2024 年 41 巻 p. 123-139
2024 年 41 巻 p. 123-139
Nowadays, the environmental crisis caused by using fossil fuels and CO2 emissions has become a universal concern in people’s lives. Photocatalysis is a promising clean technology to produce hydrogen fuel, convert harmful components such as CO2, and degrade pollutants like dyes in water. There are various strategies to improve the efficiency of photocatalysis so that it can be used instead of conventional methods, however, the low efficiency of the process has remained a big drawback. In recent years, high-pressure torsion (HPT), as a severe plastic deformation (SPD) method, has shown extremely high potential as an effective strategy to improve the activity of conventional photocatalysts and synthesize new and highly efficient photocatalysts. This method can successfully improve the activity by increasing the light absorbance, narrowing the bandgap, aligning the band structure, decreasing the electron–hole recombination, and accelerating the electron–hole separation by introducing large lattice strain, oxygen vacancies, nitrogen vacancies, high-pressure phases, heterojunctions, and high-entropy ceramics. This study reviews the recent findings on the improvement of the efficiency of photocatalysts by HPT processing and discusses the parameters that lead to these improvements.
Nowadays, serious environmental crises such as global warming and the formation of wastewater have forced humans to find alternatives like using clean fuels without carbon dioxide emissions such as hydrogen, reducing the pollutant gases such as carbon dioxide, and degrading the toxic pollutants in wastewater through clean strategies (Gaya and Abdullah, 2008). Using photocatalysts is an effective strategy to deal with these environmental crises using renewable sunlight energy (Ma et al., 2014). The function of photocatalysts is the acceleration of reduction and oxidation (redox) reactions under light irradiation. Reduction and oxidation reactions occur on the surface of a photocatalyst using the electrons and holes, respectively (Tong et al., 2012), to promote processes such as dye degradation (Akpan and Hameed, 2009), water splitting (Maeda, 2011), CO2 conversion (Tu et al., 2014), etc.
In photocatalytic dye degradation, photoexcited electrons take part in the degradation reaction of dyes (e.g. rhodamine B, methylene orange, acid red B, etc.) which normally exist in wastewaters produced by factories, laboratories and different industries (Akpan and Hameed, 2009). Photocatalytic water splitting includes the reduction to hydrogen and oxidation to oxygen (Maeda, 2011). In photocatalytic CO2 conversion, photoexcited electrons contribute to the conversion of CO2 to reactive and useful components such as carbon monoxide (CO), formic acid (HCOOH), formaldehyde (HCHO), methanol (CH3OH) and methane (CH4) (Tu et al., 2014). The mechanism of photocatalytic dye degradation, water splitting and CO2 conversion is shown in Figs. 1(a), 1(b) and 1(c), respectively.

Schematic illustration of (a) photocatalytic dye degradation, (b) photocatalytic water splitting, (c) photocatalytic CO2 conversion, and (d) the HPT process.
A photocatalyst should have some features to support the photocatalytic reactions including a narrow bandgap, appropriate band positions to cover the desired reactions, appropriate light absorbance, potential to absorb and activate the reactants, easy electron transition, and low recombination rate of electrons and holes (Akpan and Hameed, 2009; Maeda, 2011; Tu et al., 2014). Furthermore, high specific surface area, low cost, low toxicity and high stability are the other required properties that should be considered to select a photocatalyst (Tong et al., 2012). Challenging in this field is finding a photocatalyst with sufficient properties for considerable photocatalytic efficiency compared with the conventional catalytic processes. TiO2 (Ajmal et al., 2014; Ni et al., 2007; Ola and Maroto-Valer, 2015), ZnO (Chakrabarti and Dutta, 2004; Lin et al., 2012; Zhao et al., 2019), g-C3N4 (Wen et al., 2017; Ye et al., 2015; Yuan et al., 2016), WO3 (Liu Y. et al., 2010; Wang et al., 2012; Wang et al., 2019a), SrTiO3 (Huang et al., 2014a; Iwashina and Kudo, 2011; Shan et al., 2017), GaN–ZnO (Hagiwara et al., 2017; Maeda et al., 2005; Ohno et al., 2012) and BiVO4 (Gao et al., 2017; Sun et al., 2014; Zhou et al., 2010) are some of the most promising photocatalysts which have been utilized for photocatalytic dye degradation, water splitting and CO2 conversion. The efficiencies of all these photocatalysts are still low for practical applications, and thus, various strategies were utilized to enhance the optical and structural properties and accordingly the photocatalytic activity of these materials.
Vacancy introduction (Huang et al., 2014b; Lei et al., 2014; Yu H. et al., 2019), strain engineering (Di J. et al., 2020; Feng et al., 2015; Sa et al., 2014), using mesoporous structures (Li Y. et al., 2010; Pauporté and Rathouský, 2007; Puangpetch et al., 2009), formation of heterojunctions (Cao et al., 2018; Moniz et al., 2015; Uddin et al., 2012), nanosheet production (Etman et al., 2018; Tu et al., 2012; Yu J. et al., 2010) and doping with impurities (Kuvarega et al., 2011; Lee et al., 2016; Liu B. et al., 2018) are some of the reported methods which have been effective to improve the photocatalytic activity. Doping with impurities is the most common method which is used to improve the activity by narrowing the bandgap, but it usually causes recombination effects (Kuvarega et al., 2011).
High-pressure torsion (HPT) as one of the severe plastic deformation (SPD) methods has been introduced as an effective process to improve the photocatalytic efficiency of various materials by introducing vacancies (oxygen vacancy and nitrogen vacancy), dislocations, nanocrystals, lattice strain, heterojunctions, high-pressure phases and high-entropy ceramics. The HPT method is effective in reducing the recombination rate of electrons and holes which are usually observed in doped photocatalysts. The HPT method decreases the optical bandgap and enhances the photocatalytic activity of various oxides and oxynitrides including TiO2 (Razavi-Khosroshahi et al., 2016b), ZnO (Razavi-Khosroshahi et al., 2017b), Al2O3 (Edalati et al., 2019b), MgO (Fujita et al., 2020a), ZrO2 (Wang et al., 2020b), SiO2 (Wang et al., 2020a), LiTaO3 (Edalati et al., 2020a), CsTaO3 (Edalati et al., 2020a), BiVO4 (Akrami et al., 2022c), Ga6ZnON6 (Edalati et al., 2020b) for dye degradation, hydrogen production and CO2 conversion. Furthermore, HPT was successfully used to synthesize new high-entropy oxides such as TiHfZrNbTaO11 (Edalati et al., 2020c) and high-entropy oxynitrides such as TiZrHfNbTaO6N3 (Akrami et al., 2022a) for hydrogen production, oxygen evolution and CO2 conversion. Moreover, it was also utilized to form heterojunctions in binary and high-entropy composites such as TiO2–ZnO (Hidalgo-Jimenez et al., 2020) and TiZrNbTaWO12 (Edalati et al., 2022b) to improve the photocatalytic activity for hydrogen and oxygen production.
In this review paper, the influence of the HPT method on optical properties, electronic structure, electron–hole separation, migration and recombination rates and photocatalytic efficiency of various photocatalysts is discussed. The improvement of photocatalytic activity of these materials for dye degradation, hydrogen production, oxygen production and CO2 conversion is discussed by considering the generation of oxygen vacancies, nitrogen-vacancy complexes, high-pressure phases, heterojunctions and high-entropy ceramics.
