2022 年 39 巻 p. 110-118
2022 年 39 巻 p. 110-118
The synthesis of noble metals and their alloy nanoparticles by laser-induced nucleation is described. Femtosecond laser pulses with an energy on the order of mJ were tightly focused to create an intensity of 1014 W/cm2 or more in an aqueous solution of noble metal ions. The intense laser field generated solvated electrons and hydrogen radicals that have a highly reducing ability, resulting in nucleation through the reduction of the noble metal ions and particle growth through ripening. This laser-induced nucleation method can be performed without any reducing agents. Excess irradiation of chloroauric acid solution led to the formation of a stable colloidal solution of gold nanoparticles without any surfactants. Additionally, the irradiation of a mixed solution of different noble metal ions formed solid–solution alloy nanoparticles, even though these metals were immiscible in the bulk. Moreover, the laser-induced nucleation made it possible to form quinary solid-solution alloy nanoparticles of noble metals. The mechanism of superior catalytic activity found for alloy nanoparticles by using Rh–Pd–Pt solid–solution nanoparticles is discussed in terms of elemental distributions inside the particles.
Lasers have a variety of excellent features in terms of wavelength, output, pulse width, spectral width, and so on. As a result, many applications have been developed in a wide range of fields, such as communication, information technology, and medicine in both academic and industrial societies. In particular, in laser processing, lasers are rapidly expanding the application range, for example, heating, welding, cutting, drilling, marking, surface modification, particle synthesis and thin film deposition by laser ablation, and additive manufacturing. From the viewpoint of the morphological transformation of a material, these process techniques include top-down or bottom-up approaches between large- and small-scale materials. One of the merits of laser processing is the ability to sustain the crystalline structure, composition, and property of the materials during the process.
Currently, with the progress of laser technology, especially, ultrafast pulse generation and regenerative amplification, the laser output has become strong enough to generate an unprecedented ultra-intense optical field. Supposing that a laser pulse with a pulse energy of 1 mJ and a pulse width of 100 fs is focused to an area of 10 μm2 by aberration-free optics, the peak intensity at the focal point will be 1017 W/cm2, according to calculations. This value is comparable to the Coulomb field inside the hydrogen atom, which is 5.0 × 1011 V/m, corresponding to the light intensity of 7.0 × 1016 W/cm2. Such an ultrahigh electric field is not achievable by conventional electric techniques and is expected to result in a novel reaction field in materials science. The reaction in this unprecedented field would be different from those in a thermal equilibrium state at high temperature achieved by commonly used processing techniques and could offer an ultimate reaction field that has never existed on Earth. Highly intense laser science, however, has mainly focused on gas-phase reactions (Cornaggia et al., 1995; Cornaggia, 1995; Castillejo et al., 1999; Talebpour et al., 2000), where the density of atoms, ions, fragments, and molecules are dilute enough to ignore their interactions. As a result, the production of species and molecules different from the starting materials in a condensed state, such as a liquid or solid, has been outside of the scope of highly intense laser science for a long time.
As a next step, small particle synthesis by laser irradiation of a solid target immersed in liquid has been reported, which utilized the high energy density state created by the confinement effect of plasma in liquid (Wang et al., 2002; Pearce et al., 2004). In this pulsed laser ablation in liquid (PLAL), a uniformly dense reaction field was formed to avoid the generation of large debris by suppressing the laser energy. In contrast, as mentioned earlier, if ultrashort laser pulses with an energy on the order of mJ are strongly focused to a diffraction-limited small spot, an ultrahigh intense laser field can be created in condensed matter, like liquid or solid, by using a commercially available regenerative amplifier laser system. In this intense laser field, photoionization and photodissociation of materials will instantly lead to the formation of high-temperature and high-density plasma. The expansion of the plasma will be strongly suppressed, and the temperature will be rapidly cooled down by the surrounding high-density and low-temperature materials, resulting in a non-equilibrium process that is much faster than conventional processes.
