Abstract
Li-ion batteries with “nickel” as the main material or the highest ratio material on the cathode or anode electrode have attracted considerable attention. Nickel has high strength and corrosion resistance. Nickel has also been utilized successfully as the cathode and anode materials. The specific capacity and energy and power density of the material increased with increasing nickel content. However, several problems have limited the use of nickel-based Li-ion batteries. Problems such as cation mixing, the properties of nickel, and highly Ni-rich compounds leading to side reactions, influence the electrochemical performance of Li-ion batteries. The morphology is another factor affecting the electrochemical performance. Further studies will be needed to synthesize materials with the desired morphology and determine how the morphology affects the electrochemical performance. In a morphological perspective, extensive morphological adjustments are a pathway to a long and stable life cycle. In this light, nickel-based electrodes are manufactured continuously and will always be considered for next-generation secondary energy storage. The morphology of nickel-based active materials is one of the main factors determining the high-performance of Li-ion batteries.
1. Introduction
Nickel is the main highlight among researchers and industry. It is considered a versatile element because it is applied in various materials and technologies. The metal has high strength and corrosion resistance. Thus, it is often essential in construction materials, such as well-known stainless steels (Dewangan et al., 2015). Recently, nickel has found new applications in electrochemical energy storage that will permit increased mobility and flexibility. Interestingly, with current environmental issues, such as global warming and air pollution, nickel-based energy storage will be required in developing and developed countries (Ye et al., 2021).
Secondary batteries with nickel-derived active materials have been developed since the 19th century. Nickel-cadmium is the first nickel-based rechargeable battery that was successfully commercialized back in the early 20th century. Since its discovery, poor electrochemical performance and treating highly toxic spent Ni-Cd batteries have become major challenges. Its successor, Nickel-metal hydride (NiMH), also faces problems limiting its application, such as high self-discharge and low cycle life. The drawbacks of nickel-based energy storage have become alarming. Therefore, during the shift to new technology, Li-ion batteries, nickel-based electrodes were “temporally” left behind (Elbert et al., 2010; Lee et al., 2009).
Since their successful commercialization, it was predicted that Li-ion batteries would dominate the energy storage market. However, the dependence on cobalt-based materials, such as LiCoO2, cannot endure (Nitta et al., 2015; Toprakci et al., 2010). Cobalt is toxic and its exploitation has caused more harm than good. Even with relatively good stability, it has low real capacity because full Li-ion storage capacity was limited to 50 % to avoid explosions caused by thermal and design failure. In addition, cobalt is very expensive compared to other common transitional metals. These problems have shifted the concerns of researchers and industries back to nickel as an electrode material (Kalyani and Kalaiselvi, 2005).
Nickel has been used successfully utilized as both cathode and anode materials. Layered structured lithium transitional metal oxides (LTMO) were recently considered high-performance and high energy density cathode materials. Based on Fig. 1, the proportion of nickel in LTMOs has increased gradually over the past two decades, e.g., the development of NMCs (lithium nickel manganese cobalt oxide) and NCA (lithium nickel cobalt aluminum oxide) (Yan et al., 2020). While there is no classification of NCA, NMC cathode materials are classified based on the composition of the transitional metals and the types can be seen in Fig. 1. Extensive efforts to increase the nickel content in NMCs cathode materials have been reported. The specific capacity, energy density, and power density of the material increase with increasing nickel content. However, several problems are still being faced during its implementation. Nickel-rich cathode materials, such as LiNiO2, LiNi1−x−yCoxMnyO2 (x + y < 4), and LiNi0.8Co0.15Al0.05O2, are often produced with poor electrochemical properties because of cationic exchange between Li+ and Ni2+ from their lattices, where the cell suffers high irreversible capacity loss during its use (Lei et al., 2019a). Nickel-rich materials are also hygroscopic, which means they attract water molecules when not stored and handled properly. Highly reactive nickel-rich compounds at elevated working voltages also result in undesirable side reactions between the nickel-rich cathodes and the aprotic carbonate-rich electrolyte, producing unwanted side products that cause a rapid decrease in capacity or lifetime. Studies have confirmed that the cation mixing level can be reduced by the synthesis conditions and doping process in a nickel-rich cathode. Hygroscopicity can be overcome by proper storage conditions or coating of materials (Huang et al., 2014). The unwanted side reactions that occur during cell use can also be suppressed by coating the materials. However, adding a coating to a nickel-rich cathode is considered uneconomical because the coating process requires additional processing and production (Lu X. et al., 2019). Tailoring the particle morphology can also reduce the reactivity of Ni-rich cathodes. In addition, different morphologies result in different electrochemical behavior in Li-ion cells (Hwang et al., 2012).

Nickel-based anodes have undergone continuous development to replace existing carbonaceous anodes (Zuniga et al., 2019). Carbonaceous anodes have low gravimetric and volumetric capacity. To obtain commercialized artificial graphite, an extremely high heating process is necessary. Even with excellent performance, Li dendrite formation is an alarming phenomenon in graphite anodes because it can potentially cause battery failures. These concerns prove that state-of-the-art anode materials are urgently needed. Metal-based anodes, specifically nickel-based anodes, such as nickel oxides, nickel hydroxides, and nickel sulfides, are being developed continuously. The high capacity, high conductivity, easy recycling and reuse, and high availability make the metal-based anodes economically attractive. However, large capacity loss during the initial cycle often occurred because the Li storage mechanism involves a conversion and alloying-dealloying process (Wang L. et al., 2018). This problem can be overcome by morphological tailoring.
As Ni-rich Li-ion batteries are the market giant for current and future energy storage, an understanding of the factors that affect the electrochemical performance of the cells is required. Accordingly, because the morphology of the particle affects the electrochemical performance, this paper presents a review of nickel-based electrode synthesis toward its electrochemical behavior based on a morphological perspective.
2. Particle morphology for nickel-based anode material
Currently, various nickel-based materials have been applied as the anode material in Li-ion batteries. Oxides, hydroxides, sulfides, carbonates, and oxalates of nickel as anode material are representative examples. The electrochemical performance of these materials is mostly improved because of their unique morphologies. This section discusses the morphology of the nickel-based materials and their electrochemical performance.
2.1 Nanosheets nickel-based anode materials
Nanosheets Ni(OH)2 has been developed and successfully applied as an active material of Li-ion batteries. Various precursors of Ni(NO3)2·6H2O and NiCl2·6H2O provide uniform and ultra-thin nanosheet particle with particle dimension range between 20–30 μm. Particle morphology from NiSO4·6H2O showing larger particle with dimension range between 30–50 μm. The electrochemical performance of Ni(OH)2 are 1790/1157, 1945/1282, and 2019/1358 mAh g−1 with columbic efficiencies 65, 66, and 67 % (Li Yanwei et al., 2017). Modification of Ni(OH)2 by Al doping has been reported. The study showed the addition of Al slightly alter the particle dimension with specific capacity of 681 mAh g−1 at 20 % Al content and capacity retention of 50 % after 30 cycles (Li Y. et al., 2016).
Cycle ability of Ni(OH)2 can be improved by Ti3C2 lamination via hydrometallurgy method. In the study, Ni(OH)2/Ti3C2 electrode material has Li capacity of 121.3 mAh g−1, higher than pure Ni(OH)2 (117.6 mAh g−1) with stable cycle performance after 1000 cycles (Li C. et al., 2020). Double hydroxide metal transition, Nickel-cobalt, with ultrathin graphene surface layer is an alternative to a stable electrochemical cycle performance of nanosheets Ni(OH)2 (Shi et al., 2017). Ni(OH)2 blending with PANI and graphene oxide exhibited improved cycle ability with only 15.6 % capacity drop after 2000 cycles (Ma et al., 2016). This technique also successfully implemented in Ni-Co phosphates which had excellent cycling ability and stability after 9000 cycles (Li B. et al., 2017).
2.2 Nanoflakes nickel-based anode materials
Nanoflakes Ni(OH)2 was obtained via precipitation and hydrazine assisted reduction process. Blended with rGO, the material delivered high initial capacity of 1500 mAh g−1 and 1003 mAh g−1 after 40 cycles (Zhu et al., 2014). Nanoflake NiO as anode material was successfully obtained by chemical liquid deposition in Ni-foam followed by atmospheric heat treatment at 350 °C. The material delivered discharge capacity of 1.94 mAh cm−2 and capacity retention 71.1 % after 140 cycles (Ni et al., 2013). Another approach to obtain nanoflake NiO is via chemical bath deposition before being inserted into Ni foam. It exhibited initial capacity of 700 mAh g−1 and 490 mAh g−1 after 50 cycles (Huang et al., 2009). Nanoflake NiS from hydrothermally treated NiCl2 and metal alloying was also reported. The electrochemical performance test showed initial discharge capacity of 584.6 mAh g−1 and capacity retention of 34.2 % after 30 cycles (Zhu et al., 2011). Flake-shaped carbon-NiS was obtained also via hydrothermal process with addition of glucose as carbon source. The material initial and 20th discharge capacity was 575.4 mAh g−1 and 200 mAh, respectively (Wang et al., 2013).
2.3 Nanoplate nickel-based anode materials
Nanoplate Ni(OH)2/Graphene anode material was produced by hydrothermal reaction of NiNO3, ammonia and graphene at 150 °C for 12 h. It exhibited Initial specific capacity of 1318 mA/g and average capacity loss of about 0.11 % after 100 cycles (Du et al., 2018).
2.4 Nanorod nickel-based anode materials
SEM analysis of a NiS-graphene composite revealed nanorod morphological particles. NiS-graphene with a nanorod morphology was produced using the Hummers method followed by thermal reduction. The NiS to graphene mass ratio that indicates a nanorod morphology was 2:1.
The electrochemical performance test revealed an initial discharge capacity of 458 mAh g−1. This was smaller than NiS:graphene = 1:1, which had been discussed elsewhere in the sub-chapter nanosheet reported (Geng et al., 2014). The NiS nanorod morphology also could be produced using a solvothermal reaction. The raw material for the Ni-source and S-source was nitrate-based and C2H5NS, respectively. SEM showed that the concentration of the reactant solution affected the morphology of the particle. All concentrations produced a nanorod morphology, but higher concentrations resulted in a decrease in NiS particle size. The electrochemical test revealed good performance. The initial discharge capacity was 670 mAh g−1, and good cycling stability was observed after 100 cycles (Long et al., 2016). Nickel oxalate (NiC2O4·2H2O) nanorod particles were synthesized using a hydrothermal method. The temperature of the hydrothermal process influenced the particle morphology. An urchin-like nanorod morphology was observed when the reaction took place at 130 °C. On the other hand, the ratio and size of the nanorod particles increased when the reaction occurred at 170 °C. The initial discharge capacity was 1109 mAh g−1 with cycling stability of 85 % (Oh et al., 2016). Nickel oxalate was also used as a precursor material for NiO (Du et al., 2020).
2.5 Nanospike nickel-based anode materials
NiS with nano-spike morphology was obtained using a hydrothermal method in an autoclave reactor performed at a temperature of 160 °C for 24 hours. Fig. 2a shows a nano-spike particle. The electrochemical performance test was conducted on a coin cell-type cell with NiS as the cathode and Li-foil as the anode. The best initial discharge specific capacity was 550 mAh g−1. Cycling stability after 100 cycles revealed 60 % capacity retention from its initial capacity (Sonia et al., 2014). The NiS powder was modified by adding a camphoric carbon solution to the raw material to form “camphoric carbon wrapped NiS”. SEM and TEM revealed nano-spike particles. The modified-NiS was produced using a hydrothermal method in an autoclave reactor at 160 °C for 24 hours. The electrochemical performance test showed an initial discharge capacity of 500 mAh g−1. The test revealed 75 % capacity retention after 100 cycles, showing good cycling stability performance. The use of camphoric carbon increased the cyclic performance (Sebastian et al., 2014).
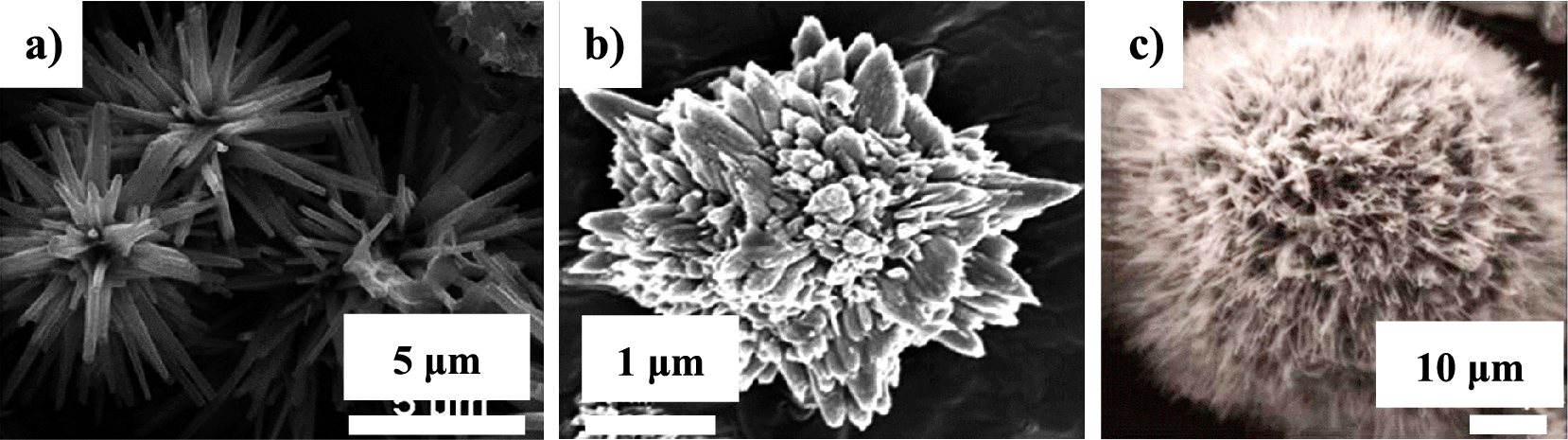
NiS material with an urchin-like morphology could be obtained by adding cetyltrimethylammonium bromide (CTAB) as an assistant. The flower-like NiS particles were synthesized using a hydrothermal method. Fig. 2b presents an SEM image of a flower-like NiS particle. The raw material for the Ni-source was a nitrate salt and NaSCN for the S-source. The highest initial discharge capacity was 6733 mAh g−1. The cycling stability was tested until 10 cycles, showing 85 % capacity retention (Ma et al., 2015). NiS with an urchin-like particle morphology was synthesized from a nickel complex as a precursor using a solvothermal method. Fig. 2c shows the NiS urchin-like particle morphology. The electrochemical performance was assessed using a coin-cell type cell. The initial discharge capacity of the battery was 597.3 mAh g−1 with a voltage window of 1.0–3.0 V (Mi et al., 2013).
