2022 年 39 巻 p. 262-269
2022 年 39 巻 p. 262-269
Nanoparticle synthesis using flow chemistry has the potential to enhance the large-scale accessibility of precision-engineered nanomaterials at lower prices. This goal has been difficult to achieve primarily due to reactor fouling and the lack of efficient reagent mixing encountered, especially in those scaled-up systems. The present study aimed to overcome the two challenges by integrating a liquid-liquid biphasic segmented flow system with static mixing. This strategy was applied to the synthesis of gold nanoparticles (AuNPs) using citrate reduction chemistry. It was demonstrated that reactor fouling was eliminated by implementing the biphasic flow strategy. As a result, the overall mean particle size of the as-synthesized AuNPs was measured to be 15.5 nm with a polydispersity index (PDI) of 0.07, and with the reproducibility of ± 6.4 %. The biphasic flow system achieved a reaction yield of 88.7 ± 1.1 % reliably with a throughput of 60 mL/hour up to 8 hours.
Nanoparticle synthesis using flow chemistry has attracted significant attention in the last decade because of its potential for automated, low-cost production of high-precision nanomaterials with minimal downtime (Nightingale et al., 2014; Stitt, 2002). The majority of the research in flow synthesis involved the study of single-phase flow in microfluidic systems, in which miscible fluids are continuously introduced into chip-based microfluidic cells under specific flow conditions (Wagner et al., 2008). However, reactor fouling (Schoenitz et al., 2015; Wagner et al., 2004), inefficient mixing (Song et al., 2003b), relatively broad residence time distribution (Trachsel et al., 2005), and relatively low throughput are some of the major challenges encountered in bench-top single-phase microfluidic reactors. They present a great barrier to the precision control over the properties of the synthesized nanoparticles, especially when the system is scaled up. These challenges must be overcome before technology transfer to the industry can occur.
For these reasons, the present work aims to develop a chemical flow reactor that allows the continuous synthesis of high-precision metal nanoparticles with higher throughput. Ultimately, the goal is to generate a knowledge base that would be valuable to flow synthesis of precision nanoparticles on a broader scale.
The chemical reaction known as the Turkevich method (Turkevich et al., 1951) was used to synthesize AuNPs. For the conventional batch reaction, a specific volume of 1.0 wt% (34.0 mM) trisodium citrate dihydrate (Na3C6H5O7 · 2H2O, or NaCt, Sigma-Aldrich) solution was injected into the solution of 0.25 mM gold chloride (HAuCl4, Sigma-Aldrich) at 100 °C under ambient pressure and with vigorous agitation. The molar ratio of NaCt to HAuCl4 is the primary factor that determines the final size and size distribution of the AuNPs (Dong et al., 2020; Frens, 1973). The effects of other parameters were studied with more details in the literature (Polte et al., 2010; Wuithschick et al., 2015).
2.2 Flow reactor design and setupThe layout of the flow system is illustrated in Fig. 1. The reagents were freshly prepared and stored in individual media bottles (FisherbrandTM Sterile PETG). The continuous synthesis was initiated by introducing the reagents into the tubular reactor using independent piston pumps (Syrris, FRX) with a precisely controlled volumetric flow rate (± 0.01 mL/min). The reagents were joined, mixed, and heated as they flow through the reactor in which the particle synthesis occurred. The reactor temperature was controlled using an oil bath on a hotplate (Syrris, FRX). The pressure inside the reactor was controlled using a needle valve (IDEX, PEEK P-447) as a back-pressure regulator at the outlet of the reactor. The absorption spectrum of the as-synthesized particles was recorded inline when the sample passes through a quartz flow cell (FireflySci, Inc.) in the light chamber of a UV-visible spectrophotometer (Ocean Optics, USB2000+ UV-VIS). Finally, the nanoparticles were collected in centrifuge tubes for further analysis.