We focused on the non-linear and non-equilibrium reaction field created near the focal point of ultrafast laser pulses with a high peak power and addressed the nanoparticle (NP) synthesis of noble metals and their alloys in the highly intense laser field. In this review, we describe the distinguishing properties found for NPs fabricated by highly intense laser irradiation of aqueous solutions of metallic ions, especially, the synthesis of stable colloidal solutions of gold (Au) NPs without any dispersants, the catalytic activity of alloy NPs, and the synthesis of solid–solution quinary alloy NPs.
A schematic drawing of the experimental setup is shown in Fig. 1. Focused femtosecond laser pulses were irradiated on aqueous solutions of metal ions held in a fused-silica cuvette for a predetermined time. The laser pulses were generated from a regenerative amplifier of a mode-locked Ti:sapphire oscillator. The center wavelength was 800 nm, the pulse width was 100 fs, the maximum pulse energy was 6 mJ, and the maximum repetition rate was 1 kHz. The laser pulses were delivered from the side of the cuvette and were focused near the center of the cuvette by an aspherical lens with a focal length of 8 mm and a numerical aperture of 0.5. The lens diameter of 10 mm was the same as the beam diameter of the laser pulses. In Fig. 1, the drawing corresponds to a stationary system, where the liquid sample was held in the cuvette, whereas a flow system was available by circulating the liquid sample with a feed pump to substantially increase the production rate of NPs (Muttaqin et al., 2015). This was because the liquid flow was able to remove the bubbles that were generated by the laser irradiation of the liquid sample, as shown in Fig. 1, and interrupted normal focusing of the laser pulses.

Schematic of the experimental setup.
The intensity of light at the focus can be calculated to be 3 × 1018 W/cm2 if we ignore the aberration of the focusing optics. However, the lens used in the experiment was not optimized for this experimental condition. The diameter of the focus was estimated to be 175 μm mainly due to the spherical aberration at the interfaces between air/glass and glass/liquid, resulting in an intensity at the focus of 2.5 × 1014 W/cm2, which was sufficiently strong enough to induce photodissociation and photoionization of the liquid sample.
The nanoparticle synthesis of noble metals and their alloys that we developed was not based on a conventional morphological change of materials but a physicochemical reaction induced by the highly intense laser field. The process is accomplished by the reduction of metal ions with solvated electrons and hydrogen radials, which are generated by photodissociation and photoionization of water molecules. Below, the synthesis of gold (Au) NPs by highly intense laser irradiation of an aqueous chloroauric acid solution is presented as a typical example. When an aqueous chloroauric acid solution was irradiated, a bright flash of plasma emission near the focal point and the generation of hydrogen and oxygen gas were observed. With increasing irradiation time, the transparent aqueous solution changed to red purple, which corresponded to localized surface plasmon resonance (LSPR) of Au NPs at approximately 520 nm. In fact, many spherical Au NPs with an average size of 10 nm were observed by transmission electron microscopy (TEM). From these observations, the following mechanism for the nanoparticle synthesis was inferred.
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
When an intense laser is used to irradiate an aqueous solution, solvated electrons (
The progress of particle formation can be monitored through the absorption peak of specific ions or the LSPR of the Au NPs. Once the absorbance of a species of interest becomes saturated, the reduction of metal ions is mostly completed; thus, we can cease laser irradiation. For the irradiation of the chloroauric acid solution, the Au NPs aggregated and precipitated sooner or later. However, when the irradiation was continued after absorbance saturation, the Au NPs showed morphological stability, and the colloidal solution remained in the dispersed state for at least several months (Nakamura et al., 2013). Next, we discuss the phenomenological change that occurred to the Au NPs with increasing irradiation time.