The SPD process is typically used to form ultrafine-grained (UFG) and nanostructured materials with enhanced mechanical and functional properties (Estrin and Vinogradov, 2013; Valiev, 2004). SPD has various methods including HPT, introduced by Bridgman in 1935 for the first time (Bridgman, 1935). In this method, torsional strain under high pressure is applied to induce large plastic strain in various ranges of materials. In the HPT process, a disc-shape sample (Bridgman, 1935) that has typically a 10 mm diameter or a ring sample (Edalati and Horita, 2009) is inserted between two anvils under high pressure and strained by rotation of anvils against each other. A schematic illustration of HPT is shown in Fig. 1(d). The applied shear strain (γ) to the sample can be calculated by the following equation (Edalati and Horita, 2016).
| (1) |
where r, N and h are the distance from the center of the disc or ring, the number of turns and the sample height, respectively.
In addition to grain refinement, the HPT method is widely used for hardening of pure metals (Edalati and Horita, 2011; Starink et al., 2013), mechanical alloying of miscible and immiscible systems (Edalati et al., 2015b; Edalati, 2019a) and plastic deformation of hard-to-deform materials (Edalati et al., 2010a; Edalati et al., 2010b; Ikoma et al., 2012). Phase transformation, consolidation of powders, the introduction of defects such as oxygen vacancies and dislocations, narrowing of the bandgap and improvement of functionalities were frequently reported after HPT processing (Edalati, 2019d). The HPT method can be utilized for plastic deformation of oxides and ceramics which are hard and brittle materials at ambient temperature (Edalati et al., 2022a). The presence of covalent or ionic bonding in ceramics results in their lower grain size after HPT processing compared to metals. Moreover, such bonding features result in the formation of large densities of defects such as vacancies and dislocations which can improve the properties and functionality of ceramics.
Despite the high potential of ceramics for various applications, there are some limited publications on the effect of HPT on the structure, properties and functionality of ceramics. A list of ceramics treated by HPT for various properties and applications and their relevant publications are given in Table 1. Table 1 indicates that these HPT-processed ceramics, including oxides, nitrides, oxynitrides and borides, have been investigated for photocatalysis, phase transformation, electrocatalysis, photocurrent generation, dielectric properties, bandgap narrowing, optical properties, mechanical properties, Li-ion batteries and microstructural features. Furthermore, the HPT method was also utilized to synthesize new ceramics for various applications. As can be seen in Table 1, ceramic photocatalysts processed or synthesized by HPT were used for water splitting (CsTaO3, LiTaO3, ZrO2, GaN–ZnO, TiO2–ZnO, TiO2, TiHfZrNbTaO11, TiZrNbTaWO12 and TiZrHfNbTaO6N3), dye degradation (ZnO, MgO, γ-Al2O3 and SiO2), CO2 conversion (TiO2, BiVO4, TiHfZrNbTaO11 and TiZrHfNbTaO6N3) and oxygen production (TiZrNbTaWO12).
Summary of major publications about ceramics treated by HPT and their major applications or features.
| Materials | Investigated properties and application | Reference |
|---|---|---|
| Photocatalysis | ||
| TiHfZrNbTaO11 | Photocatalytic activity for CO2 conversion | (Akrami et al., 2022b) |
| TiZrHfNbTaO6N3 | Photocatalytic activity for CO2 conversion | (Akrami et al., 2022a) |
| BiVO4 | Photocatalytic activity for CO2 conversion | (Akrami et al., 2022c) |
| TiO2-II | Photocatalytic activity for CO2 conversion | (Akrami et al., 2021b) |
| MgO | Photocatalytic activity for dye degradation | (Fujita et al., 2020a) |
| SiO2 | Photocatalytic activity for dye degradation | (Wang et al., 2020a) |
| γ-Al2O3 | Photocatalytic activity for dye degradation | (Edalati et al., 2019b) |
| ZnO | Photocatalytic activity for dye degradation | (Razavi-Khosroshahi et al., 2017b) |
| TiZrHfNbTaO6N3 | Photocatalytic activity for hydrogen production | (Edalati et al., 2021) |
| ZrO2 | Photocatalytic activity for hydrogen production | (Wang et al., 2020b) |
| CsTaO3, LiTaO3 | Photocatalytic activity for hydrogen production | (Edalati et al., 2020a) |
| GaN–ZnO | Photocatalytic activity for hydrogen production | (Edalati et al., 2020b) |
| TiHfZrNbTaO11 | Photocatalytic activity for hydrogen production | (Edalati et al., 2020c) |
| TiO2–ZnO | Photocatalytic activity for hydrogen production | (Hidalgo-Jimenez et al., 2020) |
| TiO2-II | Photocatalytic activity for hydrogen production | (Razavi-Khosroshahi et al., 2016b) |
| TiZrNbTaWO12 | Photocatalytic activity for oxygen production | (Edalati et al., 2022b) |
| Reviews | ||
| Oxides | Review on HPT of oxides | (Edalati, 2019b) |
| Review on HPT | (Edalati and Horita, 2016) | |
| Phase transformation | ||
| SiO2, VO2 | Phase transformation | (Edalati et al., 2019a) |
| TiO2 | Grain coarsening and phase transformation | (Edalati et al., 2019c) |
| ZrO2 | Phase transformation by ball milling and HPT | (Delogu, 2012) |
| ZrO2 | Allotropic phase transformations | (Edalati et al., 2011) |
| TiO2 | Plastic strain and phase transformation | (Razavi-Khosroshahi et al., 2016a) |
| BN | FEM modeling of plastic flow and strain-induced phase transformation | (Feng et al., 2019) |
| BN | Coupled elastoplasticity and plastic strain-induced phase transformation | (Feng and Levitas, 2017) |
| Electrocatalysis | ||
| TiO2-II | Electrocatalysis for hydrogen generation | (Edalati et al., 2019c) |
| Photocurrent | ||
| Bi2O3 | Enhanced photocurrent generation | (Fujita et al., 2020b) |
| TiO2-II | Visible light photocurrent generation | (Wang et al., 2020c) |
| Dielectric properties | ||
| BaTiO3 | Optical and dielectric properties | (Edalati et al., 2015a) |
| CuO | Dielectric properties | (Makhnev et al., 2011) |
| Bandgap investigation | ||
| ZnO | Bandgap narrowing | (Qi et al., 2021) |
| Optical properties | ||
| Y2O3 | Optical properties | (Razavi-Khosroshahi et al., 2017a) |
| CuO, Y3Fe5O12, FeBO3 | Optical properties and electronic structure | (Gizhevskii et al., 2011) |
| Cu2O, CuO | Middle infrared absorption and X-ray absorption | (Mostovshchikova et al., 2012) |
| CuO, Y3Fe5O12, FeBO3 | Optical properties | (Telegin et al., 2012) |
| Mechanical properties | ||
| α-Al2O3 | Microstructure and mechanical properties | (Edalati and Horita, 2010) |
| Fe53.3Ni26.5B20.2, Co28.2Fe38.9Cr15.4Si0.3B17.2 | Microstructure and mechanical properties | (Permyakova and Glezer, 2020) |
| Lithium-ion batteries | ||
| Fe3O4 | Lithium-ion batteries | (Qian et al., 2018) |
| Microstructural features | ||
| ZnO | Plastic flow and microstructural instabilities | (Qi et al., 2018) |
| YBa2Cu3Oy | Microstructural investigation | (Kuznetsova et al., 2017) |
| Fe71.2Cr22.7Mn1.3N4.8 | Microstructural features | (Shabashov et al., 2018) |
In photocatalysis, which is also called artificial photosynthesis, electrons in the valence band absorb the light photons, separate from the holes and transfer to the conduction band of the photocatalyst to form the electron–hole charge carriers. The charge carriers then migrate to the surface of the photocatalyst and finally take part in various chemical reactions. In this process, both reduction and oxidation reactions occur on the surface of the photocatalyst, provided that the thermodynamic and kinetic conditions are satisfied. From the thermodynamic point of view, a photocatalytic process will perform when the reduction and oxidation reaction potentials are between the valence band and the conduction band of a photocatalyst (Tong et al., 2012). For the reduction reactions, potentials lower than the valence band top are desirable, while for the oxidation reactions, potentials higher than the conduction band bottom are desirable. From the kinetic point of view, the electron–hole separation should be fast, and their recombination should be slow. Here, the fundamentals of three main photocatalytic reactions are mentioned.