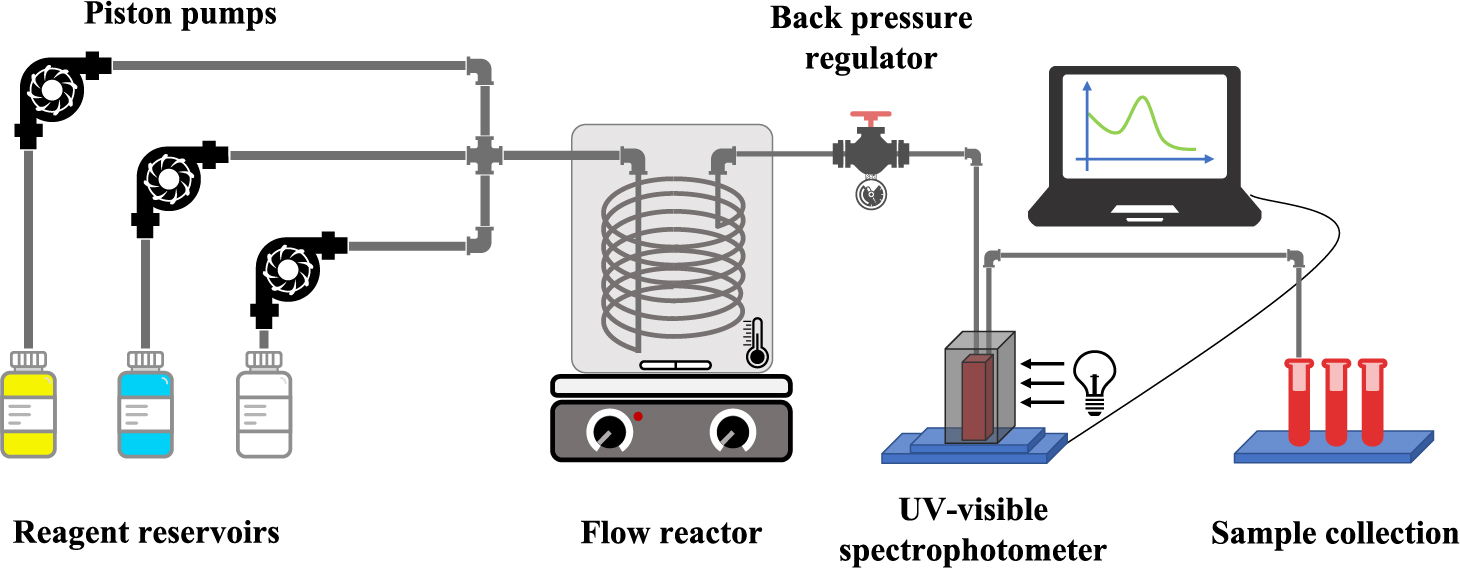
The layout of the flow reactor system. Note: the diagram is not drawn to scale. The cross junction does not represent the actual fluid junctions used.
The flow reactor integrated an aluminum framework and polymer tubing in a temperature-controlled oil bath, as illustrated in Fig. 2. The framework was made of 6061 aluminum (McMaster-Carr, sheet aluminum 6.35 mm thick 30 cm × 13 cm; threaded rod 6.35 mm diameter, 20 cm length; matching hex nuts and washer 6.35 mm screw size). Aluminum was selected because of its temperature tolerance (≤ 150 °C) for long-time service, high corrosion resistance, nonmagnetic nature, and good machinability. Perfluoroalkoxy tubing (PFA, Cole-Parmer, 3.18 mm OD, 1.59 mm ID) was selected due to its chemical compatibility, temperature tolerance (≤ 260 °C), pressure rating (≤ 40.8 bar), and better optical transparency as compared to TeflonTM, polytetrafluoroethylene (PTFE).

Schematic of the flow reactor (framework and tubing).
