pretreatment with SalB (1, 5, or 10 μM) for 1 hr, H9c2 cells were incubated with cisplatin (10 μM) and SalB for 24 hr and then total protein was collected. Western blot analysis was used to detect the expression of BAX. D-E. The representative images and quantitative analysis of TUNEL staining were observed for cell apoptosis in H9c2 cells (× 400 magnification). (n = 3 independent experiments; ###P < 0.001, vs. Vehicle group; ns, no significance, **P < 0.01, ***P < 0.001, vs. CDDP group).
Since CDDP-induced oxidative stress usually leads to cell death, we further tested the anti-oxidative effect of SalB on CDDP-induced H9c2 cells. As shown in Fig. 3A-B, CDDP increased the content of MDA and reduced the activity of SOD, but these changes were reversed by pretreatment with SalB. Nrf2, as a transcription factor regulating the gene expressions of oxidoreductase, plays a key role in antioxidation (Baird and Dinkova-Kostova, 2011; Kaspar et al., 2009). Then, we investigated the effect of SalB in the Nrf2 signaling pathway. The results of immunoblotting analysis showed that SalB induced dose-dependently an increase in the protein expression of Nrf2 (Fig. 3C). And the mRNA levels of NQO-1 and HO-1 in H9c2 cells were significantly elevated by pretreatment with SalB (Fig. 3B). Taken together, these results suggest that SalB inhibited CDDP-induced oxidative stress in H9c2 cells.
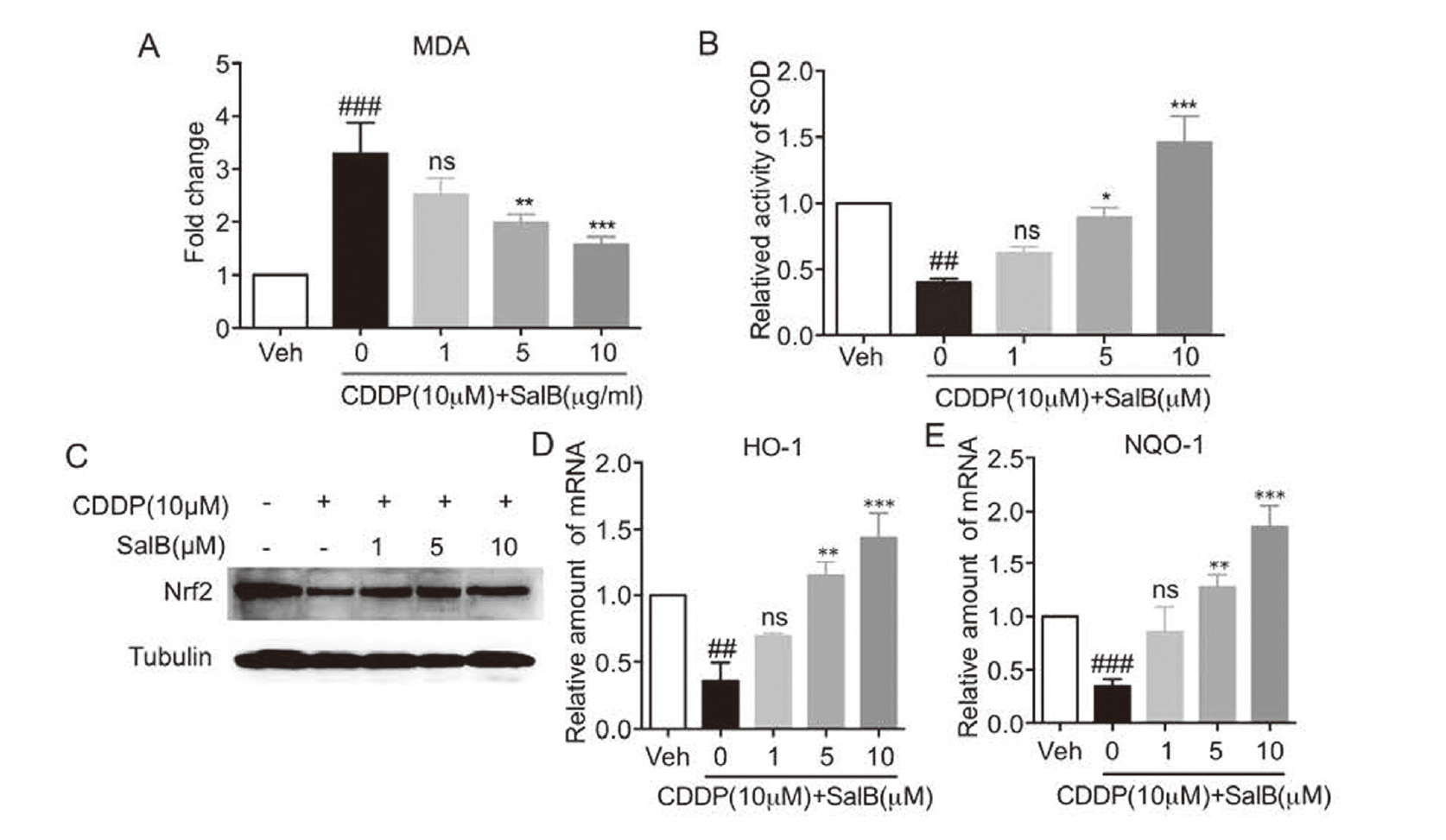