3.1 Photocatalytic dye degradationIn photocatalytic dye degradation, there are two direct and indirect pathways. In the direct pathway, the dye degrades directly by the excited electrons and holes in the conduction band and the valence band, respectively, as shown in Fig. 1(a). In the indirect pathway, which is more conventional than the direct pathway, the electrons in the conduction band react with the O2 to produce the
In photocatalytic water splitting, electrons in the conduction band take part in the reduction reaction to produce H2, while holes in the valence band take part in the oxidation reaction to produce O2 (Lu et al., 2021), as shown in Fig. 1(b). In most photocatalysts, overall water splitting does not occur, and the process is limited to either reduction or oxidation reactions thus a sacrificial agent or scavenger is usually required to produce electrons or holes. Methanol and AgNO3 are popular sacrificial agents for photocatalytic hydrogen and oxygen production, respectively.
3.3 Photocatalytic CO2 conversionIn photocatalytic CO2 conversion, electrons in the conduction band along with protons (H+) produced from water oxidation in the valence band, take part in various reduction reactions leading to the formation of CO, CH4 and some other hydrocarbons as shown in Fig. 1(c). The first step in photocatalytic CO2 conversion is the adsorption of CO2 molecules on the surface of the photocatalyst which occurs in three modes including oxygen coordination, carbon coordination and mixed coordination (Lu et al., 2021), as shown in Fig. 2. These CO2 adsorption modes determine the reaction pathway for photocatalytic CO2 conversion. For instance, the oxygen coordination mode, with the bidentate bonding of oxygen atoms with the surface of the photocatalyst, leads to the fabrication of formate anions as an intermediate product and formic acid as the final product. In the carbon coordination mode, with the monodentate binding of carbon and photocatalyst surface, the reaction results in carboxyl radical production. After the adsorption step of CO2 to the surface of the photocatalyst, the conversion reactions occur via different pathways.
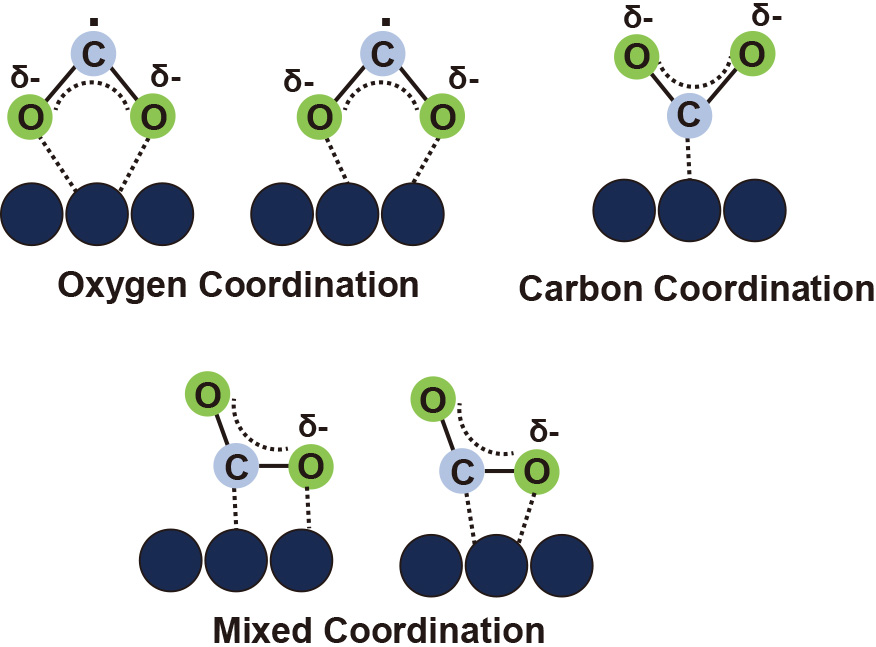
CO2 adsorption modes on the surface of photocatalysts.
It was reported that there are three main pathways for CO2 photoreduction: (i) carbene pathway (ii) formaldehyde pathway and (iii) glyoxal pathway (Habisreutinger et al., 2013; Maeda and Domen, 2007; Wang et al., 2021). The reactions for these three pathways are presented in Table 2. In all these pathways, the
CO2 photoreduction pathways (Habisreutinger et al., 2013).
| Carbene Pathway | Formaldehyde Pathway | Glyoxal Pathway |
|---|---|---|
| (1) CO2 + e− → CO2•− | (1) CO2 + e− → CO2•− | (1) CO2 + e− → CO2•− |
| (2) CO2•− + e− + H+ → CO + OH− | (2) CO2•− + H+ → •COOH | (2) CO2•− + e− + H+ → CHOO− |
| (3) CO + e− → CO•− | (3) •COOH + e− + H+ → HCOOH | (3) CHOO− + H+ → HCOOH |
| (4) CO•− + e− + H+ → C + OH− | (4) HCOOH + e− + H+ → H3OOC• | (4) HCOOH + e− → HOC• |
| (5) C + e− + H+ → CH• | (5) HCOOH2• + e− + H+ → HCOH + H2O | (5) HOC• + OH− → C2H2O2 |
| (6) CH• + e− + H+ → CH2 | (6) HCOH + e− → H2C•O− | (6) C2H2O2 + e− + H+ → H3O2C2• |
| (7) CH2 + e− + H+ → CH3• | (7) H2C•O− + H+ → H2OHC• | (7) H3O2C2• + e− + H+ → C2H4O2 |
| (8) CH3• + e− + H+ → CH4 | (8) H2OHC• + e− + H+ → CH3OH | (8) C2H4O2 + e− + H+ → H3OC2• + H2O |
| (9) CH3• + OH− → CH3OH | (9) CH3OH + e− + H+ → •CH3 + H2O | (9) H3OC2• + e− + H+ → C2H4O |
| (10) •CH3 + e− + H+ → CH4 | (10) C2H4O + h+ → H3OC2• + H+ | |
| (11) H3OC2• → CH3• + CO | ||
| (12) CH3• + e− + H+ → CH4 |
As given in Table 1, numerous studies in recent years reported that the HPT method can be used to improve the photocatalytic activity of ceramics. Bandgap narrowing for the easier transition of photoexcited electrons from the valence band to the conduction band, increasing the light absorbance in the visible region of light, electronic band structure alignment, decreasing the recombination rate of electrons and holes and accelerating the electron–hole separation and migration are some phenomena responsible for the high activity of photocatalysts processed or synthesized by HPT. The occurrence of these phenomena was attributed to the formation of oxygen vacancies, nitrogen-vacancy complexes, high-pressure phases, heterojunctions and high-entropy phases which will be discussed in detail as follows.