In a typical single-phase flow AuNP synthesis experiment, NaCt (1.7 mM) and HAuCl4 (0.54 mM) solutions were continuously introduced into the flow reactor in parallel. The solutions were merged using a Y-junction (IDEX, PEEK P-514), as shown in Fig. 3. The mixture was heated to 100 °C as it flowed through the reactor. The volumetric flow rate of each solution (1 mL/min) was kept constant so that the residence time (8.7 min) ensured that particle synthesis was complete before the sample exited the reactor. The pressure inside the reactor was maintained at 3 bar to minimize bubble formation that would impact the concentration and disrupt the steady-state flow condition. The AuNP suspension was sampled with a 30-min time interval for particle size characterization. Each experiment was continued for 4 hours, and each experiment was repeated three times on different dates.

Schematic of the Y-junction and flow field in single-phasic flow experiment. System: volumetric flow rate ratio of NaCt to HAuCl4 = 1:1; total flow rate = 2.00 ± 0.02 mL/min, reaction temperature = 100 ± 1 °C, pressure = 3 ± 1 bar, molar ratio of NaCt to HAuCl4 = 3.2, Reynolds number ≈ 90.
The liquid-liquid biphasic segmented flow system, as shown in Fig. 4, involved the parallel introduction of two immiscible liquids. The liquid that preferentially wets the surface of the channel is the continuous phase; the other liquid carried by the continuous phase is the dispersed phase. Segmented flow, also referred to as slug flow in the literature (Baroud et al., 2010; Nightingale and Demello, 2013), is different from droplet flow in which the dispersed droplets exhibit an average size smaller than the channel’s inner diameter.

Experimental setup showing the generation of biphasic segmented flow. The yellow and blue dye represent HAuCl4 and NaCt solutions, respectively. System: volumetric flow rate ratio of NaCt to HAuCl4 to silicone oil = 1:1:2; total flow rate = 2.00 ± 0.04 mL/min, reaction temperature = 100 ± 1 °C, pressure = 3 ± 1 bar, molar ratio of NaCt to HAuCl4 = 3.2, Capillary number ≈ 0.021, Reynolds number ≈ 45 in the laminar flow region.
In the biphasic flow experiments, silicone oil (Fisher ChemicalTM, viscosity = 45–55 mm2/s) was selected because of its low surface tension (21.3 ± 0.3 mN/m at ambient temperature (Svitova et al., 2002)), temperature stability (decomposition temperature > 250 °C), and it is chemically inert. As illustrated in Fig. 4, silicone oil was introduced from both sides of the cross-junction (IDEX, ETFE P-723). Droplets of aqueous reactants were generated when the aqueous solution was merged with silicone oil. Each aqueous droplet became an individual mini-reactor that was carried by silicone oil. Despite the relatively slow diffusion-limited mixing in the laminar flow region (Re ≈ 45), ninety percent of reagent mixing could be completed in the mini-reactor in approximately 5 seconds due to the presence of recirculating flow field (Baroud et al., 2010; Bringer et al., 2004; Tice et al., 2003). The detail of the mixing time calculation is provided in the Supplementary Material Section 1. The collected mixture of oil and aqueous AuNP suspension was centrifuged (Eppendorf 5810R, 2000 RCF for 20 min) to expedite the phase separation between oil and water.
2.5 Particle characterizationThe mean particle size and size distribution of the collected AuNP samples were characterized using dynamic light scattering (DLS, Malvern Zetasizer Ultra). All measurements were taken at room temperature (22 °C).
In general, the AuNP samples synthesized in the single-phase flow experiments did not agree with the reference values of particle size and size distribution (18.1 ± 3.5 nm by intensity distribution (Dong et al., 2020)) based on the DLS measurements as given in Fig. 5 (UV-vis spectrum analysis and DLS data of individual trials are provided in Supplementary Material Section 2.). The AuNP samples generally exhibited a larger mean particle size (26.9 nm) than the reference value as a consequence of fouling. Besides, the AuNP size increased and became more polydispersed after 90 min. The detection of sub-10 nm nanoparticles suggested the presence of secondary nucleation in the bulk phase. It is possibly caused by a local decrease in the molar ratio of NaCt to HAuCl4 in the bulk phase (Dong et al., 2020), which is due to the imbalanced consumption and competition of reactants from fouling.