Table 1 summarizes the summary of nickel-based anode morphology and their electrochemical behavior. A nickel-based anode with a unique morphology showed excellent performance in Li-metal batteries. With small or nanoscale size, each material can store Li, even with good cycling ability. In contrast to the graphitic or carbon-based anode, the insertion of Li-ions uses a conversion mechanism rather than an intercalation mechanism.
Table 1
Preparation of Ni-based particle for anode material of Li-ion batteries.
| Ni-based Materials |
Methods |
Modification |
Morphological feature |
Electrochemical Performance (Capacity/Cycle/Rate) |
Reference |
| Ni(OH)2 |
Precipitation |
— |
Ultra-thin nanosheet |
2019/1358 mAh g−1/0.1 C |
(Li Yanwei et al., 2017) |
| Ni(OH)2 |
Precipitation |
Al-doping 20 % w/w |
Nanosheet |
681 mAh g−1/30 cycles/0.1 C |
(Li Y. et al., 2016) |
| Ni(OH)2 |
Hydrothermal |
Ni(OH)2/delaminated-Ti3C2 |
Nanosheet |
121 mAh g−1/1000 cycles/0.1 C |
(Li C. et al., 2020) |
| Ni-Co-hydroxide |
Hydrothermal |
Combine Co-Ni hydroxide |
Nanosheet |
373 mAh g−1/500 cycles/0.1 C |
(Shi et al., 2017) |
| Ni(OH)2 |
One-step in-situ polymerization |
GO/PANI blending |
Nanosheet |
Capacity fading 15.6 % after 2000 cycles |
(Ma et al., 2016) |
| Ni(OH)2 |
precipitation |
rGO blending |
Nano-flakes |
1500/40 cycles/0.1 C |
(Zhu et al., 2014) |
| NiO |
CLD and graft growth under air condition |
— |
Nano-flakes |
1.94 mAh cm−2/140 cycles/0.1 C |
(Ni et al., 2013) |
| NiO |
CBD |
— |
Nano-flakes |
700 mAh g−1/50 cycles/0.1 C |
(Huang et al., 2009) |
| NiO |
Hydrothermal |
Graphene blending |
Nanoplate |
1318 mAh g−1/100 cycles/0.1 C |
(Du et al., 2018) |
| NiO |
Thermal decomposition |
Ni-foam as starting material |
Nanowalls |
1020/704 mAh g−1/60 cycles at various rates (0.2–10C) |
(Wang et al., 2011) |
| NiS |
Hydrothermal |
Cladding carbon (C-NiS) |
Flake-shape |
575.4 mAh g−1/30 cycles |
(Wang et al., 2013) |
| NiS/G (1:1) |
Hummers method continued with thermal reduction |
Mass ratio NiS to graphene |
Nanosheet |
887 mAh g−1/60 cycles/0.1 C |
(Geng et al., 2014) |
| NiS/G (2:1) |
Hummers method continued with thermal reduction |
Mass ratio NiS to graphene |
Nanorod |
458 mAh g−1/60 cycles/0.1 C |
(Geng et al., 2014) |
| NiS |
Solvothermal |
Reactant concentration |
Nanorod |
670 mAh g−1/100 cycles |
(Long et al., 2016) |
| NiS |
Hydrothermal |
Reaction time (24 h) |
Nano-spike |
550 mAh g−1/100 cycles/0.1 C |
(Sonia et al., 2014) |
| Camphoric carbon wrapped -NiS |
Hydrothermal |
Addition of camphoric carbon |
Nano-spike |
550 mAh g−1/100 cycles/0.1 C |
(Sebastian et al., 2014) |
| NiS |
Hydrothermal |
CTAB assistant |
Flower-like |
6733 mAh g−1/10 cycles |
(Ma et al., 2015) |
| NiS |
Solvothermal |
Nickel complex precursor |
Urchin-like |
597.3 mAh g−1 |
(Mi et al., 2013) |
| NiC2O4·2H2O |
Hydrothermal |
Reaction temperature |
Nanorod |
1109 mAh g−1 |
(Oh et al., 2016) |
Fig. 3 shows the role of morphology towards the electrochemical properties of nickel-based anode material in Li-ion batteries. Studies showed that the nanostructure property of anode material has significant roles in improving the electrochemical performance. It offers a large contact of electrolyte to the surface of the material which is beneficial for high current applications. Some of the structure such as nanorods, nanowalls, nanoflakes has a rigid structure that is able to accommodate volume change strain during prolonged cycle of charge and discharge at relatively high current. However, the capacity value is not only affected by the morphology, but also the type of material and its lithiation-delithiation mechanism which will not be discussed deeply. The cycle stability of the anode is also greatly improved by addition of 2D nano-material such as graphene, reduced graphene oxide (rGO), and Ti3C2.
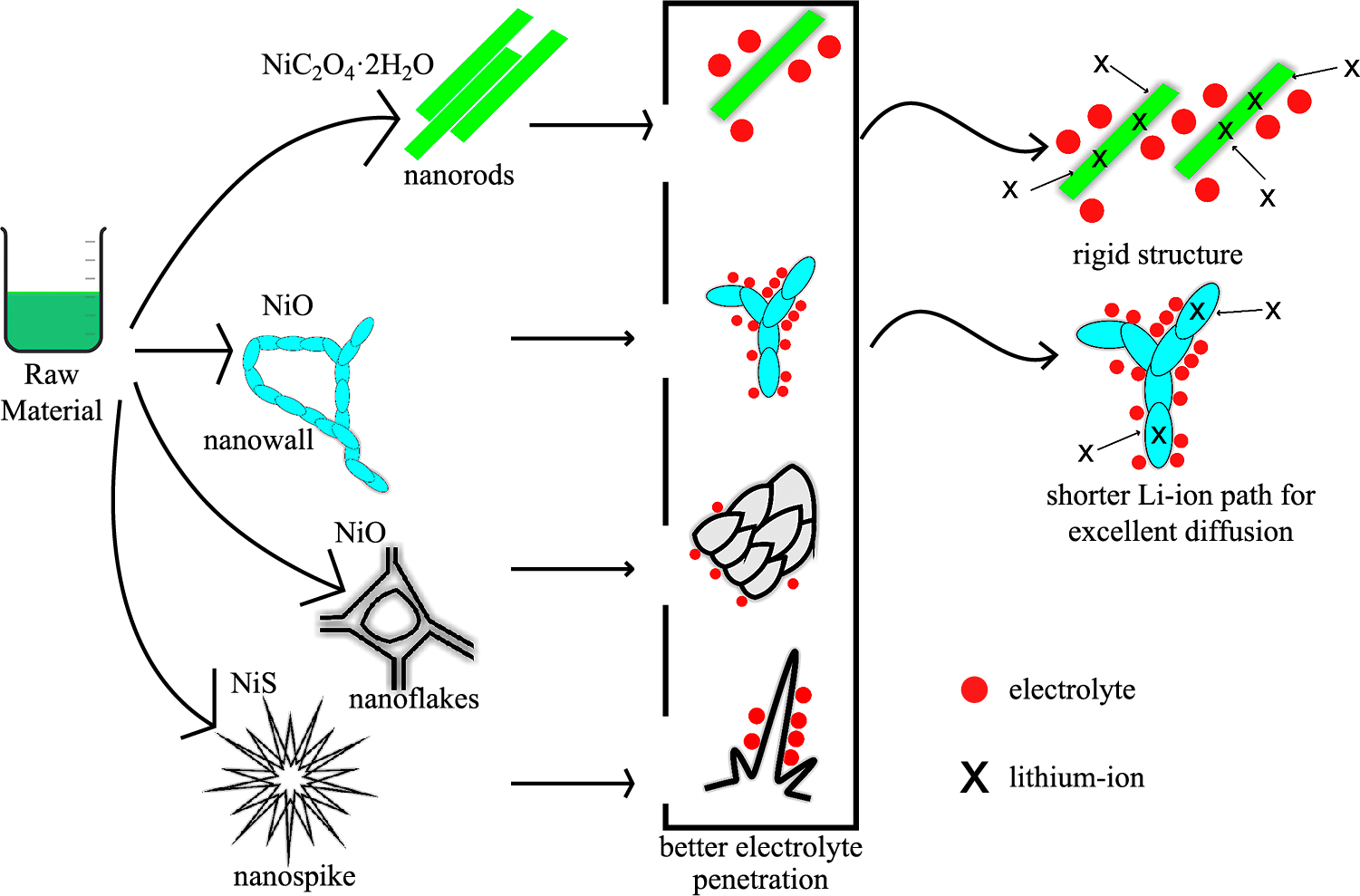
The main disadvantage of the conversion anode is the poor initial coulombic efficiency caused by a permanent Li reaction with some of the anode. In Li-metal batteries, the high Li-loss during the first cycle can be compensated for by the abundant Li source of the Li anode. In full cells, limited Li from the cathode materials can deteriorate the electrochemical performance of the cells. Even with a unique morphology, the initial lithiation capacity of Li in conversion anodes is still much larger than the de-lithiation capacity. However, efforts to increase the usability of conversion-type materials for Li-ion full cells, such as a Li-rich cathode material, material incorporation with carbon, and pre–lithiation of conversion anodes, are still being made (Qiu et al., 2018; Wei et al., 2020).
3. Nickel rich cathodes and their precursors
The synthesis of nickel-rich cathodes generally involves two processes: the formation of nickel-rich cathode precursors and high-temperature lithiation. The formation of precursors often determines the morphology of the final products, even after undergoing a long heat-treatment process. In this section, the formation of secondary particles is not discussed because the secondary particles are often related to the final morphology of the particle. The mechanism for the formation of secondary particles will be discussed in the next segment.
3.1 Nanosheets precursor to nickel-based cathode materials
Nickel manganese cobalt (NMC)-based spherical precursors were assembled using a solvothermal method. Ni(NO3)2·6H2O, Co(NO3)2·6H2O, and Mn(NO3)2 with a mol ratio of 14:3:3 were dissolved in 50 mL of ethanol. The operational conditions were RTP (Room temperature and pressure). A clear solution was placed in an autoclave and heated to 160 °C for 12 hours. The precipitate was then washed with absolute ethanol and dried at 80 °C for 8 hours in air. The morphology analysis shows microsphere flower-like particles with diameters of approximately 2–6 μm. All the microsphere flower-like particles were comprised of nanosheets (Fu et al., 2014). Fig. 4a gives an example of a nanosheet primary particle.
3.2 Nanoflakes precursor to nickel-based cathode materials
Ni-rich nano-flake particles, NMC-811 (LiNi0.8Mn0.1Co0.1O2) hydroxide precursor, can be prepared using a coprecipitation method. The raw materials for Ni, Mn, and Co sources were diluted in a mixture of NaOH and NH4OH. A calcination process was then performed under O2 atmospheric conditions at 750 °C for 12 hours. The secondary particle morphology was spherical and assembled from cross-flake particles. The precursor was mixed with LiOH and TiO2 to make a cathode material before sintering (Mo et al., 2020). Fig. 4b presents SEM images of a nano-flake particle. NMC-811 precursor synthesis is another way to improve the performance of Li-ion batteries.
3.3 Nanoplate precursor to nickel-based cathode materials
NCA (LiNi0.80Co0.15Al0.05O2) with a nanoplate morphology was obtained using sol-gel methods. The first research used raw materials: Ni(CH3COO)2·6H2O, Co(CH3COO)2·4H2O, Al(NO3)3·8H2O, and LiCH3COO with a molar ratio of 10.5:8:1.5:0.5. All the ingredients were dissolved in ethylene glycol with a concentration of 0.5 M and stirred for 8 hours at 85 °C. After the powder was formed and dried, it was ground to reduce the particle size, calcined, and sintered to prepare the cathode material (Wang Q. et al., 2018). Fig. 4c shows the nanoplate primary particles of the NCA cathode material. The second study used a nitrate-based raw material for Li, Ni, Co, and Al. C16H36O4Ti was used as the Ti doping source. All the materials were dissolved in ethylene glycol and stirred for 8 hours at 85 °C (Li J. et al., 2020).