4.1 Generation of oxygen vacanciesOxygen vacancies on the surface have a significant effect on the photocatalytic activity of materials, since they can act as active sites to adsorb and activate the reactants and as trap sites for photoexcited electrons. In contrast, oxygen vacancies in the bulk of photocatalysts may act as recombination centers and lead to decreasing photocatalytic efficiency. Oxygen vacancies can also reduce the optical bandgap by changing the electronic structure of materials via the formation of defect states between the valence band and the conduction band. The formation of oxygen vacancies after HPT processing has been evidenced by several analysis methods such as electron paramagnetic resonance (EPR), X-ray photoelectron spectroscopy (XPS), Raman spectroscopy, differential scanning calorimetry (DSC), and X-ray diffraction (XRD) (Fujita et al., 2020a; Razavi-Khosroshahi et al., 2016b; Wang et al., 2020a). Furthermore, changing the color of catalysts to the darker ones, which is frequently observed after HPT processing, indicates the light absorbance in the visible region due to the formation of oxygen vacancies as color centers (Fujita et al., 2020b; Razavi-Khosroshahi et al., 2017b; Wang et al., 2020b). Among the photocatalysts treated by HPT, Al2O3 (Edalati et al., 2019b), MgO (Fujita et al., 2020a), ZrO2 (Wang et al., 2020b), SiO2 (Wang et al., 2020a), LiTaO3 (Edalati et al., 2020a), CsTaO3 (Edalati et al., 2020a) and BiVO4 (Akrami et al., 2022c) are oxides which exhibited enhanced photocatalytic activity by oxygen vacancy formation.
Al2O3: Al2O3 is an insulator with a large bandgap (~9 eV) and does not show photocatalytic activity. First-principles calculations suggested that the formation of oxygen vacancies can reduce the optical bandgap of Al2O3 even to the visible light region (Edalati et al., 2019b). The application of HPT to Al2O3 resulted in the formation of oxygen vacancies, the reduction of optical bandgap and its photocatalytic activity. The formation of oxygen vacancies after HPT is shown in Fig. 3(a) using DSC analysis by the appearance of a peak at 530 K. After the HPT process, a narrow bandgap of 2.5 eV was achieved, in good agreement with the first-principles calculations, and the material showed photocatalytic activity for rhodamine B dye degradation under UV irradiation with an activity comparable to TiO2 (Fig. 3(b)).
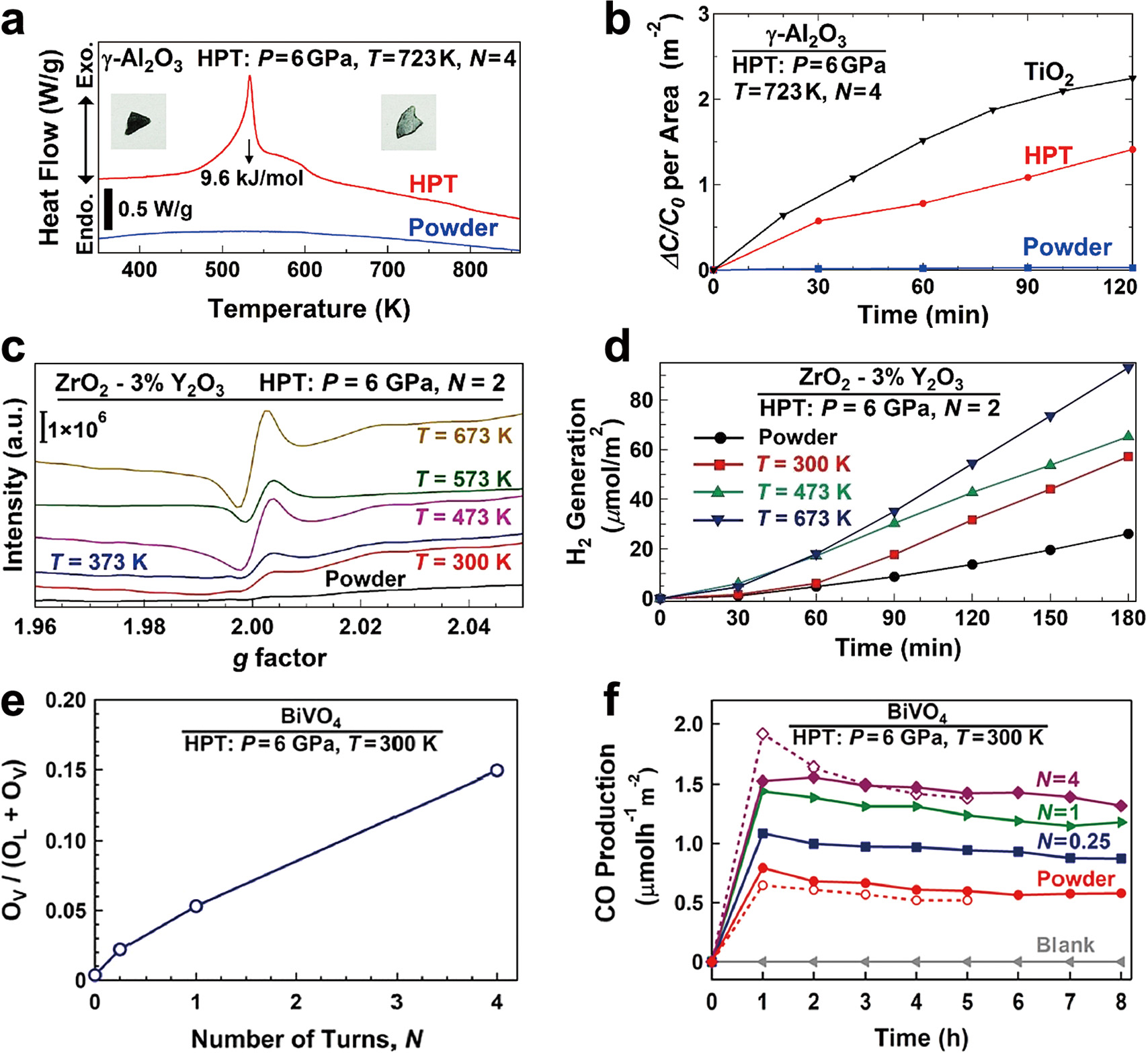
Formation of oxygen vacancies achieved by HPT processing and improvement of photocatalytic activity. (a) DSC analysis and (b) photocatalytic rhodamine B degradation for γ-Al2O3 before and after HPT processing compared with TiO2 (Edalati et al., 2019b). (c) EPR spectra and (d) corresponding photocatalytic hydrogen production for ZrO2–3 wt% Y2O3 before and after HPT processing at 300, 437, 573 and 673 K (Wang et al., 2020b). (e) Oxygen vacancy concentration and (f) photocatalytic CO2 to CO conversion for BiVO4 before and after HPT processing for N = 0.25, 1 and 4 turns (Akrami et al., 2022c). Copyright: (2019) Elsevier, (2020) Royal Society of Chemistry, (2022) Elsevier.