The DLS measurements of the AuNP samples showed varying particle size and size distribution as a function of time in the single-phase flow experiments. Note: each sample was a pool of collection over 30 min intervals, e.g., Sample 210 was collected from 210 min to 240 min. The measured values represented the particle size and size distribution of the major peak of each sample.
The increase in the mean particle size and broadening of the size distribution could be attributed to reactor fouling which is an uncontrolled material deposition or growth process on the reactor surface, as shown in Fig. 6. Reactor fouling was identified by the change in color and the decrease in optical transparency of the PFA tubing as the experiment progressed. A clean PFA tubing has a translucent and greyish appearance, as illustrated in Fig. 6(a). A black haze could be observed on the tubing 30 min after the experiment began. The transparency of the surface decreased over time. Eventually, the interior surface became covered in purple, red, or gold materials, as highlighted in Fig. 6(b). The observation agreed with Huang et al. (2018), who investigated the deposited materials using X-ray photoelectron spectroscopy and infrared spectroscopy. Their results suggested that the deposited material consisted of metallic gold, unreacted gold, and citrate-gold complex.
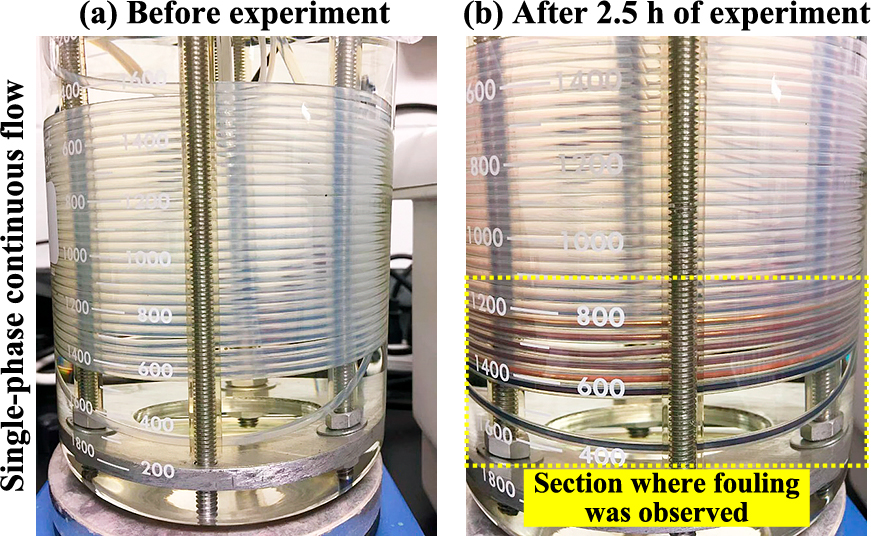
(a) The clean reactor before the experiment. (b) Reactor fouling was observed at the bottom section of the reactor in the single-phase flow experiments. Note: the fouling can be cleaned by flushing aqua regia, followed by deionized water through the reactor after it is cooled to room temperature.
The fouling was observed only at the bottom section of the reactor. It was noticed that the residence time of the fouling section matched with the extent of the chemical reaction (approx. 2.5 min at 100 °C). Based on this observation, it was hypothesized that fouling was caused by heterogeneous nucleation on the reactor surface during the synthesis reaction of AuNPs.