NMC-622 (LiNi0.6Mn0.2Co0.2O2) is another cathode material with a nanoplate morphology that was obtained from ammonia-induced coprecipitation. The first step involved mixing 12 M NH3·H2O/(Ni2+, Co2+, and Mn2+) at a molar ratio of 6:1 for 30 minutes under inert conditions (using N2). Subsequently, the metal salts were added to the first solution and stirred for four hours. Stirring was continued at 95 °C for six hours to evaporate NH3 and allow precipitation. The slurry was filtered, washed, and dried in an oven. Li2CO3, as the Li source, was added to a 7 % excess, calcined, and sintered to produce the Li-NCA cathode material (Liu et al., 2016). The NMC cathode material (LiNi0.70Mn0.15Co0.15O2) with increasing Li+ ion diffusion showed good cycling ability. The manufacturing of this material was through a coprecipitation method followed by a hydrothermal method. The Ni, Mn, and Co metal sources were acetate salts combined at a Ni:Mn:Co molar ratio of 0.7:0.15:0.15. All the materials dissolved, and NaOH was added as a precipitating agent under inert conditions (Ar). The pH of the solution was maintained at 11. The solution was then moved to another reactor and filled to 80 % of the maximum volume. PVP (polyvinylpyrrolidone) was then added for the hydrothermal process, which was performed at 150 °C for four hours. Subsequently, the temperature was decreased naturally to ambient temperature. The slurry was then filtered, washed, and dried [Ni0.70Mn0.15Co0.15O2(OH)2]. The last steps were the calcination and sintering processes (Tian et al., 2016).
3.4 Nanorods precursor to nickel-based cathode materials
The NMC-622 hydroxide precursor (Ni0.6Mn0.2Co0.2(OH)2) as cathode material of Li-ion batteries was comprised of nanorod particles (Fig. 4d). This precursor was synthesized using a controlled coprecipitation method in a continuous stirred tank reactor on a large scale with a volume of 200 L. The Ni, Co, and Mn sources were sulfate salts dissolved in deionized water. NaOH and NH3·H2O were added separately into the continuous stirred tank reactor. The ammonium concentration was controlled to 4–5 g/L, and the pH was 11.5–12. The solid/precipitant formed was separated and dried in an oven and producing Ni0.6Mn0.2Co0.2(OH)2. The precursor had a micro-spherical morphology with a surface covered with nanorod-like particles (Yang et al., 2019). A full concentration gradient (FCG) of the nanorod primary particle as a cathode material (LiNi0.54Mn0.30Co0.16O2) was made from the [(Ni0.54Mn0.30Co0.16)(OH)2] precursor material. The raw materials for Ni, Co, and Mn were NiSO4·6H2O, CoSO4·7H2O, and MnSO4·H2O, respectively. A coprecipitation method was used to manufacture the precursor. The base material with a molar ratio of Ni:Mn:Co = 0.5:0.3:0.2 was added slowly to a Ni-rich solution containing Ni:Mn = 0.7:0.3. The homogenous solution was moved into the tanks with a stirrer, and N2 was introduced to achieve inert conditions. NaOH solution and NH4OH solution were added slowly to the tank as a chelating agent. The precursor was filtered and dried at 100 °C until all moisture had been removed. (Noh et al., 2014).
3.5 Needle-like precursor to nickel-based cathode materials
Needle-like particles were identified on the morphological test results of the NCA and NMC precursor for Li-ion batteries. Ni0.815Co0.15Al0.035(OH)2 was mixed with an NH4H2PO4 solution to form a homogenous slurry and dried naturally. After the mixture had dried completely, Li2CO3 was added to the mixture. The mixture was sintered at 750 °C for 16 hours (O2 condition) to form LiNi0.815Co0.15Al0.035O2. The resulting product was comprised of needle-like particles covered with NH4H2PO4 (Tang et al., 2017). The Al-gradient composition to Ni and Co metal sources was also promising as material doping for the Ni0.815Co0.15Al0.035(OH)2 precursor. First, a Ni0.815Co0.15Al0.035(OH)2 precursor was synthesized by a coprecipitation method with NH3·H2O as a chelating agent. Al(NO3)3·9H2O was dissolved in distilled water to form a homogenous solution, followed by the addition of a 32 % NaOH solution until a clear solution had formed. Distilled water was then added to the mixture to control the concentration of [Al(OH)x]3−x. The pH of the solution was 12–13 (Duan et al., 2016).
An Al-doped Ni-Co-hydroxide precursor [Ni0.90Co0.07Al0.03(OH)2] exhibited uniform needle-like NCA particles (Fig. 4e). The Ni and Co sulfate salts sources were used to synthesize the Ni0.928Co0.072(OH)2 precursor. The Al-source was obtained from the Al-based material dissolved in a 32 % NaOH solution to form Al(NO3)3. The Ni0.928Co0.072(OH)2 precursor was then added to Al(NO3)3. The mixture was stirred at 500 rpm and 60 °C with CO2. The mixture was washed with deionized water and dried in a vacuum oven. Dried Ni0.90Co0.07Al0.03(OH)2 was mixed with LiOH·H2O and sintered in oxygen to form LiNi0.90Co0.07Al0.03O2 (Duan et al., 2018). The NCA material was modified by replacing Al with W to increase the cycling stability. The first step involved synthesizing [Ni0.9Co0.1](OH)2 using a precipitation method from the sulfate salts. WO3 was then dissolved in distilled water and added slowly to the Ni-Co precursor. Subsequently, the mixture was washed, filtered, and dried. The process took place under inert conditions (N2). LiOH was then added, and the mixture was sintered to form LiNi0.90Co0.09W0.01O2. An analysis of the surface morphology revealed needle-like particles (Ryu et al., 2019). NMC-811 needle-like primary particles could be realized using a pre-oxidized process (Zhang et al., 2018), solid-state method with controlled crystallization and high-temperature operation (Yang G. et al., 2020), and Al-doping to cover the surface of the NMC-811-hydroxide precursor (Ni0.8Mn0.1Co0.1(OH)2) (Du et al., 2021). The pre-oxidized method was performed by mixing 0.005 g of polyvinylpyrrolidone (PVP) with the NMC-81-hydroxide precursor and dissolved in distilled water. This was followed by the addition of 3 wt.% Mn(NO3)2 to the mixture. The resulting mixture was heated to 40 °C until the water had vaporized. LiOH·H2O was then added to the dried powder and sintered to produce LiNi0.54Mn0.30Co0.16O2 doped with 3 % Mn(NO3)2 (Zhang et al., 2018). The second method was controlled crystallization followed by a solid-state method. Ni, Mn, and Co sulfate salts were dissolved in distilled water and stirred in a N2 atmosphere. Subsequently, a 2M NaOH solution was added as a precipitation agent. The temperature was maintained at 50 °C and a pH of 11.2 (Yang G. et al., 2020). Al doping was then performed. A dilute Al(NO3)3·9H2O in citric acid solution with a molar ratio 1:1 was produced. The mixture was stirred and heated until 80 °C to form a sol phase. The NMC-811-hydroxide precursor was then added and stirred. The resulting solution was heated until all the solvent had evaporated. The dried precursor was mixed with LiOH·H2O and sintered to form LiNi0.80Mn0.10Co0.10O2 (Du et al., 2021). Table 2 summarizes reported morphologies of nickel-rich cathode precursors which obtained through various synthesis techniques.
Table 2
Nickel-rich cathode precursors and their morphology.
| Precursors |
Metal Sources/Modification |
Methods |
Temperature (°C)/Time/Condition |
Morphological feature |
Reference |
| NMC-OH |
Nitrates |
Solvothermal |
160 |
Nanosheet |
(Fu et al., 2014) |
| NMC-811-OH |
Sulfates |
Precipitation |
|
Nano-flake |
(Mo et al., 2020) |
| NCA |
Ni-Co-Li-acetate Al-nitrate |
Sol-gel |
85/8 h stirring |
Nanoplate |
(Li J. et al., 2020) |
| NMC-622 |
Ni-Co-nitrate Mn-Mn(Ac)2 |
Ammonia-induced and coprecipitation |
96/6 h ammonia evaporation |
Nanoplate |
(Liu et al., 2016) |
| NMC-7 1.5 1.5 |
Acetate |
Hydrothermal |
150/4 h process |
Nanoplate |
(Tian et al., 2016) |
| NMC-622 |
Sulfate |
Coprecipitation |
— |
Nanorod |
(Yang et al., 2019) |
| NMC-FCG |
Sulfate/NMC-532 solution added into NM-73 solution |
Coprecipitation |
|
Nanorod |
(Noh et al., 2014) |
| NCA-OH |
Commercial precursor-NH4H2PO4 combined |
— |
|
Needle-like |
(Tang et al., 2017) |
| Al-doped-NCA |
Sulfate/Al doping |
Coprecipitation |
|
Needle-like |
(Duan et al., 2016) |
| NCA |
Ni-Co-OH with addition of Al-nitrate |
|
60/500 rpm/CO2 condition |
Needle-like |
(Duan et al., 2018) |
| NCW |
Ni-Co-sulfate/replacement of Al with W |
Precipitation |
Inert (N2) condition |
Needle-like |
(Ryu et al., 2019) |
| NMC-811 |
Commercial precursor + PVP |
Pre-oxidized process |
40 |
Needle-like |
(Zhang et al., 2018) |
| NMC-811 |
Sulfate |
Solid state with controlled crystallization |
50 |
Needle-like |
(Yang G. et al., 2020) |
| Al-doped-NMC-811 |
Commercial precursor/Al doping from nitrate source |
|
80/sol phase |
Needle-like |
(Du et al., 2021) |
4. Nickel-rich cathode materials
4.1 Primary particles morphology of nickel-based cathode material
A nanosheet morphology is found widely in electrode materials, particularly Ni-rich material for Li-ion batteries. NMC-811 (LiNi0.8Mn0.1Co0.1O2), as a Ni-rich cathode material, showed spherical morphology and provided a high rate and excellent cycling characteristics. In some cases, however, the primary morphology represents another type of morphology. Xu et al. generated NMC-811 with a nanosheet primary morphology and a spherical secondary morphology. The modified coprecipitation method was combined with a solid-state method to make radially aligned single-crystal primary particles (RASC-NMC). This material used NMC-OH as a precursor. The crystal was approximately 50 nm in diameter with an average length of 300–1000 nm. The specific capacity based on charge-discharge analysis was 195.3 mAh g−1 at the 1st cycle. The cycling stability revealed up to 95.5 % (172.7 mAh g−1) of the initial capacity (195.3 mAh g−1) after 300 cycles (Xu et al., 2019). The nanosheet morphology of NMC-811 can also be controlled by setting the ammonia concentration during the coprecipitation method. Controlling the ammonia concentration helped improve the area of exposed {010} planes of NMC-811 (LiNi0.8Mn0.1Co0.1O2) material. The electrochemical test showed an excellent capacity of 201.6 mAh g−1 at the first cycle at a current rate of 0.2 C with capacity retention of up to 90 % after the 100th cycle (Su et al., 2018). Other NMC cathode materials were also manufactured using different precursor materials and methods. LiNi0.7Mn0.15Co0.15O2 was synthesized from a Ni-Mn-Co spherical precursor using a solvothermal method. This Ni-rich NMC cathode material exhibited a secondary flower-like morphology assembled from nanosheets. The electrochemical performance showed a specific capacity of 184.7 mAh g−1. The cycling data were taken between 1–100 cycles (Fu et al., 2014). The hydrothermal method can modify the morphology of the NMC material. The primary morphology was nanosheet particles, which were arranged into single spherical particles as second particles. The specific capacity was 189.5 mAh g−1. After the 100th cycle, the specific capacity decreased until 135.5 mAh g−1. The capacity retention decreased to 73.0 % (Liu et al., 2019).
Ni-rich, NMC-811 cathode nano-flake particles can be prepared by modifying the NMC-OH precursors with the addition of TiO2 and LiOH before sintering. TiO2 doping helps improve the electrochemical performance and reduce the internal strain and the durability of the NMC-811 structure as a cathode material. The initial specific capacity was 258 mAh g−1. The capacity decreased to 79 % when charged under a 1 C current rate for 150 cycles (Mo et al., 2020). Nanoplate NCA (LiNi0.80Co0.15Al0.05O2) showed excellent capacity. The best sintering conditions of the NCA-precursor were 750 °C for 12 hours. The highest initial discharge capacity was 210.4 mAh/g at a current rate of 0.1 C. The columbic efficiency was 88.1 %. The cell was tested only until 40 cycles, but 90 % capacity retention was achieved (Wang Q. et al., 2018). Ti doping of the LiNi0.80Co0.15Al0.05O2 cathode material improved the cycling stability considerably. The modified NCA with Sn doping was arranged chemically as LiNi0.80Co0.09Ti0.06Al0.05O2. Good performance was achieved with a capacity retention of 90.6 % after 500 cycles (Li J. et al., 2020).
NMC-622 (LiNi0.6Mn0.2Co0.2O2) with a nanoplate morphology showed excellent high-rate capability. The initial discharge capacity with a current rate of 0.2 C was 173 mAh/g. In further current rate tests at 20 °C, the capacity retention was 67 % with a value of 77 mAh/g. The cycling stability was tested until 100 cycles and reached 91 % (Liu et al., 2016). NMC-622 nano-rod morphology showing good electrochemical performance with ~81 % capacity retention after 300 cycles (Yang et al., 2019). Combination of two kinds of NMC cathode material that have different molar composition of Ni, Mn, and Co showing good cycle with 70.3 % of capacity retention after 1000 cycles. This material is made by combining LiNi0.70Mn0.25Co0.05O2 and LiNi0.58Mn0.25Co0.17O2. The final composition is LiNi0.60Mn0.25Co0.15O2. Morphology analysis shows spherical particle and the primary particle is rod-shape particle (Noh et al., 2013).