Fig. 2 shows the UV–visible absorption spectra of the chloroauric acid solution with a concentration of 2.5 × 10−4 mol/L recorded every 30 s during the laser irradiation. The repetition rate of the laser pulses was 30 Hz. All the spectra were obtained by subtracting the data for the sample before the laser irradiation. At the beginning, remarkable changes were not observed. After 3 min, the absorbance at approximately 520 nm corresponding to the LSPR of Au NPs appeared and increased with increasing irradiation time, as indicated by the arrow in the figure. This visible change of the LSPR rapidly decreased and stopped at 14 min. The peak absorbance corresponding to the LSPR is plotted as a function of the irradiation time in Fig. 3. A rapid rise of the LSPR absorbance from 3 min to 14 min is clearly visible. After 14 min of irradiation, the LSPR absorbance gradually decreased.

UV–visible absorption spectra of the chloroauric acid solution at a concentration of 2.5 × 10−4 mol/L measured every 30 s in situ during laser irradiation.

Time variation of the LSPR peak absorbance in the UV–visible absorption spectra of the chloroauric acid solution measured in situ during laser irradiation.
The termination of the rapid increase of absorbance at 14 min suggested that the auric ions in the solution were mostly exhausted by laser irradiation. Although reduction of auric ions was not further expected, a morphological change in Au NPs can be expected upon extending the laser irradiation. When laser irradiation was continued after the termination, the shape and size of the Au NPs were investigated by TEM observations. Fig. 4 shows the TEM images of the Au NPs fabricated by laser-induced nucleation with different irradiation times. The Au NPs coalesced with others until 14 min. Upon further irradiation, the particles were partly dispersed, and the particle size became smaller. At 60 min, the nanoparticles were well dispersed, and the particle size became much smaller. The particle size variation with irradiation time is shown in Fig. 5. The particle size variation is clearly observable in the TEM images. Specifically, the dispersion and the mean size of the particles became smaller with irradiation time. The UV–visible absorption spectra showed no significant difference after 14 min of irradiation, whereas the morphology of the Au NPs drastically changed, as shown in the TEM images. These findings suggested that the electronic property of the Au NPs was affected by the excess laser irradiation.

TEM images of the Au NPs fabricated by laser-induced nucleation with irradiation times of (a) 9, (b) 14, (c) 20 and (d) 60 min.

Mean particle size of the fabricated nanoparticles as a function of irradiation time.
We analyzed the long-time dispersion stability because the Au NPs were well dispersed after 60 min, as shown in Fig. 4(d). In Fig. 6, we show the absorption spectra of the colloidal solution of Au NPs 1 day and 2 weeks after preparation with different irradiation times. Each inset shows photographs of the solutions. For the irradiation times of 9 and 14 min, the LSPR peak became predominant 1 day after irradiation. At this irradiation time, the reduction of auric ions was not completed, and the Au NPs grew via a self-catalytic effect. The particles were aggregated 2 weeks later, as shown in Figs. 6(a) and (b). For the irradiation time of 20 min, the increase in the LSPR peak 1 day after preparation became small likely because the reduction of auric ions was almost competed, and further growth of the particles was inhibited.

Long-time variation of the UV–vis. absorption spectra of the colloidal solutions of Au NPs prepared by laser-induced nucleation for different irradiation times of (a) 9, (b) 14, (c) 20, and (d) 60 min. Insets: photographs of the colloid solution (i) just after irradiation, (ii) 1 day later, and (iii) 2 weeks later.
In contrast, the UV–visible absorption spectra of the colloidal solution prepared with 60 min of irradiation time showed no significant change even after 2 weeks, as shown in Fig. 6(d). This stable dispersion state was maintained for at least several months. The zeta potential of the Au NPs in suspension was approximately −30 mV, meaning that the particle surface was negatively charged. A similar result was been reported for Au NPs fabricated by PLAL (Sylvestre et al., 2004), where the colloidal dispersion stability of the Au NPs was attributed to the partial surface oxidation and the transfer of negative charge from the chloride ions during the intense laser ablation of the bulk Au in water. Additionally, fragmentation by the prolonged irradiation, which effectively reduced the particle size, should have contributed to the dispersion stability through the increase of the specific surface area of the NPs.