The following experiments were conducted to investigate the proposed hypothesis. First, the flow rate was doubled while keeping other parameters constant. The result showed that the length of the section that exhibited fouling was doubled. In the second experiment, the flow direction in the reactor was changed by switching the inlet and the outlet of the reactor. As expected, the section that exhibited fouling was relocated near the new inlet (upper section) with the same length. In the third experiment, 500 mL of colloidal AuNPs (15 nm in diameter) were synthesized in a batch reactor. The AuNP suspension was then circulated in the flow reactor at the same temperature (100 °C), pressure (3 bar), and flow rate (2 mL/min) for 12 hours. The absorption spectrum of the suspension was continuously monitored; it showed minimal change during the experiment, and no fouling was observed. The colloidal concentration of AuNPs was estimated using the Beer-Lambert law in Fig. 7, which suggested less than 2 % loss of nanoparticles due to adsorption on the reactor surface. The results indicated that once the AuNP synthesis reaction was complete, the citrate stabilized AuNPs would not cause fouling.

The colloidal concentration of the fully synthesized and stabilized AuNPs exhibited minimal change while circulating in the flow reactor for 12 hours. System: temperature (100 °C), flow rate (2 mL/min), pressure (3 bar), initial concentration (45.1 ppm).
Various strategies were reported in the literature to mitigate reactor fouling (Schoenitz et al., 2015), for instance, increasing the flow rate, i.e., shear force at the solid-liquid interface (Wagner and Köhler, 2005; Yang et al., 2007); increasing the electrostatic repulsion between the surface and the depositing materials by tuning pH (Wagner and Köhler, 2005); modifying the wettability of the reactor surface (Wagner and Köhler, 2005); and implementing coaxial fluid injection (Baber et al., 2015). A few of these strategies were investigated, including the test of different reactor materials (glass vs. TeflonTM), different surface roughness, and increasing the flow rate up to 5 mL/min. However, these methods were found to be ineffective in the present study. An alternative strategy (Shestopalov et al., 2004) involving the biphasic flow system was investigated to overcome fouling.
3.2 Biphasic flow AuNP synthesisWhen silicone oil was introduced in the flow system, it was noticed that fouling was no longer visible during the AuNP synthesis, as shown in Fig. 8. The reason was suspected to be the elimination of direct physical contact between the aqueous phase and the reactor surface due to the presence of the oil phase (Shestopalov et al., 2004).

Reactor fouling was not observed within (a) 2.5 hours or (b) 8 hours in the biphasic flow experiments (aqueous reactants dispersed in silicone oil).
The AuNP synthesis in the biphasic segmented flow system resulted in much better reproducibility than the single-phase flow system, as demonstrated in Fig. 9. The overall mean particle size from the biphasic experiments was 15.5 ± 4.1 nm (from the intensity distribution of DLS measurements), which agreed with the reference value (18.1 ± 3.5 nm (Dong et al., 2020), p ≈ 0.26). The particle size distribution, represented by the error bar in Fig. 9, also showed a significant improvement in the precision of the nanoparticles (tabular data is provided in the Supplementary Material Section 2 and on J-STAGE Data website).

The DLS measurements of the mean particle size of the AuNPs synthesized in biphasic flow experiments was reproducible and consistent with the reference value (18.1 ± 3.5 nm). The particle size distribution is presented for each trial by showing the highest standard deviation of the data points of that trial. The raw DLS data is available publicly at https://doi.org/10.50931/data.kona.15022002
The reaction yield was calculated from the experimental and the theoretical colloidal concentration. The experimental concentration of the AuNP samples was estimated based on the Beer-Lambert law and the calibration curve using UV-vis absorption spectrum and ICP-MS data (Dong et al., 2020). The theoretical concentration was calculated with the assumption of 100 % conversion from the reactant to the product in the AuNP synthesis reaction.
In the biphasic flow experiments, the colloidal concentration of AuNPs resulted in a constant value of 46.5 ± 0.5 ppm, which was approximately 88 % of the theoretical value compared to a maximum of 83 % in the single-phase experiments, as shown in Fig. 10. The decrease in the yield in the single-phase reaction systems can be attributed to reactor fouling that alters the physical and chemical nature of the surface (PFA) so that heterogeneously nucleation on the reactor surface becomes more favorable.