MgO: MgO, as another ceramic insulator, also exhibited high light absorbance and bandgap narrowing (from 7.8 eV to 3.9 eV) after HPT processing (Fujita et al., 2020a). The HPT-processed MgO showed photocatalytic activity for methylene blue dye degradation under UV irradiation (55 % decomposition after 3 h). The formation of oxygen vacancies in this material was evidenced by XPS analysis of Mg 2p, which showed a shift to lower energy, and such a shift became more significant by increasing the applied shear strain (i.e. by increasing the number of HPT turns).
ZrO2: ZrO2 is another ceramic that was processed by HPT to introduce a high concentration of oxygen vacancies (Wang et al., 2020b). The optical bandgap of this material decreased from 5.1 to 4.0 eV due to the formation of oxygen vacancies after HPT. The formation of oxygen vacancies after HPT processing at various temperatures is shown in Fig. 3(c) using the EPR analysis. The appearance of a pair peak with a g factor of ~2 indicates the formation of oxygen vacancies, while the formation of oxygen vacancies is enhanced by increasing the HPT processing temperature. Improvement of photocatalytic activity of ZrO2 using HPT for hydrogen evolution is shown in Fig. 3(d), indicating that the photocatalytic activity is improved by increasing the concentration of oxygen vacancies.
SiO2: SiO2 quartz sand also was examined for photocatalytic activity after processing using the HPT method (Wang et al., 2020a). This material with the initially reported bandgap of ~9 eV and negligible light absorbance exhibited a bandgap narrowing to 2.8 eV, with a small activity for rhodamine B dye degradation under UV light irradiation.
LiTaO3 and CsTaO3: LiTaO3 and CsTaO3 are two tantalate perovskites that show activity for photocatalytic hydrogen production. After oxygen vacancy generation by HPT processing, both materials exhibited bandgap narrowing (4.7 to 4.2 eV for LiTaO3 and 4.7 to 3.6 eV for CsTaO3) and enhanced photocatalytic activity for hydrogen generation (Edalati et al., 2020a).
BiVO4: BiVO4 is a popular photocatalyst for CO2 conversion due to its low bandgap, but it suffers from a high recombination rate of electrons and holes and a low position of the conduction band. HPT could successfully solve these two problems by simultaneously introducing the oxygen vacancies and lattice strain (Akrami et al., 2022c). Increasing the oxygen vacancy concentration by increasing the number of HPT turns, calculated from XPS, is shown in Fig. 3(e). These structural modifications by HPT processing resulted in significant improvement of photocatalytic CO2 conversion on BiVO4, as shown in Fig. 3(f).
4.2 Generation of nitrogen-vacancy complexesOxynitrides are promising photocatalysts due to their low bandgap compared with oxide photocatalysts. It was reported that nitrogen vacancies, which are formed during the synthesis of these materials, can act as recombination centers, and reduce photocatalytic activity (Maeda and Domen, 2007). However, it was observed that HPT processing of Ga6ZnON6 led to the formation of nitrogen-based vacancies, decreasing the electron and hole recombination and enhancement of photocatalytic activity (Edalati et al., 2020b). Fig. 4(a) shows the EPR spectra of Ga6ZnON6 before and after HPT processing. A g factor of 1.958 for powder is a piece of evidence for the formation of nitrogen monovacancies during the synthesis of oxynitrides, while a g factor of 2.006 indicates the presence of nitrogen-based vacancy complexes by HPT processing. Introducing the nitrogen-based vacancy complexes to this material by HPT, led to reducing the bandgap from 2.7 eV to 2.4 eV, enhancing the light absorbance and improvement of photocatalytic hydrogen production under UV irradiation. This study showed that in addition to the location of vacancies (surface or bulk), their arrangement (monovacancy or vacancy complex) can affect photocatalytic activity. These vacancy complexes can act as active sites for reactions rather than the recombination centers for electrons and holes. Decreasing the recombination of electrons and holes after HPT processing for this oxynitride is presented in Fig. 4(b), in which the photoluminescence intensity is suppressed effectively after the HPT process.

Formation of nitrogen-based vacancy complexes and suppression of recombination in oxynitride processed by HPT. (a) EPR spectra and (b) photoluminescence spectra for Ga6ZnON6 before and after HPT processing (r: radial distance from center of HPT-processed disc) (Edalati et al., 2020b). Copyright: (2020) Elsevier.
Due to high pressure and high strain in HPT, the method can lead to the formation of metastable and high-pressure phases. These phases usually form with fast kinetics at lower pressures during HPT processing compared to the transition pressures under static conditions due to the presence of high concentrations of nanograin boundaries and defects. It was suggested that the formation of these defects and their interaction increase localized pressure and result in the formation and stability of high-pressure phases (Edalati, 2019b). BaTiO3 (tetragonal to cubic) (Makhnev et al., 2011), TiO2 (anatase-tetragonal to orthorhombic TiO2-II) (Razavi-Khosroshahi et al., 2016b), Y2O3 (cubic to monoclinic) (Razavi-Khosroshahi et al., 2017a), ZnO (wurtzite-hexagonal to rocksalt-cubic) (Razavi-Khosroshahi et al., 2017b) and SiO2 (quartz to coesite) (Wang et al., 2020a) are some oxides which showed transition to high-pressure phases after HPT processing. Among these materials, the formation of high-pressure phases in TiO2 and ZnO was reported to lead to the improvement of photocatalytic activity for hydrogen production, CO2 conversion and dye degradation. Figs. 5(a) and 5(b) illustrate the pressure–temperature phase diagrams of TiO2 and ZnO, respectively; Figs. 5(c) and 5(d) show the formation of TiO2-II and rocksalt-ZnO high-pressure phases after HPT processing, respectively; and Figs. 5(c) and 5(d) show the influence of HPT processing on the enhancement of the photocatalytic activity of TiO2 and ZnO, respectively. Since TiO2 and ZnO are the most common photocatalysts, the reports on the application of HPT to these two photocatalysts are of significance as they suggested new solutions to enhance the activity by nanostructure and polymorphic control and without dopant or impurity addition (Razavi-Khosroshahi et al., 2016b; Razavi-Khosroshahi et al., 2017b).

Improved photocatalytic activity by introducing high-pressure phases. (a) Phase diagram of TiO2 (Akrami et al., 2021b), (b) phase diagram of ZnO (Razavi-Khosroshahi et al., 2017b), (c) high-resolution lattice image of TiO2 including TiO2-II high-pressure phase (Akrami et al., 2021b), (d) XRD profile of ZnO including rocksalt high-pressure phase (Razavi-Khosroshahi et al., 2017b), (e) photocatalytic CO2 conversion on TiO2 before and after HPT processing and after annealing under UV light (Akrami et al., 2021b), and (f) photocatalytic rhodamine B dye degradation for ZnO before and after HPT processing under visible light (Razavi-Khosroshahi et al., 2017b). Copyright: (2021) Elsevier, (2017) Royal Society of Chemistry.