SalB attenuated cisplatin-induced oxidative stress. A-B. Pretreatment with SalB (1, 5, or 10 μM) for 1 hr, H9c2 cells were incubated with cisplatin (10 μM) and SalB for 24 hr and then total cells were collected. The contents of MDA (A) and SOD (B) were determined. C. Pretreatment with SalB (1, 5, or 10 μM) for 1 hr, H9c2 cells were incubated with cisplatin (10 μM) and SalB for 12 hr and then total protein was collected. Western blot analysis was used to detect the expression of Nrf2. D-E. Pretreatment with SalB (1, 5, or 10 μM) for 1 hr, H9c2 cells were incubated with cisplatin (10 μM) and SalB for 12 hr and then total RNA was collected. RT-qPCR was used to detect the mRNA levels of HO-1 (D) and NQO-1 (E). (n = 3 independent experiments; ## P < 0.01, ### P < 0.001, vs. Vehicle group; * P < 0.05, **P < 0.01, ***P < 0.001, vs. CDDP group).
Next, we investigated the cardiotoxic effect of CDDP in vivo. Figure 4A-B shows that compared with saline group, EF and FS of mice in the CDDP-treated group were lowered without altering body weight and heart weight. And serum LDH and CK-MB were significantly increased by CDDP (Fig. 4C-D). Histopathological analysis showed that increased myocardial disorders were observed in cardiac tissues of CDDP-induced mice (Fig. 4E). Furthermore, immunoblotting analysis showed that CDDP induced an increased expression of pro-apoptotic BAX in cardiac tissues (Fig. 4F). These results indicated that CDDP induced cardiac injury, dysfunction and cardiomyocyte apoptosis in mice.