The colloidal concentration of the AuNP samples from the biphasic flow experiments was maintained constantly and reproducibly at 46.5 ± 0.5 ppm, which was approx. 88 % of the theoretical value.
A biphasic flow experiment was continuously run for over 8 hours without reactor fouling, as shown in Fig. 8(b). The DLS data plotted in Fig. 11 shows that the mean particle size of the AuNP samples was maintained at 16.9 ± 3.7 nm, which was consistent with the reference value (18.1 ± 3.5 nm (Dong et al., 2020), p ≈ 0.59) and the previous biphasic experiment results (15.5 ± 4.1 nm). The colloidal concentration was also maintained at the same value, 46.4 ± 0.7 ppm, equivalent to a percentage yield of 87.5 ± 1.3 %.

The DLS measurements of particle size and size distribution of the AuNP samples collected in an uninterrupted biphasic flow experiment. System: molar ratio of citrate to gold (3.2), temperature (100 °C), pressure (3 bar), flow rate (2 mL/min), volumetric flow rate ratio of oil to water (1:1), residence time (8.7 min).
The precision of nanoparticles, i.e., size distribution, was quantified using the polydispersity index (PDI) (ISO13321, 1996; Malvern, 2011). PDI can be calculated using Eq. (1) (NanoComposix, 2015). The average PDI of the samples synthesized in biphasic flow experiments (0.12 ± 0.02) was lower by nearly one order of magnitude than the single-phase flow experiments (1.02 ± 0.43).
| (1) |
The improvement of particle size precision in the biphasic flow system can be attributed to the enhanced reagents mixing efficiency due to the recirculating flow field inside each aqueous segment (Song et al., 2003a). The time required to complete the reagent mixing in each droplet depends on the channel size, the droplet size, and the traveled distance. The average mixing time was estimated to be approximately 5 seconds. Detailed calculation is provided in the Supplementary Material Section 1.
The mixing by diffusion generally becomes less efficient as the reactor channel size increases (Stitt, 2002). To overcome this challenge, an inline static mixer was implemented. Based on the concept of packed bed column reactors, a packed bed static mixer (PBSM) was fabricated by filling yttria-stabilized zirconia beads (80 μm in diameter) in the PFA tubing. The PBSM was installed ahead of the fluid junction that introduced silicone oil, as illustrated in the graphic abstract. Statistical analysis indicated that the PDI of the AuNP samples was reduced by an additional 40 % with the implementation of the PBSM (p ≈ 2E-9), as shown in Table 1.
Comparison of polydispersity index (PDI) of the AuNP samples synthesized using the biphasic flow system under specific conditions.
| PDI | SD | |
|---|---|---|
| Reference value, batch method | 0.065 | 0.009 |
| Without static mixing, Fresh oil | 0.119 | 0.021 |
| With static mixing, Fresh oil | 0.068 | 0.008 |
| With static mixing, Recycled oil | 0.079 | 0.018 |
For the purposes of developing a greener and more sustainable process, preliminary experiments using recycled silicone oil were conducted. The used silicone oil was separated from the aqueous phase using a separation funnel, then cleaned by centrifugation and vacuum filtration, and reused in the subsequent experiments. Using the recycled silicone oil resulted in a 16 % increase in PDI (p ≈ 4E-3) while the mean particle size remained unaffected (p ≈ 0.37). It was noticed that the samples generated from the experiments using the recycled oil had a higher degree of oil contamination possibly due to thermal degradation of the silicone oil.