NH4H2PO4-doped NCA (LiNi0.815Co0.15Al0.035O2) with a needle-like morphology electrochemical performance was tested. The initial discharge capacity of NCA with a 1 % mass was 195.3 mAh/g. This capacity was greater than that of the pure NCA material with an initial discharge capacity of 172.3 mAh/g. The capacity retention was 70.2 % from the initial discharge specific capacity after 100 cycles (Tang et al., 2017). Another study of LiNi0.815Co0.15Al0.035O2 doped with an Al-gradient doped solution showing excellent electrochemical performance. The initial discharge capacity was 187.5 mAh/g, and 93.4 % capacity retention was achieved after 200 cycles (Duan et al., 2016). Al doping was also performed to manufacture LiNi0.90Co0.07Al0.03O2. The initial discharge capacity was 224.6 mAh/g and after 100 cycles, 93.4 % capacity retention was observed (Duan et al., 2018). Tungsten (W) was the alternative metal source to replace Al. The presence of W greatly increased the cycling stability. LiNi0.90Co0.09W0.01O2 (NCW) exhibited needle-like primary particles. The initial discharge capacity was 231.2 mAh/g−1, which is greater than the NCA cathode material (223.5 mAh/g). The cycling stability test showed that the capacity of the NCW material was 92 % of the initial capacity. This result is more significant than the NCA material, with only 63 % of capacity retention (Ryu et al. (2019).). The effect of primary particles shapes towards their electrochemical performance are summarized in Table 3.
Table 3
The effect of nickel-rich cathode primary particles toward its electrochemical properties.
| Material |
Precursor/Modification |
Morphological feature |
Electrochemical Properties (Capacity/Cycle/Rate) |
Reference |
| NMC-811 |
NMC-811-OH/Modified coprecipitation and solid-state |
Nanosheet |
195 mAh g−1/300 cycles/0.1 C |
(Xu et al., 2019) |
| NMC-811 |
NMC-OH/Modification of ammonia concentration controlled |
Nanosheet |
201 mAh g−1/100 cycles/0.2 C |
(Su et al., 2018) |
| NMC-7 1.5 1.5 |
NMC precursor with solvothermal method |
Nanosheet |
184.7 mAh g−1/100 cycles/0.1 C |
(Fu et al., 2014) |
| NMC-8.5 0.75 0.75 |
NMC precursor with hydrothermal method |
Nanosheet |
189.5 mAh g−1/300 cycles/0.2 C |
(Liu et al., 2019) |
| NMC-811 |
NMC-OH/TiO2 doping |
Nano-flake |
258 mAh g−1/150 cycles/1 C |
(Mo et al., 2020) |
| NCA |
NCA-OH |
Nano-flake |
210.4 mAh g−1/40 cycles/0.1 C |
(Wang Q. et al., 2018) |
| NMC-622 |
NMC-OH |
Nanoplate |
173 mAh g−1/100 cycles/0.2 C |
(Liu et al., 2016) |
| NMC-622 |
NMC-OH |
Nanorod |
193.6 mAh g−1/300 cycles |
(Yang et al., 2019) |
| NMC-6 2.5 1.5 |
Combination of NMC-7 2.5 0.5-OH and NMC-5.8 2.5 1.7-OH |
Rod-shape |
206 mAh g−1/1000 cycles |
(Noh et al., 2013) |
| NMC-5.4 3 1.6 |
Modification of Ni/Mn/Co concentration gradient (FCG) |
Nanorod |
183.7 mAh g−1/100 cycles |
(Noh et al., 2014) |
| NCA-8.15 1.5 0.35 |
NCA-OH/NH4H2PO4 doping |
Needle-like |
195.3 mAh g−1/200 cycles/0.1 C |
(Tang et al., 2017) |
| NCA-8.15 1.5 0.35 |
NCA-OH/Al-gradient-doped |
Needle-like |
187.5 mAh g−1/200 cycles |
(Duan et al., 2016) |
| NCA-9 0.7 0.3 |
NCA-OH/Al-doped |
Needle-like |
224.6 mAh g−1/100 cycles |
(Duan et al., 2018) |
| NCW-9 0.9 0.1 |
NCW-OH/Replacing Al with W |
Needle-like |
231.2 mAh g−1/200 cycles/0.1 C |
(Ryu et al., 2019) |
| NCA |
Pre-oxidized method |
Needle-like |
175 mAh g−1/100 cycles |
(Zhang et al., 2018) |
| NCA |
Solid state with controlled crystallization |
Needle-like |
187.7 mAh g−1/100 cycles |
(Yang G. et al., 2020) |
| NCA |
Al-doping |
Needle-like |
171.4 mAh g−1/1000 cycles/1 C |
(Du et al., 2021) |
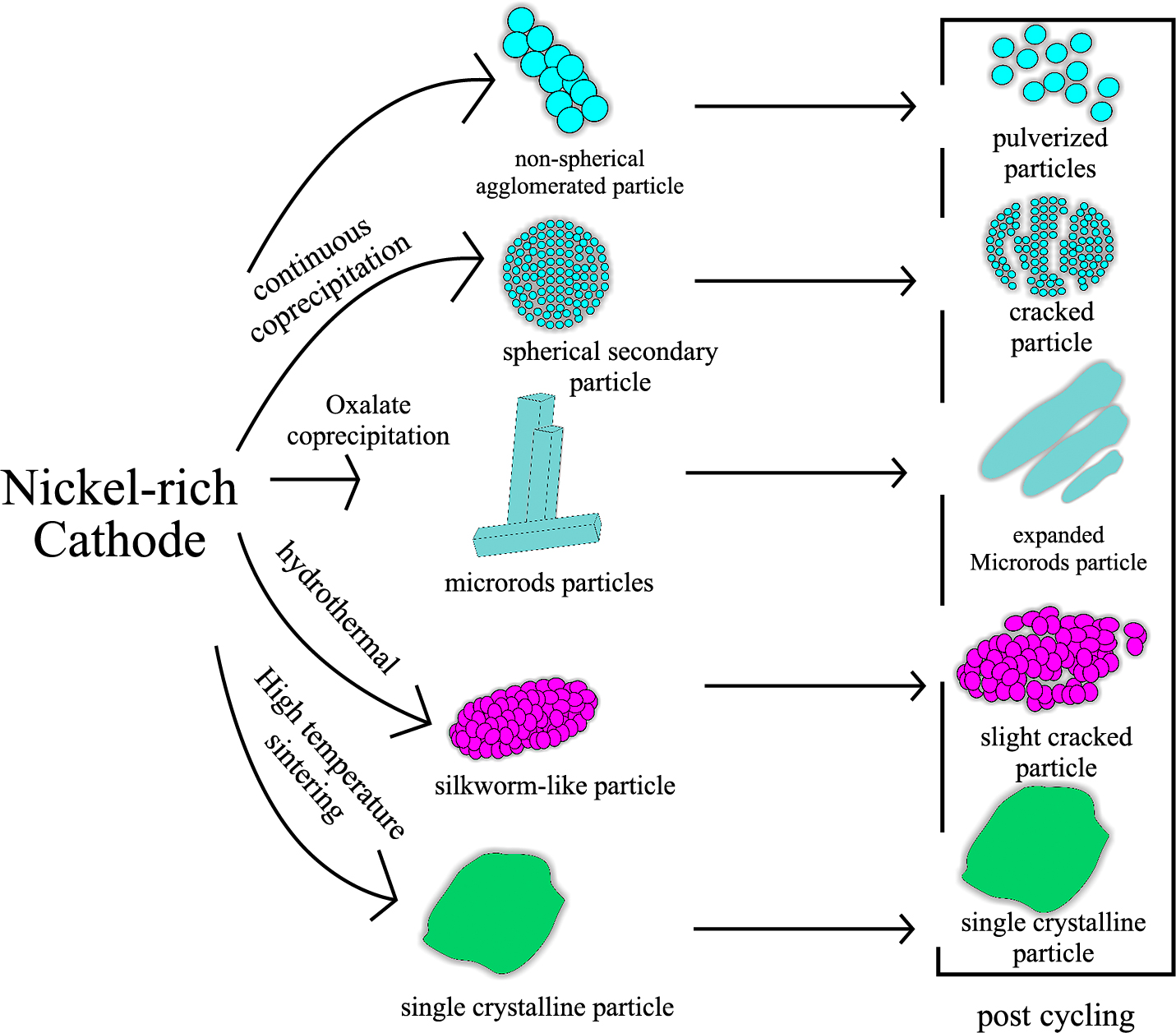
The secondary particles of a Ni-rich cathode material consist of agglomerated primary particles that form a larger and denser particle. Secondary particle formation reduces the high surface area of primary particles if they are present in singular particles (Barai et al., 2019). The reduction was proven efficient in hindering unwanted reactions between the active materials with the electrolyte (Qiu et al., 2017b; Yudha et al., 2019). The morphology of the nickel-rich cathode materials strongly depends on the synthesis process and the presence of additives that help control the secondary particle shape.
4.2.1 Microsphere / spherical Particles
Coprecipitation of transitional metal with high-nickel content is the most used technique to obtain micron-sized spherical particles of the nickel-rich cathode. The spherical morphology of a particle was formed from the agglomeration of crystal nuclei into larger particles. The formation of large or secondary spherical particles is in accordance with the Ostwald ripening mechanism, where the rate of crystal formation is in balance with the rate of crystal dissolution (Seo and Lee, 2017). Extensive studies focused on the effects of the synthesis parameters on the morphology, such as pH, concentration, mixing speed, stirrer type, reaction time, and precipitating agents (Zhu Q. et al., 2019). Hydroxide coprecipitation uses dissolved hydroxide compounds, such as sodium hydroxide, lithium hydroxide, or potassium hydroxides, as the precipitating agent. An ammonia solution is often introduced during the process to reduce the reaction rate avoid uneven particle growth. For hydroxide coprecipitation, the optimal pH to form high-density spherical secondary particles is 10–11 (Chen et al., 2018; Purwanto et al., 2018). The temperature during the coprecipitation process also is a factor determining the final morphology. In most cases, hydroxide and carbonate coprecipitation of nickel-rich cathode materials produced dense micron-sized secondary particles, while oxalate precipitation showed rock-like particles. Various chelating agents were reported to replace ammonia during hydroxide and carbonate coprecipitation. Acid or base chelating agents, such as EDTA, citric acid, sulfosalicylic acid, lactic acid, or their conjugates, such as EDTA-2Na, and sodium lactate, have been used as greener alternatives to ammonia. The excessive use of ammonia can cause environmental issues and health risks for humans and animals (Nam et al., 2015).
Quanzhong Zhu et al. (Zhu Q. et al., 2019) proposed a formation mechanism for spherical or microspheres secondary particle of Ni-rich cathode material in coprecipitation techniques. This began with crystal nuclei formation during the initial precipitation of single-crystal primary particles. With time, these single-crystal particles agglomerate into polycrystalline grains. These polycrystalline grains form initial secondary particles with rough surfaces from the aggregated particles. Because the formation of single-crystal nuclei occurs continuously, these primary particles are adsorbed onto the surface of the initial secondary particles. The process of single-crystal adsorption to the initial secondary particle continues with time. The primary particles become smooth, and the secondary particles grow with dense properties due to a decrease in particle porosity. In the nickel-rich cathode, the residence time to obtain smooth micro-spherical particles increases with increasing nickel content. The resulting microsphere particles also need to be treated delicately during the lithiation process because microsphere particles can be broken easily due to mechanical forces, such as the milling process.
In addition to coprecipitation, it was reported that the sol-gel, spray drying technique, and spray pyrolysis techniques can be adapted to produce micro-spherical particles. For spray methods, spherical particles were formed by the formation of spherical droplets using a droplet maker, such as nebulizer and spray nozzles (Kadota and Shirakawa, 2021). The resulting droplets can be tuned easily. Thus, a more homogenous and consistent particle morphology can be obtained. With the addition of additives to the spray solution, the produced particles can have a smooth morphology. Table 4 lists the effects of chelating agents for nickel-rich cathode materials on their morphology and electrochemical performance.
Table 4
Summary of Ni-rich cathode synthesis techniques toward its properties.