Laser-induced nucleation can produce not only noble metal nanoparticles (Nakamura et al., 2008; 2011) but also alloy nanoparticles of noble metals from mixed aqueous solutions with different noble metal ions, e.g., gold (Au) and silver (Ag) (Herbani et al., 2011). The compositional distribution in the synthesized nanoparticles was uniform, meaning that the nanoparticles were a solid solution (Herbani et al., 2012; Sarker et al., 2013). Most noble metals (rhodium (Rh), palladium (Pd), Ag, iridium (Ir), platinum (Pt), and Au) crystallize in a face-centered cubic (fcc) structure, except for ruthenium (Ru) and osmium (Os), which have a hexagonal closely packed (hcp) structure. Accordingly, their alloys can be solid solutions, but some alloys are phase-separated. For example, the Au–Pt alloy exhibits phase separation in a wide composition range in bulk, as shown by its binary phase diagram. Despite this physical inclination in the bulk, laser-induced nucleation can synthesize all-proportional solid–solution Au–Pt alloy NPs from a mixed ion solution of chloroauric acid and chloroplatinic acid (Nakamura et al., 2012). The solid–solution of the Au–Pt alloy is considered to be a metastable state. Accordingly, it is presumed that the synthesis of solid–solution Au–Pt NPs is possible not by the conventional thermal equilibrium process but by laser-induced nucleation, which accompanies rapid cooling of the reaction field. The synthesis of all-proportional solid–solution alloy NPs has been achieved for other combinations of noble metals (Sarker et al., 2015; Nakamura and Sato, 2015). Not only binary but also ternary alloy NPs were synthesized (Sarker et al., 2014).
Because the properties of solid–solution alloy NPs of noble metals are not well known, it is attractive to determine the unknown properties of those NPs. Below, we focus on Rh–Pd–Pt NPs, which have been utilized as a three-way catalyst for emission gas purification of automobiles, and show the catalytic activity of solid–solution Rh–Pd–Pt alloy NPs produced by laser-induced nucleation (Sarker et al., 2019).
A mixed aqueous solution of rhodium (III) chloride trihydrate, palladium (II) chloride, and hydrogen hexachloroplatinate (IV) hexahydrate at a concentration of 2.5 × 10−4 mol/L was prepared. In this case, citric acid at 10 wt% was added to improve the particle size dispersion for improved evaluation of the catalytic activity. The prepared solution was irradiated by laser pulses at a repetition rate of 100 Hz for 30 min, which was much longer than the time required for the reduction of noble metal ions in the solution. The structure and composition of the NPs obtained were analyzed by a scanning TEM with energy dispersive X-ray spectroscopy (STEM–EDS).
The STEM images and elemental mappings of the fabricated NPs are shown in Fig. 7(a). Three elements distributed uniformly in the entire particle. The EDS analysis revealed that the elemental composition was Rh29Pd35Pt36, which was close to the initial mixing ratio of metallic ions of Rh:Pd:Pt = 1:1:1 in the mixed aqueous solution. These observations suggested that solid–solution alloy NPs were formed and their composition reflected the initial mixing ratios of noble metal ions in the aqueous solution.

STEM–EDS mappings for (a) the Rh–Pd–Pt alloy NPs prepared by laser-induced nucleation from a mixed ion solution and (b) the Rh–Pd–Pt alloy NPs after heat treatment.
The catalytic activity of the Rh–Pd–Pt alloy NPs was evaluated through oxidation of carbon monoxide (CO). The conversion ratio from CO to carbon dioxide (CO2) was measured in a reaction chamber with 1 % CO and 0.5 % O2 in helium gas for pure metal and their alloy NPs supported on γ–alumina particles. Fig. 8 shows the CO conversion ratios as a function of reaction temperature for the pure metal and their binary and ternary alloy NPs prepared by laser-induced nucleation. Among the pure metal NPs, Rh was most active at low temperature. In contrast, the catalytic activity of the Pt NPs was low owing to the well-known surface poising and the adsorption of CO on the particle surface.