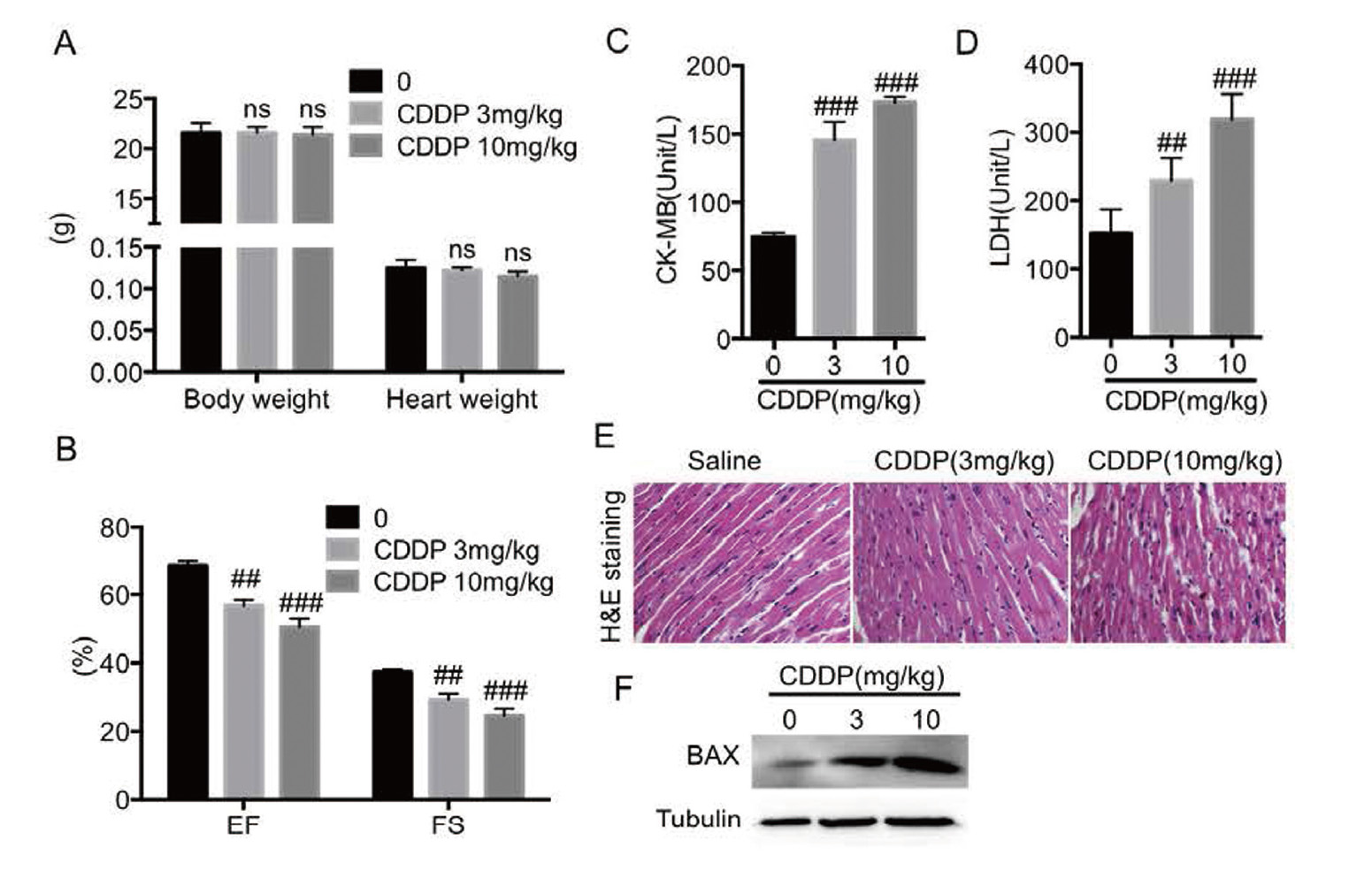
Cisplatin induced cardiac dysfunction and injury in vivo. A. The body weight and heart weight. B. Eject fraction and Fractional shortening were measured by echocardiography. C-D. The levels of serum CK-MB and LDH were detected by the corresponding kits. E. The representative images of H&E staining in heart tissues were observed for myocardial fiber disorder (× 400 magnification). F. Western blot analysis was used to detect the expression of BAX in heart tissues. (n = 6 in each group; ns, no significance, ## P < 0.01, ### P < 0.001, vs. Saline group).