| Cathode materials |
Methods |
Chelating agents/Additives |
Morphological feature |
Electrochemical Performance (Capacity/Cycle/Rate) |
Reference |
| NCA |
Coprecipitation |
Ammonia |
Dense spherical particles, 5–12 μm |
196 mAh/g/95 % after 50 cycles/150 mAh/g at 2 C |
(Hu et al., 2012) |
| Coprecipitation |
Ammonia |
Dense spherical particle, 4–6 μm |
206 mAh/g/80 % after 50 cycles at 55 °C/~130 mAh/g at 5 C 55 °C |
(Kim et al., 2014) |
| Coprecipitation |
5-Sulfosalicylic acid |
Dense spherical particles, ~4–8 μm |
203 mAh/g/90 % after 200 cycles at 1 C/174 mAh/g at 1 C |
(Xie et al., 2015) |
| Coprecipitation |
EDTA |
Dense spherical particles, ~10 μm |
193 mAh/g/90 % after 100 cycles at 1 C/- |
(Xie et al., 2016) |
| Coprecipitation |
Ammonia |
Dense spherical particles, ~10 μm |
183 mAh/g/91 % after 100 cycles at 1 C/140 mAh/g at 5 C |
(Seo and Lee, 2017) |
| Spray pyrolysis |
Citric acid-Ethylene glycol-DCCA |
Spherical particles, ~1 μm |
218 mAh/g/100 % after 20 cycles at 0.1 C/- |
(Ju et al., 2010) |
| Spray pyrolysis |
|
Spherical/yolk shell particles, 1.1–1.6 μm |
225 mAh/g/89 % after 49 cycles at 0.2 C/129 mAh/g at 1 C |
(Yang et al., 2014) |
| Flame spray pyrolysis |
Urea |
Quasi-spherical |
124 mAh/g/81 % after 200 cycle at 0.5 C/- |
(Nurcahyani et al., 2020) |
| Spray drying |
Glycerol |
Spherical, 10–20 μm |
200 mAh/g/91 % after 200 cycles at 1 C/130 mAh/g at 5 C |
(Zhang J. et al., 2020) |
| Spray drying |
Citric acid |
Spherical and hollow particles, 10–50 μm |
188 mAh/g/94 % after 100 cycles at 0.5 C/- |
(Park and Chan Kang, 2014) |
| NMC-622 |
Coprecipitation |
Ammonia |
Dense spherical particles, 10–20 μm |
172 mAh/g/94 % after 100 cycles at 1 C/143 mAh/g at 10 C |
(Liang et al., 2014) |
| Coprecipitation |
Sodium lactate |
Quasi-spherical particles, ~4 μm |
201 mAh/g/78 % after 100 cycles at 0.1 C/~120 mAh/g at 5 C |
(Xu et al., 2018) |
| Coprecipitation |
Ammonia |
Quasi-spherical particles, < 8 μm |
155 mAh/g/96 % after 30 cycles at 0.5 C/- |
(Fu et al., 2011) |
| Coprecipitation |
Ammonia |
Quasi-spherical particles, < 1.2 μm |
186 mAh/g/95 % after 10 cycles at 0.5 C/~50 mAh/g at 5 C |
(Ronduda et al., 2020) |
| Sol-gel |
Citric acid |
Spherical particles, 800 nm |
174 mAh/g/87 % after 100 cycles at 1 C/~70 mAh/g at 10 C |
(Lee et al., 2016) |
| Spray pyrolysis |
|
Spherical particles, 5–13 μm |
— |
(Tian et al., 2018) |
| NMC-7 1.5 1.5 (LiNi0.7Mn0.15Co0.15O2) |
Coprecipitation |
Ammonia |
Dense spherical particles, 6–21 μm |
185 mAh/g/95 % after 50 cycles at 0.2 C/- |
(Lu et al., 2014) |
| Coprecipitation |
Ammonia |
near-spherical particles, 200–500 nm |
164 mAh/g/-/- |
(Zhang et al., 2012) |
NMC-712 (LiNi0.7Mn0.1Co0.2O2)
NMC-811 |
Sol-gel |
Citric acid |
Quasi-spherical with 300–800 nm primary particle |
205 mAh/g/86.24 % after 100 cycles/137 mAh/g at 5 C |
(Dong et al., 2019) |
| Coprecipitation |
Ammonia |
Dense spherical particles, 14–16 μm |
202 mAh/g/96 % after 200 cycles at 1 C/~54 mAh/g at 10 C |
(Duan et al., 2020) |
| Coprecipitation |
Ammonia + NH4F |
Dense spherical particles, ~10 μm |
202 mAh/g/94 % after 100 cycles at 2 C/~141 mAh/g at 10 C |
(Yue et al., 2013) |
| Coprecipitation-Spray Dry |
Ammonia |
Dense spherical particles, ~12 μm |
~175 mAh/g/86 % after 100 cycles at 1 C/~130 mAh/g at 10 C |
(Huang et al., 2019) |
| Flame spray pyrolysis |
|
Spherical particles, 1 μm |
178 mAh/g/85 % after 25 cycles at 1 C/- |
(Abram et al., 2019) |
| Hydro/Solvothermal |
Ethylene glycol |
Spherical particles, 6–8 μm |
201 mAh/g/81 % after 100 cycles at 0.1 C/142 mAh/g at 10 C |
(Gu et al., 2021) |
| Spray pyrolysis |
|
Submicron particles from spherical particle breakage |
197 mAh/g/93 % after 80 cycles at 1 C/173 mAh/g at 5 C |
(Li Yan et al., 2018) |
| NFA (LiNi0.8Fe0.15Al0.05O2) |
Coprecipitation |
Ammonia |
Spherical particle, ~8–10 μm |
190 mAh/g/88 % after 100 cycles at 0.33 C/110 mAh/g at 1 C |
(Muralidharan et al., 2020) |
| LNMO (LiNi0.9Mn0.1O2) |
Coprecipitation |
Ammonia |
Spherical particle, ~10 μm |
~220 mAh/g/86 % after 100 cycles at 0.5 C/~145 mAh/g at 10 C |
(Sun et al., 2013) |
| Coprecipitation |
Ammonia |
Spherical particle, ~3 μm |
197 mAh/g/94 % after 100 cycles at 0.2 C/- |
(Ma et al., 2020) |
| LNO (LiNiO2) |
Coprecipitation |
Ammonia |
Spherical particle |
216 mAh/g/80 % after 200 cycles at 1 C/121 mAh/g at 5 C |
(Mu et al., 2020) |
Based on Table 4 spherical shaped nickel-rich cathode materials have good electrochemical performances. However, the perfectly shaped spherical particle with high density and relatively large particle size has higher specific capacity, higher cycle retention and improved rate-ability. A High density and large secondary particle size of nickel-rich cathode material has better stability toward undesired chemical reaction between electrolyte, which sometimes has HF content, with the surface of the cathode particle that is related to its surface area. High density spherical particles with narrow particle size distribution also improve the overall density of the particle due to better packing factor. Thus, this type of particle has multiple benefits compared to the other shape particles.
According to multiple researches, spherical particle with narrow distribution can be obtained via coprecipitation method which tightly controlled its process parameters. Spherical particle with high tap density often achieved by a strict control of pH level, which depends on the composition of the metals, temperature of coprecipitation, flow rates, retention time and more importantly ageing process. Extensively large secondary particles can cause poor Li-ion transfer during the charge and discharge process. Thus, product optimization via control of particle size distribution is highly required knowledge for the development of nickel-rich cathode materials.
4.2.2 Non-spherical/agglomerated particles
Several studies have reported the formation of spherical Ni-rich cathode materials, as listed in Table 4. In microsphere particles, however, micro-cracks in secondary particles are often occurring. These micro-cracks increase exposure to the internal areas of the particles, which promote unwanted reactions causing rapid capacity fading (Kleiner et al., 2015; Yoon et al., 2017). Furthermore, they also increase the bulk density of the overall particles. Some studies stated that non-spherical particles are preferred to avoid the formation of particle grain cracks. However, non-spherical particles with a low density are not preferred because they will also suffer from particle breakage during the electrochemical charge-discharge process.
The formation of non-spherical/agglomerated nickel-rich cathode material particles is often found in the literature (Purwanto et al., 2020). Qiu et al. reported that polyhedral-shaped particles could be produced by oxalic acid crystallization of NCA cathode material produce (Qiu et al., 2017a; 2017b). Using the same crystallization/precipitating agent, Wu (Wu et al., 2015) claimed that micro-rod particles of NCA have better compaction factors than spherical particles, which can avoid micro-cracks even when it was cycled at high temperature. Zheng et al. precipitated Ni, Co, and Mn using ammonium oxalate, producing a rod-shaped precursor. After high sintering lithiation, however, the morphology of the precursor changed from rod to rock-like particles (Zeng and Zhang, 2020) A urea-assisted hydrothermal technique was developed to produce silkworm-like NMC811 particles, but slight particle cracking was observed (Fig. 5a,b,c) after the material was cycled 100 times (Lei et al., 2019b; Zhang et al., 2017). Therefore, micro-cracking and particle breakage would likely occur when repeatedly used especially at high rate and high operating temperature.

Micron-sized single crystals of Ni-rich cathode have been successfully developed. Single crystal has a large energy density owing to its pore-free and high strength (Duan et al., 2017; Li et al., 2018). This particle was pioneered because polycrystalline materials are prone to intergranular fracture during battery cell operation (Qian et al., 2020).
Although the polycrystalline particles are randomly oriented, this intergranular fracture is caused by primary particles pushing against each other, causing significant pressure (Langdon and Manthiram, 2021). The methods were used for the synthesis of precursors and cathode materials including co-precipitation, molten salt and spray pyrolysis (Table 5). Xing Xu et al. succeeded in producing radially aligned single-crystal hydroxide precursors of NMC 811. The two aspects examined were optimizing the ammonia concentration as a precipitating agent and the stirring speed. A high ammonia concentration (8 mol/L in this study) can adjust and promote the direction of growth of the primary particles. A high stirring speed can result in strong and homogeneous mechanical agitation, which prevents the nucleation and re-agglomeration of small secondary particles (Xu et al., 2019). Xinming Fan et al. examined the morphology of the precursor and NMC 831106 material after sintering, as shown in Fig. 6.
Table 5
Single crystal nickel-rich cathode materials.
| Sample |
Synthesis method |
Precursor |
T (°C) |
Particle size (μm) |
Specific capacity |
Ref. |
| NMC-761410 |
Coprecipitation and molten salt |
Hydroxide precursor |
900 |
3 |
182.3 mAh/g at 0.1 C |
(Bi et al., 2020) |
| NMC-811 |
Coprecipitation |
Hydroxide precursor |
740 |
— |
197.9 mAh/g at 0.1 C |
(Xu et al., 2019) |
| NMC-811 |
Spray pyrolysis |
Oxide precursor |
900 |
1–5 |
211 mAh/g at 0.1 C |
(Zhu et al., 2020) |
| NMC-811 |
Coprecipitation or molten salt |
|
850 |
1 |
— |
(Zhu and Chen, 2019) |
| NMC-811 |
Coprecipitation |
Hydroxide precursor |
830 |
1–4 |
— |
(Yang C. et al., 2020) |
| NMC-811 |
Commercial |
Hydroxide precursor |
800–960 |
1–1.5 |
194 mAh/g at 0.1 C |
(Zhao et al., 2019) |
| NMC-910603 |
Coprecipitation |
Hydroxide precursor |
820 |
1–2.5 |
203.8 mAh/g at 0.1 C |
(Lee et al., 2020) |
| NCA-880903 |
Coprecipitation |
Hydroxide precursor |
840 |
4–5 |
240 mAh/g at 0.1 C |
(Zhang H. et al., 2020) |
| LNO |
Commercial |
Hydroxide precursor |
800 |
— |
— |
(Weber et al., 2020) |

The NMC precursors showed a good secondary flower morphology. After sintering, the particles became micron-sized particles with a smooth surface (Fan et al., 2020). Another study, the synthesis of single-crystal Ni-rich with NaCl flux was carried out. NaCl functions as a growth flux for primary particles, reducing the temperature and sintering time (Bi et al., 2020; Lee et al., 2020). Jie Zhu et al. used spray pyrolysis method to produce a single crystal NMC 811. The precursor was synthesized from a chloride salt solution that was then aerosolized using an ultrasonic nebulizer and passed through a furnace tube at 800 °C. The microsphere, fine size, and mesoporous precursors were obtained from this method (Zhu et al., 2020).
Fig. 7 shows the effect of morphology of nickel-rich cathode towards its likability to pulverized during prolonged charge-discharge process. In contrast to nickel-based anode, over exposure of electrolyte with the surface of nickel-rich cathode materials can promote the formation of inactive phases due to the reaction of nickel with electrolyte. On the exposed surface created due to particle pulverization, a new SEI layer also occur which increase the impedance and Li-ion mobility resistance, thus lower the electrochemical properties. In Fig. 7, microcracks often occurs in a particle with visible agglomeration of primary particles.
5. Current advances and future projection
Applications of lithium-ion batteries that require high specific energy coupled with lower prices have encouraged the development of nickel-based electrodes. The successful of NCA and NMC materials, especially for electric vehicles, has made researchers constantly develop these materials. But, cobalt, which was originally doped for the material, is reconsidered because of its relatively high price. Thus, cobalt-free and nickel-rich materials were raised to be popular in the future. Various attempts were made to obtain superior material, especially for the morphology of the material. Currently, several efforts were determined to directly control the morphology of nickel containing particles using scalable, continuous and rapid method such as via fast drying, flash spray pyrolysis, vapor phase deposition, laser techniques, plasma assisted techniques, and fast crystallization. Thus, it opens a new opportunity to directly tune the morphology of nickel-based material that concern towards the overall feasibility and economical aspect (Purwanto et al., 2018; L. Wang et al., 2018).
It has been mentioned in the previous section that the morphology of the cathode material directly affects the electrochemical performance. Thus, efforts to get the most favorable morphology need to be done by modifying the synthesis parameters. First, doping modifications are carried out in order to make the morphology more regular (Ryu et al., 2019). Some dopant can affect the primary particle thereby increasing the stability of the cycle, previously assigned to cobalt. Second, the morphology for both cobalt-free and Ni-rich materials is single crystal. Materials with high nickel content have a limited life cycle due to their greater surface-electrolyte reactivity and lower structural integrity. Single-crystal proved to be able to limit the surface reactivity and cracking of these particles (Langdon and Manthiram, 2021). However, single crystal materials still face challenges in terms of irreversible structural degradation and longer ionic transport pathways. Small cracks in single crystal can also block Li+ transport (Wang et al., 2021). These challenges may be interesting to research for the future.
6. Conclusion
Thorough morphological analysis of nickel-based electrodes for Li-ion batteries was performed. Materials, such as nickel oxide, nickel hydroxide, nickel oxalates, and nickel sulfide, can be applied to Li-ion batteries. However, unique morphological features, such as shapes and nanoscale sizes of such materials, are strongly required. The nano-scaled unique morphology of the nickel-based anode can suppress the large lithium irreversibility and increase its electrical and electrochemical features.
Numerous studies on the morphological tuning of nickel-rich cathode materials have been reported. Among other morphologies, micron-sized spherical particles are largely applied and used, even commercialized in Li-ion batteries. However, spherical particles suffer from micro-cracking, which can cause rapid capacity decay and cell chemistry failures. Non-spherical secondary polycrystalline particles have also been studied; however, the particles still have micro-cracking problems. A crack-free single-crystalline morphology is currently the focus of research, and techniques to obtain such particles in a nickel-rich cathode are still under development due to several problems:
-
(1)
High-temperature sintering, which favors the formation of the rock salt phase and large Li-loss;
-
(2)
Flux induced synthesis, which reduces the contact between the oxidative atmosphere and is crucial for stable nickel-rich cathodes formation;
-
(3)
Post-production handling, such as milling and storage, is necessary in low humidity areas for a high-nickel content cathode.