TiO2: TiO2 light absorbance improved significantly after the formation of the high-pressure TiO2-II (columbite) phase by HPT processing (Akrami et al., 2021b; Razavi-Khosroshahi et al., 2016b). The color of TiO2 changed from white to gray or green and its bandgap decreased from 3.1 eV to 2.5 eV, which resulted in photocatalytic activity of the material under visible light. To solve the recombination issue due to the presence of bulk defects after HPT processing, the HPT-processed sample was processed by annealing which resulted in a further increase in the photocatalytic activity and photocurrent generation. It was shown that the formation of TiO2-II results in photocatalytic activity for hydrogen production under visible light and CO2 conversion under UV light (Fig. 5(e)), although the high activity is influenced by other factors such as the presence of oxygen vacancies and interphase boundaries.
ZnO: HPT processing of ZnO led to the formation of a high-pressure rocksalt phase and accordingly to the improvement of light absorbance in the visible region with a bandgap narrowing from 3.0 eV to 1.8 eV (Razavi-Khosroshahi et al., 2017b). This bandgap narrowing was also predicted by first-principles calculations. The HPT-processed ZnO, containing a high-pressure phase with a large fraction of oxygen vacancies and interphase boundaries, showed photocatalytic rhodamine B dye degradation under visible light, while its activity improved with increasing the processing pressure (Fig. 5(f)).
4.4 Formation of heterojunctionsHeterojunctions are the space-charge regions that are formed by the combination of two different phases or different semiconductors. Charge carriers can penetrate heterojunctions which results in the formation of an electronic field in this region (Moniz et al., 2015). Heterojunctions accelerate the electron and hole separation and improve their migration to the surface of the photocatalyst for the reactions. Furthermore, they significantly suppress recombination (Cao et al., 2018; Uddin et al., 2012). As mentioned earlier, among HPT-processed materials, some of them exhibit phase transformations resulting in the formation of heterojunctions between two or more phases such as cubic-rhombohedral phases in Al2O3 (Edalati et al., 2019b), monoclinic-tetragonal phases in ZrO2 (Wang et al., 2020b) and coesite-quartz phases in SiO2. (Wang et al., 2020a). There is also an application of HPT to TiO2–ZnO composite to generate heterojunctions for enhanced photocatalytic hydrogen production (Hidalgo-Jimenez et al., 2020). There is another attempt to generate 10 different heterojunctions in a high-entropy oxide TiZrNbTaWO12 for visible-light-driven photocatalytic oxygen production (Edalati et al., 2022b). In all these cases, the interphase boundaries formed by HPT can act as sites to accelerate the electron–hole separation to effectively improve the photocatalytic activity, as will be discussed below for the TiO2–ZnO composite.
TiO2–ZnO: A recent study generated heterojunctions using HPT by making a composite of TiO2 and ZnO as two typical catalysts for photocatalytic hydrogen production (Hidalgo-Jimenez et al., 2020). This work was performed to ease electron–hole separation as shown in Fig. 6(a). In addition to TiO2–ZnO heterojunctions, HPT processing led to the formation of high-pressure phases and formation of various kinds of heterojunctions. XRD profiles of TiO2–ZnO composite show the presence of five different phases of TiO2 and ZnO in Fig. 6(b). After HPT processing, the light absorbance of the composite significantly increased in the visible light region and a bandgap narrowing was observed from 3.2 eV to 2.6 eV and 1.6 eV for N = 3 and 15 HPT turns, respectively. The recombination rate of electrons and holes was also suppressed effectively after HPT processing. Although electronic structure, light absorbance and recombination suppression improved by increasing the number of HPT turns to N = 15, photocatalytic hydrogen production for N = 15 was lower compared to the powder mixture and sample processed by N = 3 due to the formation of very small crystals and poor crystallinity (Fig. 6(c)). The composite processed with N = 3 had a hydrogen production amount of 2.5 time better than initial powders mixture under UV irradiation.
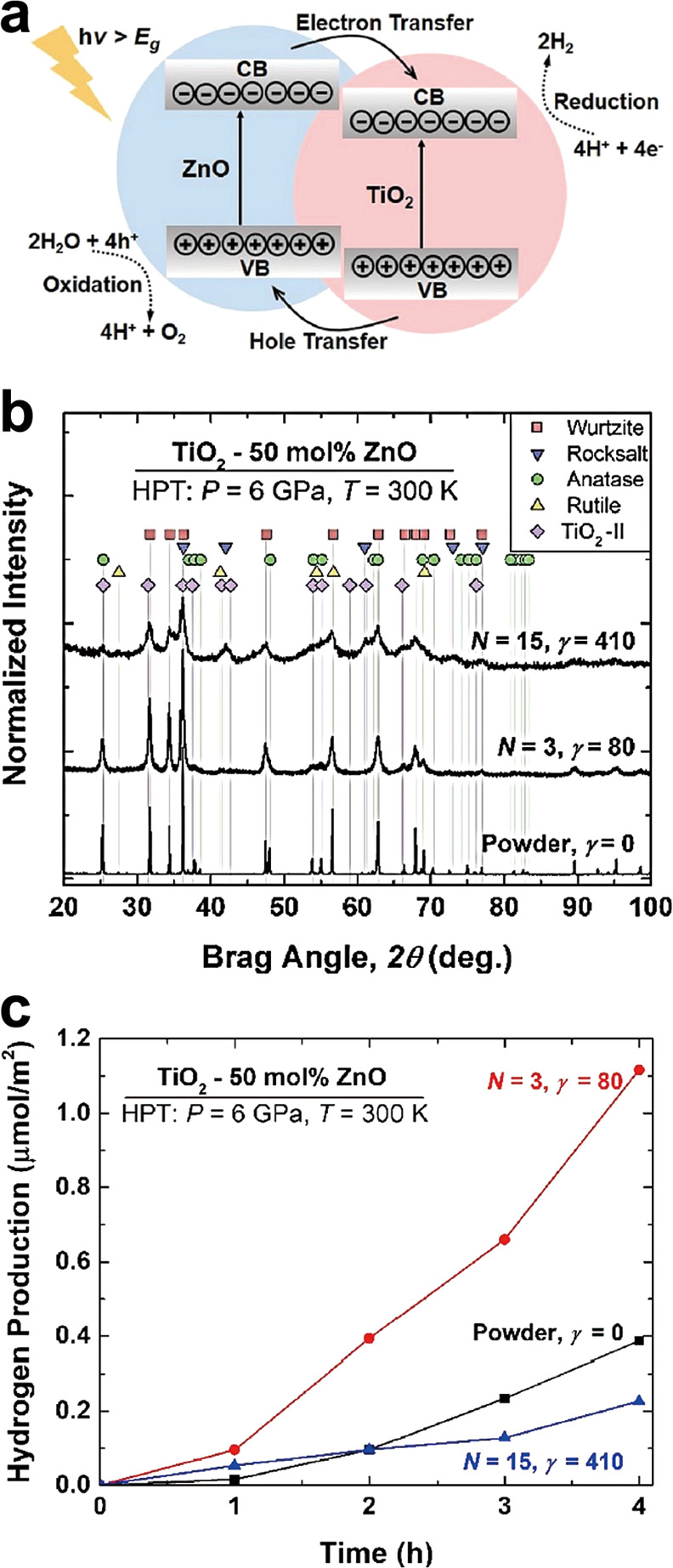
Enhanced photocatalytic hydrogen production by heterojunction formation. (a) Schematic illustration of electronic band structure of TiO2–ZnO composite including heterojunctions. (b) XRD profile and (c) photocatalytic hydrogen production on TiO2–ZnO composite before and after HPT processing for N = 3 and 15 turns (Hidalgo-Jimenez et al., 2020). Copyright: (2020) Elsevier.