To further study the cardioprotective effect of SalB in CDDP-treated mice, we investigated the serum LDH and CK-MB, cardiac function, and cardiac pathological changes. As shown in Fig. 5A-B, reduced EF and FS induced by CDDP were reversed by SalB without altering body weight and heart weight. Increased levels of serum LDH and CK-MB were reduced by pretreatment with SalB (Fig. 5C-D). And H&E staining showed that compared with saline group, the myocardial fibers in the CDDP group were disordered and discontinuous, while those in the SalB-treated group were orderly and continuous (Fig. 5E). Moreover, SalB reduced CDDP-induced expression of BAX and attenuated CDDP-induced cardiomyocyte apoptosis in cardiac tissues (Fig. 5F-G). These results indicated that SalB could alleviate cardiac dysfunction and injury induced by CDDP.
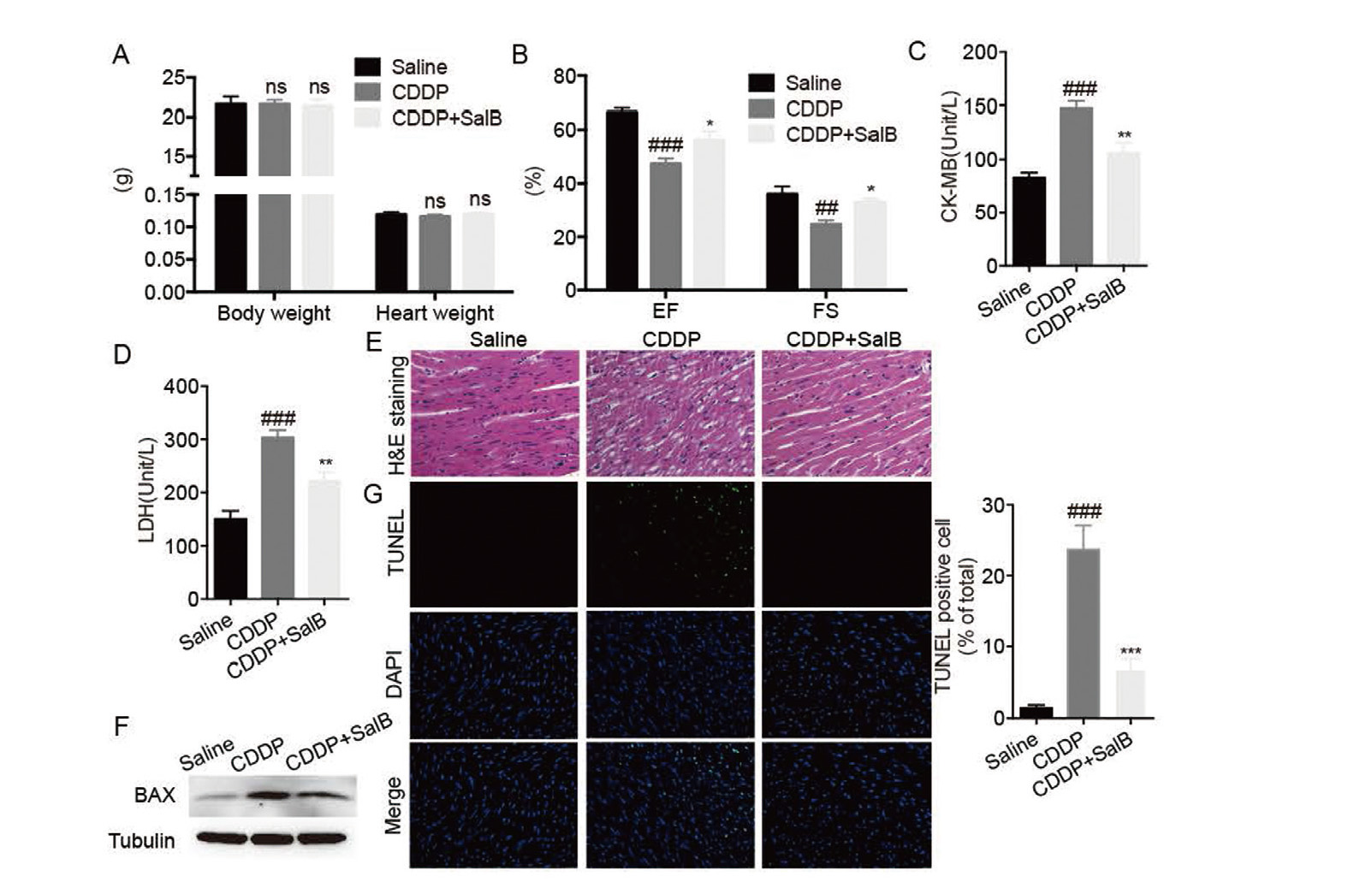
SalB attenuated cisplatin-induced cardiac dysfunction and injury. A. The body weight and heart weight. B. Eject fraction and Fractional shortening were measured by echocardiography. C-D. The levels of serum CK-MB and LDH were detected by the corresponding kits. E. The representative images of H&E staining in heart tissues were observed for myocardial fiber disorder (× 400 magnification). F. Western blot analysis was used to detect the expression of BAX in heart tissues. G. The representative