In a morphological perspective, extensive morphological tuning for the race toward stable, long life cycle, nickel-based electrodes are being performed continuously and will always be considered for the next generation of secondary energy storage. The morphology of nickel-based active materials is one of the major determining factors for high-performance Li-ion batteries.
Acknowledgment
This paper is supported by Indonesia Endowment Fund for Education (LPDP / Lembaga Pengelola Dana Pendidikan) through Pendanaan Riset Inovatif Produk (Rispro) Invitasi grant no.PRJ-6/LPDP/2020.
References
- Abram C., Shan J., Yang X., Yan C., Steingart D., Ju Y., Flame aerosol synthesis and electrochemical characterization of Ni-rich layered cathode materials for Li-ion batteries, ACS Applied Energy Materials, 2 (2019) 1319–1329. DOI: 10.1021/acsaem.8b01892
- Barai P., Feng Z., Kondo H., Srinivasan V., Multiscale computational model for particle size evolution during coprecipitation of Li-ion battery cathode precursors, Journal of Physical Chemistry B, 123 (2019) 3291–3303. DOI: 10.1021/acs.jpcb.8b12004
- Bi Y., Tao J., Wu Y., Li L., Xu Y., Hu E., Wu B., Hu J., Wang C., Zhang J.G., Qi Y., Xiao J., Reversible planar gliding and microcracking in a single-crystalline Ni-rich cathode, Science, 370 (2020) 1313–1318. DOI: 10.1126/science.abc3167
- Chen T., Li X., Wang H., Yan X., Wang L., Deng B., Ge W., Qu M., The effect of gradient boracic polyanion-doping on structure, morphology, and cycling performance of Ni-rich LiNi0.8Co0.15Al0.05O2 cathode material, Journal of Power Sources, 374 (2018) 1–11. DOI: 10.1016/j.jpowsour.2017.11.020
- Dewangan A., Patel A., Bhadania A., Stainless steel for dairy and food industry: a review, Journal of Material Science & Engineering, 4 (2015) 1–4. DOI: 10.4172/2169-0022.1000191
- Dong S., Zhou Y., Hai C., Zeng J., Sun Y., Shen Y., Li X., Ren X., Qi G., Zhang X., Ma L., Ultrathin CeO2 coating for improved cycling and rate performance of Ni-rich layered LiNi0.7Co0.2Mn0.1O2 cathode materials, Ceramics International, 45 (2019) 144–152. DOI: 10.1016/j.ceramint.2018.09.145
- Du F., Li X., Wu L., Hu D., Zhou Q., Sun P., Xu T., Mei C., Hao Q., Fan Z., Zheng J., Tailoring the Al distribution in secondary particles for optimizing the electrochemical performance of LiNi0.8Co0.1Mn0.1O2, Ceramics International, (2021). DOI: 10.1016/j.ceramint.2021.01.161
- Du M., Li Q., Pang H., Oxalate-derived porous prismatic nickel/nickel oxide nanocomposites toward lithium-ion battery, Journal of Colloid and Interface Science, 580 (2020) 614–622. DOI: 10.1016/j.jcis.2020.07.009
- Du Y., Ma H., Guo M., Gao T., Li H., Random oriented hexagonal nickel hydroxide nanoplates grown on graphene as binder free anode for lithium ion battery with high capacity, Chemical Physics Letters, 699 (2018) 167–170. DOI: 10.1016/j.cplett.2018.03.056
- Duan J., Dong P., Wang D., Li X., Xiao Z., Zhang Y., Hu G., A facile structure design of LiNi0.90Co0.07Al0.03O2 as advanced cathode materials for lithium ion batteries via carbonation decomposition of NaAl(OH)4 solution, Journal of Alloys and Compounds, 739 (2018) 335–344. DOI: 10.1016/j.jallcom.2017.12.236
- Duan J., Hu G., Cao Y., Tan C., Wu C., Du K., Peng Z., Enhanced electrochemical performance and storage property of LiNi0.815Co0.15Al0.035O2 via Al gradient doping, Journal of Power Sources, 326 (2016) 322–330. DOI: 10.1016/j.jpowsour.2016.07.008
- Duan J., Wu C., Cao Y., Huang D., Du K., Peng Z., Hu G., Enhanced compacting density and cycling performance of Ni-riched electrode via building mono dispersed micron scaled morphology, Journal of Alloys and Compounds, 695 (2017) 91–99. DOI: 10.1016/j.jallcom.2016.10.158
- Duan J., Zhang R., Zhu Q., Xiao H., Huang Q., The effect of controlling strategies of pH and ammonia concentration on preparing full concentration gradient Ni0.8Co0.1Mn0.1(OH)2 via coprecipitation in a pilot-scale reactor, Energy Technology, 8 (2020) 1–12. DOI: 10.1002/ente.201901437
- Elbert P., Onder C., Gisler H.J., Capacitors vs. batteries in a serial hybrid electric bus, IFAC Proceedings Volumes (IFAC-PapersOnline), 43 (2010) 252–257. DOI: 10.3182/20100712-3-DE-2013.00020
- Fan X., Hu G., Zhang B., Ou X., Zhang J., Zhao W., Jia H., Zou L., Li P., Yang Y., Crack-free single-crystalline Ni-rich layered NCM cathode enable superior cycling performance of lithium-ion batteries, Nano Energy, 70 (2020) 104450. DOI: 10.1016/j.nanoen.2020.104450
- Fu C., Li G., Luo D., Li Q., Fan J., Li L., Nickel-rich layered microspheres cathodes: lithium/nickel disordering and electrochemical performance, ACS Applied Materials and Interfaces, 6 (2014) 15822–15831. DOI: 10.1021/am5030726
- Fu C., Zhou Z., Liu Y., Zhang Q., Zheng Y., Li G., Synthesis and electrochemical properties of Mg-doped LiNi0.6Co0.2Mn0.2O2 cathode materials for li-ion battery, Journal Wuhan University of Technology, Materials Science Edition, 26 (2011) 211–215. DOI: 10.1007/s11595-011-0199-z
- Geng H., Kong S.F., Wang Y., NiS nanorod-assembled nanoflowers grown on graphene: morphology evolution and Li-ion storage applications, Journal of Materials Chemistry A, 2 (2014) 15152–15158. DOI: 10.1039/c4ta03440f
- Gu H., Wang J., Wang Z., Tong J., Qi N., Han G., Zhang M., Self-assembled porous LiNi0.8Co0.1Mn0.1O2 cathode materials with micro/nano-layered hollow morphologies for high-power lithium-ion batteries, Applied Surface Science, 539 (2021) 148034. DOI: 10.1016/j.apsusc.2020.148034
- Hu G., Liu W.M., Peng Z., Dua K., Cao Y.B., Du K., Cao Y.B., Synthesis and electrochemical properties of LiNi0.8Co0.15Al0.05O2 prepared from the precursor Ni0.8Co0.15Al0.05OOH, Journal of Power Sources, 198 (2012) 258–263. DOI: 10.1016/j.jpowsour.2011.09.101
- Huang B., Li X., Wang Z., Guo H., Xiong X., Synthesis of Mg-doped LiNi0.8Co0.15Al0.05O2 oxide and its electrochemical behavior in high-voltage lithium-ion batteries, Ceramics International, 40 (2014) 13223–13230. DOI: 10.1016/j.ceramint.2014.05.029
- Huang B., Liu D., Zhang L., Qian K., Zhou K., Cai X., Kang F., Li B., An efficient synthetic method to prepare high-performance Ni-rich LiNi0.8Co0.1Mn0.1O2 for lithium-ion batteries, ACS Applied Energy Materials, 2 (2019) 7403–7411. DOI: 10.1021/acsaem.9b01414
- Huang X.H., Tu J.P., Xia X.H., Wang X.L., Xiang J.Y., Zhang L., Zhou Y., Morphology effect on the electrochemical performance of NiO films as anodes for lithium ion batteries, Journal of Power Sources, 188 (2009) 588–591. DOI: 10.1016/j.jpowsour.2008.11.111
- Hwang I., Lee C.W., Kim J.C., Yoon S., Particle size effect of Ni-rich cathode materials on lithium ion battery performance, Materials Research Bulletin, 47 (2012) 73–78. DOI: 10.1016/j.materresbull.2011.10.002
- Ju S.H., Kim J.H., Kang Y.C., Electrochemical properties of LiNi0.8Co0.2−xAlxO2 (0 ≤ x ≤ 0.1) cathode particles prepared by spray pyrolysis from the spray solutions with and without organic, Metals and Materials International, 16 (2010) 299–303. DOI: 10.1007/s12540-010-0421-0
- Kadota K., Shirakawa Y., Particle preparation and morphology control with mutual diffusion across liquid-liquid interfaces, KONA Powder and Particle Journal, 38 (2021) 122–135. DOI: 10.14356/kona.2021006
- Kalyani P., Kalaiselvi N., Various aspects of LiNiO2 chemistry: a review, Science and Technology of Advanced Materials, 6 (2005) 689–703. DOI: 10.1016/j.stam.2005.06.001
- Kim C., Park T.-J., Min S.-G., Yang S.-B., Son J.-T., Effects of iron doping at 55 °C on LiNi0.85Co0.10Al0.05O2, Journal of the Korean Physical Society, 65 (2014) 243–247. DOI: 10.3938/jkps.65.243
- Kleiner K., Dixon D., Jakes P., Melke J., Yavuz M., Roth C., Nikolowski K., Liebau V., Ehrenberg H., Fatigue of LiNi0.8Co0.15Al0.05O2 in commercial Li ion batteries, Journal of Power Sources, 273 (2015) 70–82. DOI: 10.1016/j.jpowsour.2014.08.133
- Langdon J., Manthiram A., A perspective on single-crystal layered oxide cathodes for lithium-ion batteries, Energy Storage Materials, 37 (2021) 143–160. DOI: 10.1016/j.ensm.2021.02.003
- Lee J.H., Kim H.H., Wee S.B., Paik U., Effect of additives on the dispersion properties of aqueous based C/LiFePO4 paste and its impact on lithium ion battery high power properties, KONA Powder and Particle Journal, 27 (2009) 239–245. DOI: 10.14356/kona.2009022
- Lee S.H., Sim S.J., Jin B.S., Kim H.S., High performance well-developed single crystal LiNi0.91Co0.06Mn0.03O2 cathode via LiCl-NaCl flux method, Materials Letters, 270 (2020) 127615. DOI: 10.1016/j.matlet.2020.127615
- Lee S.W., Kim H., Kim M.S., Youn H.C., Kang K., Cho B.W., Roh K.C., Kim K.B., Improved electrochemical performance of LiNi0.6Co0.2Mn0.2O2 cathode material synthesized by citric acid assisted sol-gel method for lithium ion batteries, Journal of Power Sources, 315 (2016) 261–268. DOI: 10.1016/j.jpowsour.2016.03.020
- Lei Y., Ai J., Yang S., Jiang H., Lai C., Xu Q., Effect of flower-like Ni(OH)2 precursors on Li+/Ni2+ cation mixing and electrochemical performance of nickel-rich layered cathode, Journal of Alloys and Compounds, 797 (2019a) 421–431. DOI: 10.1016/j.jallcom.2019.05.065
- Lei Y., Ai J., Yang S., Lai C., Xu Q., Nb-doping in LiNi0.8Co0.1Mn0.1O2 cathode material: Effect on the cycling stability and voltage decay at high rates, Journal of the Taiwan Institute of Chemical Engineers, 97 (2019b) 255–263. DOI: 10.1016/j.jtice.2019.02.006
- Li B., Gu P., Feng Y., Zhang G., Huang K., Xue H., Pang H., Ultrathin nickel–cobalt phosphate 2D nanosheets for electrochemical energy storage under aqueous/solid-state electrolyte, Advanced Functional Materials, 27 (2017) 1605784. DOI: 10.1002/adfm.201605784
- Li C., Xue Z., Qin J., Sawangphruk M., Yu P., Zhang X., Liu R., Synthesis of nickel hydroxide/delaminated-Ti3C2 MXene nanosheets as promising anode material for high performance lithium ion battery, Journal of Alloys and Compounds, 842 (2020) 155812. DOI: 10.1016/j.jallcom.2020.155812
- Li F., Kong L., Sun Y., Jin Y., Hou P., Micron-sized monocrystalline LiNi1/3Co1/3Mn1/3O2 as high-volumetric-energy-density cathode for lithium-ion batteries, Journal of Materials Chemistry A, 6 (2018) 12344–12352. DOI: 10.1039/c8ta03363c
- Li J., Liu M., An J., Tian P., Tang C., Jia T., Butt F.K., Yu D., Bai W., Cao C., Feng X., The synergism of nanoplates with habit-tuned crystal and substitution of cobalt with titanium in Ni-rich LiNi0.80Co0.15Al0.05O2 cathode for lithium-ion batteries, Journal of Alloys and Compounds, 829 (2020) 154555. DOI: 10.1016/j.jallcom.2020.154555
- Li Yan, Li X., Wang Z., Guo H., Wang J., Spray pyrolysis synthesis of nickel-rich layered cathodes LiNi1−2x Cox Mnx O2 (x = 0.075, 0.05, 0.025) for lithium-ion batteries, Journal of Energy Chemistry, 27 (2018) 447–450. DOI: 10.1016/j.jechem.2017.11.025
- Li Y., Pan G., Xu W., Yao J., Zhang L., Effect of Al substitution on the microstructure and lithium storage performance of nickel hydroxide, Journal of Power Sources, 307 (2016) 114–121. DOI: 10.1016/j.jpowsour.2015.12.129
- Li Yanwei, Xu W., Xie Z., Zhang L., Yao J., Structure and lithium storage performances of nickel hydroxides synthesized with different nickel salts, Ionics, 23 (2017) 1625–1636. DOI: 10.1007/s11581-017-1983-3