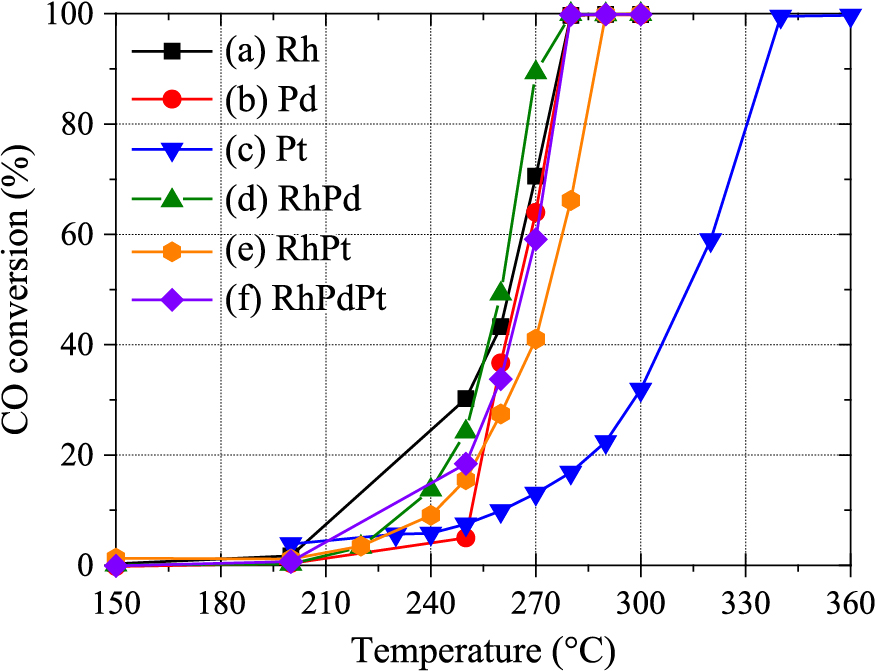
Catalytic CO conversion of the Rh, Pd, Pt, Rh–Pd, Rh–Pt, Rh–Pt, and Rh–Pd–Pt NPs as a function of temperature. Reprinted from Ref. (Sarker et al., 2019) under the terms of the CC-BY-NC 3.0 license. Copyright: (2019) The Authors, published by The Royal Society of Chemistry.
The catalytic activities of binary and ternary alloy NPs were improved compared with the Pt NPs but almost the same as those of Rh and the Pd NPs. It has been reported that Rh–Pt (Alayoglu and Eichhorn., 2008) and Pd–Ru (Kusada et al., 2014) alloy NPs show higher activity than their pure metal NPs. This catalytic enhancement of the alloy NPs was attributed to the electron displacement owing to the core–shell structure in the Rh–Pt NPs and the defects due to the mismatch of crystal structures of bulk Rh (fcc) and Ru (hcp) for Rh–Ru NPs. In contrast, the homogeneous structure of the alloy NPs prepared by laser-induced nucleation could not contribute to the activity. This encouraged us to compare homogeneous NPs with inhomogeneous solid–solution alloy NPs, which can be formed by heat treatment of solid–solution alloy NPs.
The STEM images of the heat-treated NPs and the elemental mappings are shown in Fig. 7(b). The elemental components, especially Pt, were inhomogeneously distributed in the NPs after heat treatment compared to the as-prepared NPs, as shown in Fig. 7(a). Fig. 9 shows the catalytic activities of the NPs with heat treatment. Clearly, the catalytic activity of the heat-treated NPs increased at low temperature as expected. This result explicitly verified that the enhanced catalytic activities of the alloy NPs in the preceding studies were intimately related to the inhomogeneity of the elemental distribution inside the NPs.

Catalytic CO conversion of the alloy NPs before and after heat treatment. Reprinted from Ref. (Sarker et al., 2019) under the terms of the CC-BY-NC 3.0 license. Copyright: (2019) The Authors, published by The Royal Society of Chemistry.