images and quantitative analysis of TUNEL staining in heart tissues were observed for cardiomyocyte apoptosis (× 400 magnification). (n = 6 in each group; ns, no significance, ## P < 0.01, ### P < 0.001, vs. Saline group; * P < 0.05, **P < 0.01, ***P < 0.001,vs. CDDP group).
Oxidative stress has been reported to drive the occurrence and development of cardiac hypertrophy and heart failure (Korge et al., 2015). Here, we found that CDDP induced an increased in MDA of cardiac tissues, but that was reduced by pretreatment with SalB (Fig. 6A). In addition, the protein expression of Nrf2 in cardiac tissues was reduced in CDDP-treated mice, but that was elevated by pretreatment with SalB (Fig. 6B). Similarly, the mRNA levels of NQO-1 and HO-1 were elevated by pretreatment with SalB (Fig. 6C-D). These results suggest that SalB could alleviate CDDP-induced oxidative stress.
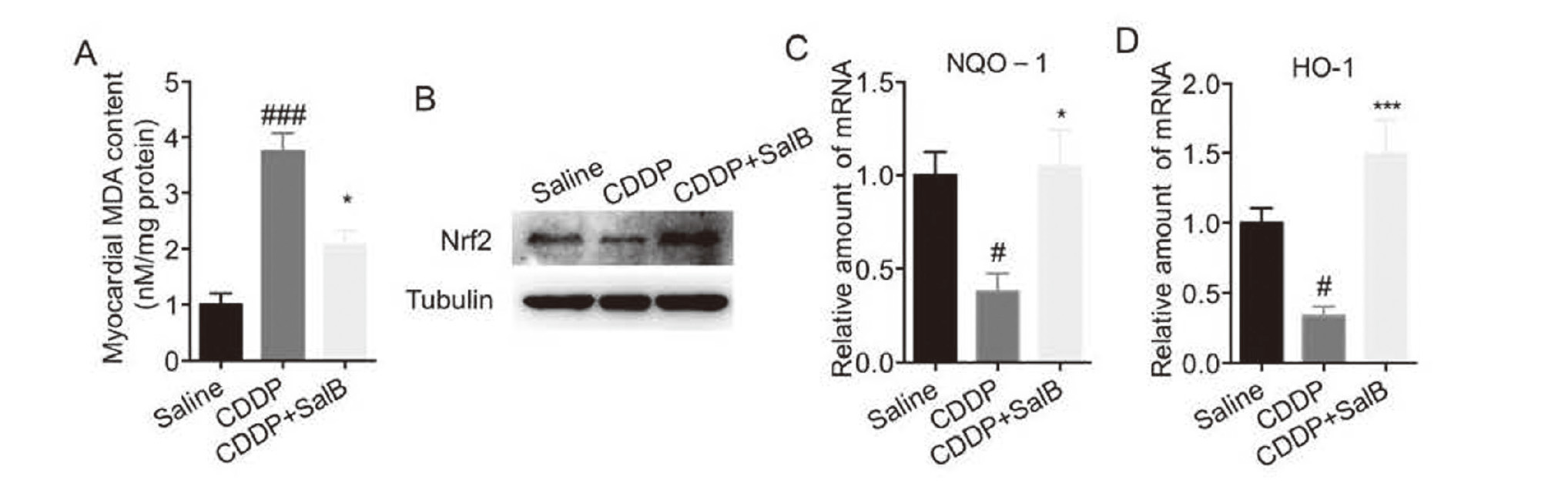
KPF significantly attenuated oxidative stress induced by cisplatin in myocardial tissue. A. The content of MDA was determined by the corresponding kit. B. Western blot analysis was used to detect the expression of Nrf2 in heart tissues. C-D. RT-qPCR was used to detect the mRNA levels of NQO-1 (C) and HO-1 (D) in heart tissues. (n = 6 in each group; #P < 0.05, ### P < 0.001, vs. Saline group; * P < 0.05, ***P < 0.001, vs. CDDP group).