- Liang L., Du K., Peng Z., Cao Y., Duan J., Jiang J., Hu G., Co-precipitation synthesis of Ni0.6Co0.2Mn0.2(OH)2 precursor and characterization of LiNi0.6Co0.2Mn0.2O2 cathode material for secondary lithium batteries, Electrochimica Acta, 130 (2014) 82–89. DOI: 10.1016/j.electacta.2014.02.100
- Liu W., Hua W., Zheng Z., Zhong B., Zhang Z., Facile synthesis of hierarchical porous Ni-rich LiNi0.6Co0.2Mn0.2O2 cathode material with superior high-rate capability, Ionics, 22 (2016) 1781–1790. DOI: 10.1007/s11581-016-1710-5
- Liu Y., Yao W., Lei C., Zhang Q., Zhong S., Yan Z., Ni-rich oxide LiNi0.85Co0.05Mn0.1O2 for lithium ion battery: effect of microwave radiation on its morphology and electrochemical property, Journal of The Electrochemical Society, 166 (2019) A1300–A1309. DOI: 10.1149/2.0151908jes
- Long P., Xu Q., Peng G., Yao X., Xu X., NiS nanorods as cathode materials for all-solid-state lithium batteries with excellent rate capability and cycling stability, ChemElectroChem, 3 (2016) 764–769. DOI: 10.1002/celc.201500570
- Lu X., Zhang N., Jahn M., Pfleging W., Improved capacity retention of SiO2 -coated LiNi0.6Mn0.2Co0.2O2 cathode material for lithium-ion batteries, Applied Sciences, 9 (2019) 3671. DOI: 10.3390/app9183671
- Lu Z.G., Tan X.X., Tang Y.G., Zhou K.C., LiNi0.7Co0.15Mn0.15O2 microspheres as high-performance cathode materials for lithium-ion batteries, Rare Metals, 33 (2014) 608–614. DOI: 10.1007/s12598-013-0097-y
- Ma L., Su L., Zhang J., Zhao D., Qin C., Jin Z., Zhao K., A controllable morphology GO/PANI/metal hydroxide composite for supercapacitor, Journal of Electroanalytical Chemistry, 777 (2016) 75–84. DOI: 10.1016/j.jelechem.2016.07.033
- Ma R., Zhao Z., Fu J., Lv H., Li C., Wu B., Mu D., Wu F., Tuning cobalt-free nickel-rich layered LiNi0.9Mn0.1O2 cathode material for lithium-ion batteries, ChemElectroChem, 7 (2020) 2637–2642. DOI: 10.1002/celc.202000443
- Ma Z., Yuan X., Zhang Z., Mei D., Li L., Ma Z.F., Zhang L., Yang J., Zhang J., Novel flower-like nickel sulfide as an efficient electrocatalyst for non-aqueous lithium-air batteries, Scientific Reports, 5 (2015) 1–9. DOI: 10.1038/srep18199
- Mi L., Chen Y., Wei W., Chen W., Hou H., Zheng Z., Large-scale urchin-like micro/nano-structured NiS: controlled synthesis, cation exchange and lithium-ion battery applications, RSC Advances, 3 (2013) 17431–17439. DOI: 10.1039/c3ra42859a
- Mo Y., Guo L., Jin H., Du B., Cao B., Chen Yigao, Li D., Chen Yong, Building nickel-rich cathodes with large concentration gradient for high performance lithium-ion batteries, Journal of Power Sources, 468 (2020) 228405. DOI: 10.1016/j.jpowsour.2020.228405
- Mu L., Kan W.H., Kuai C., Yang Z., Li L., Sun C.J., Sainio S., Avdeev M., Nordlund D., Lin F., Structural and electrochemical impacts of mg/mn dual dopants on the LiNiO2 cathode in Li-metal batteries, ACS Applied Materials and Interfaces, 12 (2020) 12874–12882. DOI: 10.1021/acsami.0c00111
- Muralidharan N., Essehli R., Hermann R.P., Parejiya A., Amin R., Bai Y., Du Z., Belharouak I., LiNixFeyAlzO2, a new cobalt-free layered cathode material for advanced Li-ion batteries, Journal of Power Sources, 471 (2020) 228389. DOI: 10.1016/j.jpowsour.2020.228389
- Nam K.M., Kim H.J., Kang D.H., Kim Y.S., Song S.W., Ammonia-free coprecipitation synthesis of a Ni-Co-Mn hydroxide precursor for high-performance battery cathode materials, Green Chemistry, 17 (2015) 1127–1135. DOI: 10.1039/c4gc01898b
- Ni S., Li T., Lv X., Yang X., Zhang L., Designed constitution of NiO/Ni nanostructured electrode for high performance lithium-ion battery, Electrochimica Acta, 91 (2013) 267–274. DOI: 10.1016/j.electacta.2012.12.113
- Nitta N., Wu F., Lee J.T., Yushin G., Li-ion battery materials: present and future, Materials Today, 18 (2015) 252–264. DOI: 10.1016/j.mattod.2014.10.040
- Noh H.J., Chen Z., Yoon C.S., Lu J., Amine K., Sun Y.K., Cathode material with nanorod structure—an application for advanced high-energy and safe lithium batteries, Chemistry of Materials, 25 (2013) 2109–2115. DOI: 10.1021/cm4006772
- Noh H.J., Ju J.W., Sun Y.K., Comparison of nanorod-structured Li[Ni0.54Co0.16Mn0.30]O2 with conventional cathode materials for li-ion batteries, ChemSusChem, 7 (2014) 245–252. DOI: 10.1002/cssc.201300379
- Nurcahyani C., Anjani A.E., Purwanto A., Yudha C.S., Hasanah L.M., Dyartanti E.R., Nur A., Flame-assisted spray pyrolysis of lithium nickel cobalt aluminum oxide leaching stream, AIP Conference Proceedings, 2219 (2020) 030003. DOI: 10.1063/5.0003156
- Oh H.J., Jo C.H., Yoon C.S., Yashiro H., Kim S.J., Passerini S., Sun Y.K., Myung S.T., Nickel oxalate dihydrate nanorods attached to reduced graphene oxide sheets as a high-capacity anode for rechargeable lithium batteries, NPG Asia Materials, 8 (2016) e270. DOI: 10.1038/am.2016.59
- Park G.D., Chan Kang Y., Characteristics of precursor powders of a nickel-rich cathode material prepared by a spray drying process using water-soluble metal salts, RSC Advances, 4 (2014) 44203–44207. DOI: 10.1039/c4ra08524h
- Purwanto A., Yudha C.S., Ikhwan Muhammad K., Algifari B.G., Widiyandari H., Sutopo W., Synthesis of LiNi0.8Co0.15Al0.05O2 cathode material via flame-assisted spray pyrolysis method, Advanced Powder Technology, 31 (2020) 1674–1681. DOI: 10.1016/j.apt.2020.01.035
- Purwanto A., Yudha C.S., Ubaidillah U., Widiyandari H., Ogi T., NCA cathode material: synthesis methods and performance enhancement efforts NCA cathode material: synthesis methods and performance enhancement efforts, Materials Research Express, 5 (2018) 122001. DOI: 10.1088/2053-1591/aae167
- Qian G., Zhang Y., Li L., Zhang R., Xu J., Cheng Z., Xie S., Wang H., Rao Q., He Y., Shen Y., Chen L., Tang M., Ma Z., Single-crystal nickel-rich layered-oxide battery cathode materials: synthesis, electrochemistry, and intra-granular fracture, Energy Storage Materials, 27 (2020) 140–149. DOI: 10.1016/j.ensm.2020.01.027
- Qiu H., Du T., Wu J., Wang Y., Liu J., Ye S., Liu S., Towards deriving Ni-rich cathode and oxide-based anode materials from hydroxides by sharing a facile co-precipitation method, Dalton Transactions, 47 (2018) 6934–6941. DOI: 10.1039/c8dt00893k
- Qiu Z., Zhang Y., Wang D., Xia S., A ternary oxide precursor with trigonal structure for synthesis, Solid State Electrochem, 21 (2017a) 3037–3046. DOI: 10.1007/s10008-017-3643-y
- Qiu Z., Zhang Y., Xia S., Yao Y., A facile method for synthesis of LiNi 0.8Co0.15Al0.05O2 cathode material, Solid State Ionics, 307 (2017b) 73–78. DOI: 10.1016/j.ssi.2017.04.011
- Ronduda H., Zybert M., Szczęsna-Chrzan A., Trzeciak T., Ostrowski A., Szymański D., Wieczorek W., Raróg-Pilecka W., Marcinek M., On the sensitivity of the Ni-rich layered cathode materials for li-ion batteries to the different calcination conditions, Nanomaterials, 10 (2020) 1–21. DOI: 10.3390/nano10102018
- Ryu H.H., Park K.J., Yoon D.R., Aishova A., Yoon C.S., Sun Y.K., Li[Ni0.9Co0.09W0.01]O2: a new type of layered oxide cathode with high cycling stability, Advanced Energy Materials, 9 (2019) 1–7. DOI: 10.1002/aenm.201902698
- Sebastian S.T., Jagan R.S., Rajagoplan R., Paravannoor A., Menon L.V., Subramanian K.R.V., Nair S.V., Balakrishnan A., Lithium-ion storage performance of camphoric carbon wrapped NiS nano/micro-hybrids, RSC Advances, 4 (2014) 11673–11679. DOI: 10.1039/c4ra00176a
- Seo J., Lee J., Fast growth of the precursor particles of Li(Ni0.8Co0.16Al0.04)O2 via a carbonate co-precipitation route and its electrochemical performance, Journal of Alloys and Compounds, 694 (2017) 703–709. DOI: 10.1016/j.jallcom.2016.10.062
- Shi J., Du N., Zheng W., Li X., Dai Y., He G., Ultrathin Ni-Co double hydroxide nanosheets with conformal graphene coating for highly active oxygen evolution reaction and lithium ion battery anode materials, Chemical Engineering Journal, 327 (2017) 9–17. DOI: 10.1016/j.cej.2017.06.080
- Sonia T.S., Anjali P., Roshny S., Lakshmi V., Ranjusha R., Subramanian K.R.V., Nair S.V., Balakrishnan A., Nano/micro-hybrid NiS cathodes for lithium ion batteries, Ceramics International, 40 (2014) 8351–8356. DOI: 10.1016/j.ceramint.2014.01.041
- Su Y., Chen G., Chen L., Li W., Zhang Q., Yang Z., Lu Y., Bao L., Tan J., Chen R., Chen S., Wu F., Exposing the {010} planes by oriented self-assembly with nanosheets to improve the electrochemical performances of Ni-Rich Li[Ni0.8Co0.1Mn0.1] O2 microspheres, ACS Applied Materials and Interfaces, 10 (2018) 6407–6414. DOI: 10.1021/acsami.7b18933
- Sun H.H., Weeks J.A., Heller A., Mullins C.B., Nanorod gradient cathode: preventing electrolyte penetration into cathode particles, ACS Applied Energy Materials, 2 (2019) 6002–6011. DOI: 10.1021/acsaem.9b01116
- Sun Y.K., Lee D.J., Lee Y.J., Chen Z., Myung S.T., Cobalt-free nickel rich layered oxide cathodes for lithium-ion batteries, ACS Applied Materials and Interfaces, 5 (2013) 11434– 11440. DOI: 10.1021/am403684z
- Tang Z.F., Wu R., Huang P.F., Wang Q.S., Chen C.H., Improving the electrochemical performance of Ni-rich cathode material LiNi0.815Co0.15Al0.035O2 by removing the lithium residues and forming Li3PO4 coating layer, Journal of Alloys and Compounds, 693 (2017) 1157–1163. DOI: 10.1016/j.jallcom.2016.10.099
- Tian C., Nordlund D., Xin H.L., Xu Y., Liu Y., Sokaras D., Lin F., Doeff M.M., Depth-dependent redox behavior of LiNi0.6Mn0.2Co0.2O2, Journal of The Electrochemical Society, 165 (2018) A696–A704. DOI: 10.1149/2.1021803jes
- Tian J., Su Y., Wu F., Xu S., Chen F., Chen R., Li Q., Li J., Sun F., Chen S., High-rate and cycling-stable nickel-rich cathode materials with enhanced Li+ diffusion pathway, ACS Applied Materials and Interfaces, 8 (2016) 582–587. DOI: 10.1021/acsami.5b09641
- Toprakci O., Toprakci H.A.K., Ji L., Zhang X., Fabrication and electrochemical characteristics of LiFePO4 powders for lithium-ion batteries, KONA Powder and Particle Journal, 28 (2010) 50–73. DOI: 10.14356/kona.2010008
- Wang L., Sun Juncai, Sun Jing, Hydrothermal synthesis of nanostructured NiS and its optimisation as cathode material for rechargeable lithium battery, International Journal of Nanomanufacturing, 9 (2013) 303–310. DOI: 10.1504/IJNM.2013.056056
- Wang L., Zhang G., Liu Q., Duan H., Recent progress in Zn-based anodes for advanced lithium-ion batteries, Materials Chemistry Frontiers, 2 (2018) 1414–1435. DOI: 10.1039/c8qm00125a
- Wang Q., Zhang L., Zhao P., Du Z., Facile synthesis of a high-capacity LiNi0.8Co0.15Al0.05O2 nanoplate cathode with a 010 orientation for lithium-ion batteries, International Journal of Electrochemical Science, 13 (2018) 10382–10389. DOI: 10.20964/2018.11.15
- Wang X., Li X., Sun X., Li F., Liu Q., Wang Q., He D., Nanostructured NiO electrode for high-rate Li-ion batteries, Journal of Materials Chemistry, 21 (2011) 3571–3573. DOI: 10.1039/c0jm04356g
- Wang Y., Wang E., Zhang X., Yu H., High-voltage “single-crystal” cathode materials for lithium-ion batteries, Energy and Fuels, 35 (2021) 1918–1932. DOI: 10.1021/acs.energyfuels.0c03608
- Weber R., Li H., Chen W., Kim C.-Y., Plucknett K., Dahn J.R., In situ XRD studies during synthesis of single-crystal LiNiO2, LiNi0.975Mg0.025O2, and LiNi0.95Al0.05O2 cathode materials, Journal of The Electrochemical Society, 167 (2020) 100501. DOI: 10.1149/1945-7111/ab94ef
- Wei S., Di Lecce D., Brescia R., Pugliese G., Shearing P.R., Hassoun J., Electrochemical behavior of nanostructured NiO@C anode in a lithium-ion battery using LiNi⅓Co⅓Mn⅓O2 cathode, Journal of Alloys and Compounds, 844 (2020) 155365. DOI: 10.1016/j.jallcom.2020.155365