High-entropy ceramics are materials with at least five principal cations and a mixing entropy higher than 1.5R (R: gas constant) (Akrami et al., 2021a; Oses et al., 2020). These materials have been employed for various applications due to their high stability, lattice strain, inherent defects and high entropy of mixing (Zhang and Reece, 2019; Xiang et al., 2021). They have potential to be used as Li-ion batteries (Lun et al., 2021; Nguyen et al., 2020; Wang et al., 2019b), catalysts (Albedwawi et al., 2021; Qiao et al., 2021; Xu et al., 2020), photocatalysts (Akrami et al., 2022a; Akrami et al., 2022b; Edalati et al., 2020c), supercapacitors (Jin et al., 2018; Liang et al., 2020; Talluri et al., 2021), dielectrics (Bérardan et al., 2016; Zhou et al., 2020; Zhou et al., 2022), thermal barrier coatings (Li F. et al., 2019; Ren et al., 2020; Zhang et al., 2022), etc. Utilizing the high-entropy ceramics as photocatalysts, which was performed for the first time by the authors of the current work, is a new functional aspect of these materials which is in its initial research stage. The high stability of high-entropy ceramics and the formation of defects and strain in these materials were the main motivations to use them as photocatalysts. As will be discussed below, the oxides TiZrHfNbTaO11 and TiZrNbTaWO12 and the oxynitride TiZrHfNbTaO6N3 are high-entropy ceramics synthesized by HPT and used for photocatalytic hydrogen production, CO2 conversion and O2 evolution. Since oxides and oxynitrides with d0 and d10 electronic configurations show good photocatalytic activity, five d0 cations were selected to design these photocatalysts.
TiZrHfNbTaO11: TiZrHfNbTaO11 high-entropy oxide (HEO) with two monoclinic and orthorhombic phases which was synthesized by the HPT method and high-temperature oxidation, showed better light absorbance compared to relevant binary oxides including TiO2, ZrO2, HfO2, Nb2O5 and Ta2O5 (Fig. 7(a)) (Edalati et al., 2020c). This material contained defects such as oxygen vacancies and dislocations as shown in Fig. 8(a). Furthermore, it demonstrated lower photoluminescence intensity compared to anatase TiO2 and BiVO4 as two typical photocatalysts, indicating the low recombination rate of electrons and holes in this HEO (Akrami et al., 2022b). Moreover, this material showed the potential to generate photocurrent, produce hydrogen and convert CO2 to CO under UV light. The rate of hydrogen and CO production for TiZrHfNbTaO11 was higher than anatase TiO2 and BiVO4 and almost the same as P25 TiO2 as a benchmark photocatalyst. This high photocatalytic activity was attributed to the formation of defects, interphases and high-entropy phases which led to the acceleration of electron–hole separation and diminishing their recombination.
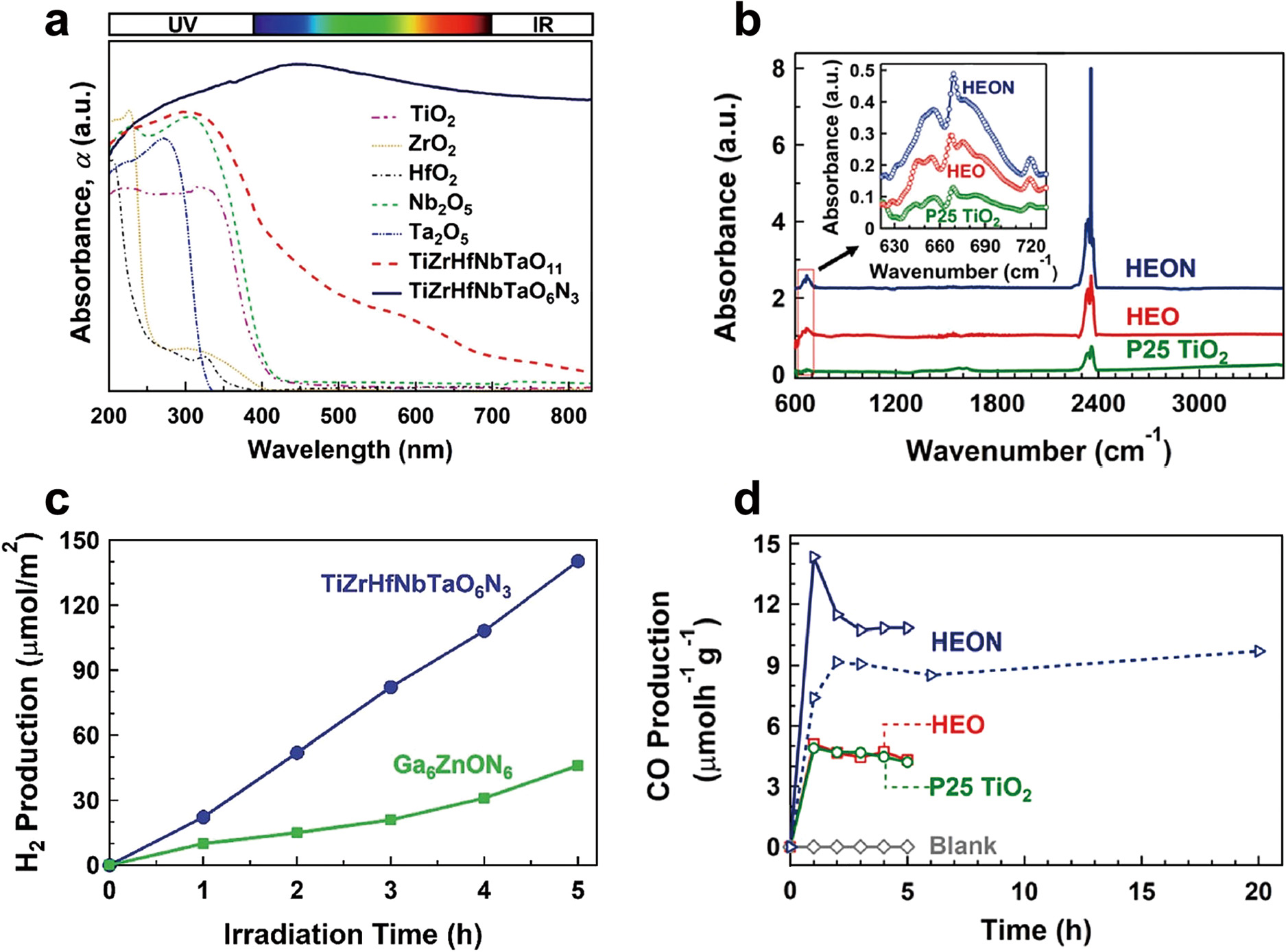
High light absorbance and significant photocatalytic activity of high-entropy ceramics. (a) UV-vis spectra of TiZrHfNbTaO11, TiZrHfNbTaO6N3 and relevant binary oxides including TiO2, ZrO2, HfO2, Nb2O5 and Ta2O5 (Edalati et al., 2021), (b) diffuse reflectance infrared Fourier transform spectra for TiZrHfNbTaO11 and TiZrHfNbTaO3N6 compared with P25 TiO2 in which the peaks at 665 cm−1 and 2350 cm−1 represent chemisorption and physisorption of CO2 on surface (Akrami et al., 2022a), (c) photocatalytic hydrogen production on TiZrHfNbTaO6N3 compared with Ga6ZnON6 (Edalati et al., 2021), and (d) photocatalytic CO2 to CO conversion on TiZrHfNbTaO11 and TiZrHfNbTaO6N3 compared with P25 TiO2 (Akrami et al., 2022a). Copyright: (2021) Royal Society of Chemistry, (2022) Elsevier.