In our study, cardiac function was significantly decreased, and serum LDH and CK-MB were significantly increased in cisplatin -induced mice; moreover, myocardial fiber disorder and myocardial cell apoptosis were increased (Fig. 4). In addition, in the in vitro experiment cisplatin decreased cell viability and increased cell apoptosis in H9c2 cells (Fig. 1). However, these changes were reversed by pretreatment with SalB (Figs. 2 and 5). The results obtained from two different species experimental models indicated that the pathological mechanism of cisplatin-induced cardiotoxicity in humans might be consistent with rat and mice. SalB is the main active component of a traditional Chinese medicine, Salvia miltiorrhiza. Its biosafety has been fully confirmed in the history of traditional Chinese medicine and our results showed that SalB protected against cardiotoxicity induced by cisplatin, suggesting that SalB might be a novel clinical therapeutic strategy for treating cisplatin-induced cardiotoxicity.
Recently, it has been found that oxidative stress response could lead to cell death (Nabeebaccus et al., 2011). Emerging evidence showed that the generation of ROS induced by cisplatin triggered oxidative stress, resulting in lipid peroxidation of the cell membrane, and damage of proteins and DNA (Ma et al., 2010; Topal et al., 2018). Our results found that cisplatin induced oxidative stress in vitro and in vivo (Figs. 3A-B and 6A). However, pretreatment with SalB attenuated cisplatin-induced oxidative stress (Figs. 3A-B and 6A). As is known to all, the Nrf2 signaling pathway plays a key role in endogenous antioxidant defense against myocardial injury (Ma, 2013). SalB has been reported to regulate Nrf2 expression against myocardial ischemia-reperfusion injury (Calvert et al., 2009; Kaspar et al., 2009). In the present study, SalB increased the protein expression of Nrf2 and mRNA expression of its downstream genes in cisplatin-induced mice and H9c2 cells (Figs. 3C-E and 6B-D). These results indicated that SalB could alleviate oxidative stress induced by cisplatin, which might be related to the regulation of Nrf2 expression.
The current study found that SalB could effectively attenuate cisplatin-induced cardiomyocyte apoptosis and cardiac dysfunction, accompanied by inhibition of oxidative stress, which fully indicated that SalB could exert protective effects in cisplatin-induced cardiotoxicity. However, we failed to report the effects of cisplatin on other molecular modifications (i.e., mitochondrial dysfunction, DNA damage, endoplasmic reticulum stress, and inflammation) and the anti-apoptotic activity of high dose of SalB, so that it remained obscure whether SalB entirely attenuated cisplatin-induced apoptosis via inhibiting oxidative stress. In conclusion, SalB may be considered to be a protective candidate in cardiovascular complications associated with conventional cancer chemotherapy and targeting oxidative stress may be a feasible therapeutic strategy against cisplatin-induced cardiotoxicity.
Financial support was provided by the Zhejiang Medical Health Technology Platform Project (2018KY183).
Conflict of interestThe authors declare that there is no conflict of interest.