- Wu N., Wu H., Yuan W., Liu S., Liao J., Zhang Y., Facile synthesis of one-dimensional LiNi0.8Co0.15Al0.05O2 microrods as advanced cathode materials for lithium ion batteries, Journal of Materials Chemistry A, 3 (2015) 13648–13652. DOI: 10.1039/C5TA02767E
- Xie H., Du K., Hu G., Duan J., Peng Z., Zhang Z., Cao Y., Synthesis of LiNi0.8Co0.15Al0.05O2 with 5-sulfosalicylic acid as a chelating agent and its electrochemical properties, Journal of Materials Chemistry A, 3 (2015) 1–21. DOI: 10.1039/C5TA05266A
- Xie H., Hu G., Du K., Peng Z., Cao Y., An improved continuous co-precipitation method to synthesize LiNi0.80Co0.15Al0.05O2 cathode material, Journal of Alloys and Compounds, 666 (2016) 84–87. DOI: 10.1016/j.jallcom.2016.01.064
- Xu L., Zhou F., Kong J., Chen Z., Chen K., Synthesis of Li(Ni0.6Co0.2Mn0.2)O2 with sodium DL-lactate as an eco-friendly chelating agent and its electrochemical performances for lithium-ion batteries, Ionics, 24 (2018) 2261–2273. DOI: 10.1007/s11581-017-2363-8
- Xu X., Huo H., Jian J., Wang L., Zhu H., Xu S., He X., Yin G., Du C., Sun X., Radially oriented single-crystal primary nanosheets enable ultrahigh rate and cycling properties of LiNi0.8Co0.1 Mn 0.1O2 cathode material for lithium-ion batteries, Advanced Energy Materials, 9 (2019) 1–9. DOI: 10.1002/aenm.201803963
- Yan W., Yang S., Huang Y., Yang Y., Guohui Yuan, A review on doping/coating of nickel-rich cathode materials for lithium-ion batteries, Journal of Alloys and Compounds, 819 (2020) 153048. DOI: 10.1016/j.jallcom.2019.153048
- Yang C., Zhu Z., Wei W., Zhou L., Superior cycle stability of single crystal nickel-rich layered oxides with micron-scale grain size as cathode material for lithium-ion batteries, International Journal of Electrochemical Science, 15 (2020) 5031–5041. DOI: 10.20964/2020.06.03
- Yang G., Qin X., Wang B., Cai F., Gao J., Well-ordered spherical LiNi0.8Co0.1Mn0.1O2 cathode material for lithium-ion batteries, Journal of Materials Research, 35 (2020) 51–57. DOI: 10.1557/jmr.2019.307
- Yang H., Liu P., Chen Q., Liu X., Lu Y., Xie S., Ni L., Wu X., Peng M., Chen Yanbin, Tang Y., Chen Yanfeng, Fabrication and characteristics of high-capacity LiNi0.8Co0.15Al0.05O2with monodisperse yolk–shell spherical precursors by a facile method, RSC Advances, 4 (2014) 35522. DOI: 10.1039/c4ra04122d
- Yang X., Tang Y., Zheng J., Shang G., Wu J., Lai Y., Li J., Zhang Z., Tailoring structure of Ni-rich layered cathode enable robust calendar life and ultrahigh rate capability for lithium-ion batteries, Electrochimica Acta, 320 (2019) 134587. DOI: 10.1016/j.electacta.2019.134587
- Ye Z., Qiu L., Yang W., Wu Z., Liu Y., Wang G., Song Y., Zhong B., Guo X., Nickel-rich layered cathode materials for lithium-ion batteries, Chemistry – A European Journal, 27 (2021) 4249–4269. DOI: 10.1002/chem.202003987
- Yoon C.S., Jun D.W., Myung S.T., Sun Y.K., Structural stability of LiNiO2 cycled above 4.2 v, ACS Energy Letters, 2 (2017) 1150–1155. DOI: 10.1021/acsenergylett.7b00304
- Yudha C.S., Muzayanha S.U., Hendri W., Iskandar F., Sutopo W., Purwanto A., Widiyandari H., Synthesis of LiNi0.85Co0.14Al0.01O2 cathode material and its performance in an NCA / graphite full-battery, Energies, 12 (2019) 1886. DOI: 10.3390/en12101886
- Yue P., Wang Z., Wang J., Guo H., Xiong X., Li X., Effect of fluorine on the electrochemical performance of spherical LiNi0.8Co0.1Mn0.1O2 cathode materials via a low temperature method, Powder Technology, 237 (2013) 623–626. DOI: 10.1016/j.powtec.2012.12.061
- Zeng T., Zhang C., An effective way of co-precipitating Ni2+, Mn2+ and Co2+ by using ammonium oxalate as precipitant for Ni-rich Li-ion batteries cathode, Journal of Materials Science, 55 (2020) 11535–11544. DOI: 10.1007/s10853-020-04753-w
- Zhang C., Qi J., Zhao H., Hou H., Deng B., Tao S., Su X., Wang Z., Qian B., Chu W., Facile synthesis silkworm-like Ni-rich layered LiNi0.8Co0.1Mn0.1O2 cathode material for lithium-ion batteries, Materials Letters, 201 (2017) 1–4. DOI: 10.1016/j.matlet.2017.04.121
- Zhang H., Yang S., Huang Y., Xianhua H., Synthesis of non-spherical LiNi0.88Co0.09Al0.03O2 cathode material for lithium-ion batteries, Energy & Fuels, 34 (2020) 9002–9010. DOI: 10.1021/acs.energyfuels.0c01692
- Zhang J., Xu S., Hamad K.I., Jasim A.M., Xing Y., High retention rate NCA cathode powders from spray drying and flame assisted spray pyrolysis using glycerol as the solvent, Powder Technology, 363 (2020) 1–6. DOI: 10.1016/j.powtec.2019.12.057
- Zhang L., Dong T., Yu X., Dong Y., Zhao Z., Li H., Synthesis and electrochemical performance of LiNi0.7Co0.15Mn0.15O2 as gradient cathode material for lithium batteries, Materials Research Bulletin, 47 (2012) 3269–3272. DOI: 10.1016/j.materresbull.2012.08.002
- Zhang Q., Su Y., Chen L., Lu Y., Bao L., He T., Wang J., Chen R., Tan J., Wu F., Pre-oxidizing the precursors of Nickel-rich cathode materials to regulate their Li+/Ni2+ cation ordering towards cyclability improvements, Journal of Power Sources, 396 (2018) 734–741. DOI: 10.1016/j.jpowsour.2018.06.091
- Zhao Z., Huang B., Wang M., Yang X., Gu Y., Facile synthesis of fluorine doped single crystal Ni-rich cathode material for lithium-ion batteries, Solid State Ionics, 342 (2019) 115065. DOI: 10.1016/j.ssi.2019.115065
- Zhu J., Chen G., Single-crystal based studies for correlating the properties and high-voltage performance of Li[NixMnyCo1−x−y]O2 cathodes, Journal of Materials Chemistry A, 7 (2019) 5463–5474. DOI: 10.1039/c8ta10329a
- Zhu J., Zheng J., Cao G., Li Y., Zhou Y., Deng S., Hai C., Flux-free synthesis of single-crystal LiNi0.8Co0.1Mn0.1O2 boosts its electrochemical performance in lithium batteries, Journal of Power Sources, 464 (2020) 228207. DOI: 10.1016/j.jpowsour.2020.228207
- Zhu L.Z., Han E.S., Cao J.L., Synthesis and characteristics of NiS for cathode of lithium ion batteries, Advanced Materials Research, 236–238 (2011) 694–697. DOI: 10.4028/www.scientific.net/AMR.236-238.694
- Zhu Q., Xiao H., Zhang R., Geng S., Huang Q., Effect of impeller type on preparing spherical and dense Ni1−x−yCoxMny(OH)2 precursor via continuous co-precipitation in pilot scale: a case of Ni0·6Co0·2Mn0·2(OH)2, Electrochimica Acta, 318 (2019) 1–13. DOI: 10.1016/j.electacta.2019.06.008
- Zhu X., Zhong Y., Zhai H., Yan Z., Li D., Nanoflake nickel hydroxide and reduced graphene oxide composite as anode materials for high-capacity lithium ion batteries, Electrochimica Acta, 132 (2014) 364–369. DOI: 10.1016/j.electacta.2014.03.132
- Zuniga L., Gonzalez G., Chavez R.O., Myers J.C., Lodge T.P., Alcoutlabi M., Centrifugally spun α-Fe2O3/TiO2/carbon composite fibers as anode materials for lithium-ion batteries, Applied Sciences, 9 (2019) 4032. DOI: 10.3390/app9194032
Authors’ Short Biographies
Agus Purwanto

Agus Purwanto is a Professor at Chemical Department Sebelas Maret University, Indonesia. He received his Ph.D. degree in Chemical Engineering from Hiroshima University, Japan. His research interest is in the synthesis and characterization of functional materials for energy storage and conversion. One of the research interests is the development li-ion battery materials, optimization and its application.
Shofirul Sholikhatun Nisa

Shofirul Sholikhatun Nisa received her bachelor’s degree in 2019 from the Chemical Engineering Department, Universitas Sebelas Maret. She continued her master’s studies at the same department and university and is now in final year. She is very interested in the field of energy storage especially Li-ion batteries. Currently, her research focus is on the synthesis of NMC materials, especially Ni-rich materials.
Ike Puji Lestari

Ike Puji Lestari is a master student at the Department of Chemical Engineering, Universitas Sebelas Maret. Previously she received her bachelor’s degree in the same department in 2019. Currently she is working on a novel silicon based anode material for Li-ion batteries.
Muhammad Nur Ikhsanudin

Muhammad Nur Ikhsanudin received his bachelor’s degree in 2020 from the Chemical Engineering Department, Universitas Sebelas Maret. He continued his master’s studies at the same department and university and is now in first year. He is very interested in the field of energy storage especially Li-ion batteries. Currently, His research focus is on the anode-free Li-ion batteries.
Cornelius Satria Yudha

Cornelius Satria Yudha received his bachelor degree (2015) and master degree (2019) in chemical engineering, Universitas Sebelas Maret. Currently, He is a young researcher at University Centre of Excellence for Electrical Storage Technology (CE-FEEST), Universitas Sebelas Maret. His research interest is hydrometallurgical processing of novel material for state-of-the-art secondary energy storage development.
Hendri Widiyandari

Hendri Widiyandari is a lecturer at Physics Department Sebelas Maret University, Indonesia, since 2018. Before that, she was a lecturer at the Department of Physics, Diponegoro University, Indonesia, from 1999 until 2017. She received her Ph.D. degree in Chemical Engineering from Hiroshima University, Japan. Her research interest is in the synthesis and characterization of functional materials for energy storage and conversion. One of the research interests is the development of active materials for Li-ion batteries.
 https://orcid.org/0000-0002-8044-7835
https://orcid.org/0000-0002-8044-7835
 https://ror.org/021hq5q33 Centre of Excellence for Electrical Energy Storage Technology, Universitas Sebelas Maret, Indonesia
https://ror.org/021hq5q33 Centre of Excellence for Electrical Energy Storage Technology, Universitas Sebelas Maret, Indonesia  https://ror.org/021hq5q33
https://ror.org/021hq5q33
 https://ror.org/021hq5q33 Centre of Excellence for Electrical Energy Storage Technology, Universitas Sebelas Maret, Indonesia
https://ror.org/021hq5q33 Centre of Excellence for Electrical Energy Storage Technology, Universitas Sebelas Maret, Indonesia  https://ror.org/021hq5q33
https://ror.org/021hq5q33
 https://ror.org/021hq5q33 Centre of Excellence for Electrical Energy Storage Technology, Universitas Sebelas Maret, Indonesia
https://ror.org/021hq5q33 Centre of Excellence for Electrical Energy Storage Technology, Universitas Sebelas Maret, Indonesia  https://ror.org/021hq5q33
https://ror.org/021hq5q33
 https://ror.org/021hq5q33 Centre of Excellence for Electrical Energy Storage Technology, Universitas Sebelas Maret, Indonesia
https://ror.org/021hq5q33 Centre of Excellence for Electrical Energy Storage Technology, Universitas Sebelas Maret, Indonesia  https://ror.org/021hq5q33
https://ror.org/021hq5q33
 https://ror.org/021hq5q33 Centre of Excellence for Electrical Energy Storage Technology, Universitas Sebelas Maret, Indonesia
https://ror.org/021hq5q33 Centre of Excellence for Electrical Energy Storage Technology, Universitas Sebelas Maret, Indonesia  https://ror.org/021hq5q33
https://ror.org/021hq5q33
 https://ror.org/021hq5q33 Physics Department, Faculty of Mathematics and Natural Science, Universitas Sebelas Maret, Indonesia
https://ror.org/021hq5q33 Physics Department, Faculty of Mathematics and Natural Science, Universitas Sebelas Maret, Indonesia  https://ror.org/021hq5q33
https://ror.org/021hq5q33