In the present study, gold nanoparticles (AuNPs) were continuously synthesized in a flow reactor using citrate reduction chemistry. Reactor fouling was a major problem in the single-phase experiments, which affected the consistency, reproducibility, and precision control of the AuNP size, size distribution, and reaction yield. The main reason for fouling was ascribed to the occurrence of the heterogeneous nucleation reaction in the vicinity of the reactor surface, leading to growth and accumulation of materials there. The fouling problem was solved by introducing a water-immiscible silicone oil to the system that preferentially wetted the reactor surface. Compared with the single-phase flow system, the AuNPs produced in the biphasic flow experiments exhibited significantly narrower size distribution (PDI: 0.07 ± 0.01), higher and more consistent yield (approx. 88 %), and reproducibility of ± 6.4 % to the mean particle size.
Future research is suggested for inline particle functionalization using secondary reactors and further scale-up of the present reactor system. It could be achieved by increasing the overall flow rate, increasing the chemical concentration, and utilizing parallel channels. One of the major challenges encountered in the scaled-up flow system was inefficient reagent mixing. For precision AuNP synthesis, it was critical to ensure that the mixing was completed before the reaction began. Other challenges could be maintaining the flow stability at a higher flow velocity, maintaining the stability of AuNPs, and homogenizing the heat and mass transfer and pressure across parallel channels. Overall, the knowledge base developed in the present study should enable designing scalable systems for commercially viable larger-scale production of precisely engineered nanomaterials.
This work was primarily supported by the National Science Foundation (NSF Award No. 1602032) and our industrial partner OndaVia, Inc. Any opinions, findings, and conclusions or recommendations expressed in this material were those of the author(s) and did not necessarily reflect the views of the NSF or OndaVia, Inc. The authors want to acknowledge and express gratitude to Paul Carpinone (formerly University of Florida, UF), Gary Scheiffele (UF), and Bahar Basim (formerly UF) for their advice and assistance.
The raw DLS data is available publicly in J-STAGE Data (https://doi.org/10.50931/data.kona.15022002).
Jiaqi Dong
Jiaqi Dong received his B.S. in Materials Science and Engineering from the Illinois Institute of Technology in 2013. He then attended the University of Florida as a graduate student and received his M.S. in Materials Science and Engineering in 2016. Currently, he is a Ph.D. candidate under the supervision of Dr. Brij Moudgil and Dr. Bahar Basim at the Center for Particulate and Surfactant Systems (CPaSS). His current research focus is metallic nanoparticle synthesis and functionalization using flow chemistry.
Jonathan Lau
Jonathan Lau is currently a student at the University of Florida. He is expecting to complete a B.S. in Mechanical Engineering in 2022. He has conducted undergraduate research under the supervision of Ph.D. candidate Jiaqi Dong and Dr. Brij Moudgil at the Center for Particulate and Surfactant Systems (CPaSS). His current research interests are fluid flow and computational fluid dynamics. He has previous industry experience with GE Aviation in 2019, Cummins in 2021, and Texas Instruments in 2021.
Spyros A. Svoronos
Dr. Spyros A. Svoronos is the Harry & Bertha Bernstein Professor of Chemical Engineering at the University of Florida. He received his B. A in Chemistry from Oberlin College and his M.S. and Ph.D. degrees in Chemical Engineering from the University of Minnesota. His current research interests are in modeling and optimization of a variety of processes, including bioreactors and nanoparticle synthesis. In addition, he is developing low-cost Arduino-controlled experimental equipment for teaching laboratories. He has published more than 70 technical papers and has been awarded four patents.
Brij M. Moudgil
Dr. Brij M. Moudgil is a Distinguished Professor of Materials Science and Engineering at the University of Florida. He received his B.E from the Indian Institute of Science, Bangalore, India, and his M.S. and Eng.Sc.D degrees from Columbia University, New York. His current research interests are in surfactant and polymer adsorption, dispersion and aggregation of fine particles, adhesion, and removal of microbes from surfaces, synthesis of functionalized nanoparticles, anti-scaling and surfactant mediated corrosion inhibitors, photocatalytic degradation of hazardous microbes, and nanotoxicity. He has published more than 400 technical papers and has been awarded over 25 patents. He is a member of the U.S National Academy of Engineering.