This method was able to form solid–solution alloy NPs simply by mixing ion solutions of different noble metals. In addition to binary and ternary alloy NPs, the formation of quaternary and quinary alloy NPs was achieved by laser-induced nucleation. Fig. 10(a) shows a STEM–HAADF image of the NPs fabricated in the mixed metal ion solutions of Rh, Pd, Ir, Au, and Pt. Corresponding elemental maps by EDS are depicted in Figs. 10(b)–(f). Every element was uniformly distributed inside the particles, suggesting that quinary solid–solution alloy NPs were formed. Observations using high-resolution transmittance electron microscopy revealed that the NPs were polycrystalline. According to the definition (Miracle, 2019), the nanoparticles synthesized here were of a high-entropy alloy because they contained five elements. Because the crystalline structures of Rh, Pd, Ir, Au, and Pt are unexceptionally fcc, as mentioned before, it is easy to assume that their solid–solution alloy NPs can be formed. However, their binary phase diagrams suggest that six out of ten combinations are immiscible. Miscible combinations are limited to the Au–Pd, Ir–Rh, Pd–Pt, and Pt–Rh systems, indicating the difficulties involved in the formation of their solid–solution alloy NPs. Thus, the non-thermal equilibrium process achieved by the laser-induced nucleation may be a promising tool for the formation of high-entropy alloy NPs.

(a) STEM–HAADF image of nanoparticles formed by laser-induced nucleation using the mixed metal ion solutions of Rh, Pd, Ir, Au and Pt. (b)–(f) corresponding elemental EDS maps.
We demonstrated the nanoparticle formation of noble metals and their alloys in the highly intense laser field generated by tightly focusing ultrashort optical pulses in aqueous solution. This “laser-induced nucleation” is free from a reducing agent because the highly intense laser irradiation of the aqueous solution was able to produce solvated electrons and hydrogen radials directly from water molecules, which act as strong reducing agents for metal ions. Prolonged irradiation of chloroauric acid solution resulted in the formation of an Au colloidal solution with a stable dispersive state over several months. Owing to the ultrafast and non-thermal nature of the intense laser process, solid–solution alloy NPs were formed even though the elements were immiscible in the bulk. The solid–solution alloy NPs with a homogeneous elemental distribution contributed to the superior catalytic activity of the alloy NPs, which were formed by a conventional chemical process and had an inhomogeneous elemental distribution.
With the progress of laser technology, the intense laser field will offer an extremely non-linear, non-equilibrium, high-temperature and high-pressure field that has never existed on Earth. We are expecting that this unprecedented state opens the door to ultimate technologies in both science and industry.
Energy dispersive X-ray spectroscopy
fccface-centered cubic
HAADFHigh angle annular dark field
hcphexagonal close-packed
LSPRLocalized surface plasmon resonance
NPNanoparticle
PLALPulsed laser ablation in liquid
STEMScanning transmission electron microscopy
TEMTransmission electron microscopy
krate constant of reaction (dm3 mol−1 s−1)
λwavelength of light (m)
VSHEredox potential for standard hydrogen electrode (V)
Takahiro Nakamura
Associate Professor T. Nakamura received his Bachelor’s degree in Engineering in 1999, Master’s degree in Engineering in 2001, and Doctorate in Engineering in 2004 from the Tohoku University. He started his academic career with laser materials processing. He worked as an Assistant Professor (from 2005 to 2015) and as an Associate Professor (from 2015 to now) in Institute of Multidisciplinary Research for Advanced Materials, Tohoku University. His research topics cover broad area in laser materials processing.
Yuki Yamazaki
Yuki Yamazaki received his Bachelor’s and Master’s degrees in Engineering from the Tohoku University in 2016 and 2018, respectively. He is now working in IBM Japan Ltd.
Shunichi Sato
Professor S. Sato received his Bachelor’s degree in Science in 1981, Master’s degree in Engineering in 1983, and Doctorate in Engineering in 1992 from the Tohoku University. He started his academic career with laser application to bioscience and medicine, and expanded his interest to materials science. He became Professor of Institute of Multidisciplinary Research for Advanced Materials, Tohoku University in 2003. He has believed in the extreme potential of light and is trying to explore its hidden peculiarities.