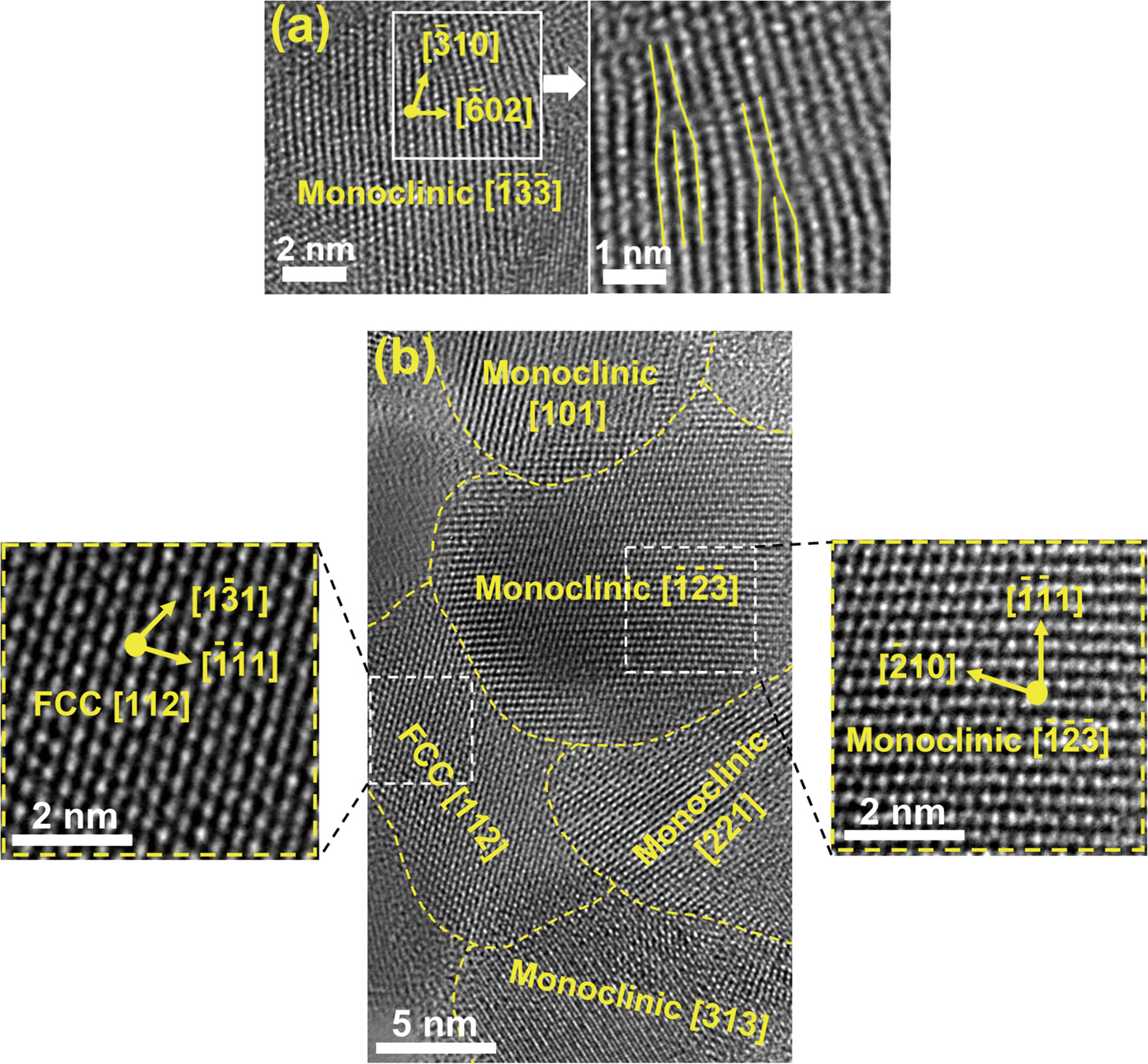
Formation of nanograins, grain boundaries, interphases, and dislocation defects in high-entropy ceramics synthesized by HPT. High-resolution lattice images of (a) TiZrHfNbTaO11 including dislocations (Akrami et al., 2022b) and (b) TiZrHfNbTaO6N3 including monoclinic and FCC phases and their heterojunctions (Edalati et al., 2021). Copyright: (2022) Elsevier, (2021) Royal Society of Chemistry.
TiZrHfNbTaO6N3: Low-bandgap high-entropy oxynitride (HEON) TiZrHfNbTaO6N3 was produced by HPT processing followed by oxidation and nitriding (Edalati et al., 2021). This HEON showed significantly higher light absorbance compared to corresponding high-entropy and binary oxides (Fig. 7(a)) with a narrow bandgap of 1.6 eV, which is lower than the bandgap of most reported oxynitride photocatalysts in the literature. This HOEN could successfully generate photocurrent with a significantly low recombination rate of electrons and holes compared to HEO and P25 TiO2 as a benchmark photocatalyst (Akrami et al., 2022a). Furthermore, it showed a high potential to adsorb the CO2 molecules on the surface as shown in Fig. 7(b). This material produced hydrogen under UV irradiation with better activity than Ga6ZnON6 as one of the most popular oxynitride photocatalysts (Fig. 7(c)). It also showed a higher photocatalytic activity per surface area for CO2 conversion compared to HEO and P25 TiO2 (Fig. 7(d)) and all reported photocatalysts in the literature. Improved photocatalytic activity on this HEON was attributed to the high light absorbance and easy electron–hole separation, low recombination rate, and high surface activity for adsorption and conversion of water and CO2. The presence of two phases in this HEON and the formation of interphases, as shown in Fig. 8(b), were also believed to contribute to its high activity.
TiZrNbTaWO12: The HEO, TiZrNbTaWO12, was produced by HPT and consequent high-temperature oxidation (Edalati et al., 2022b). It had five different phases including one orthorhombic, two monoclinic and two tetragonal phases and ten heterojunctions. This material showed a low bandgap of 2.3–2.8 eV and a light absorbance higher than relevant binary oxides. Introducing the heterojunctions in this material resulted in successful photocatalytic oxygen production under viable light irradiation.
Photocatalytic production of hydrogen from water, conversion of CO2 to value-added compounds and degradation of toxic materials in wastewater under sunlight, are clean processes that have high potential to be used instead of conventional chemical methods. Photocatalysts used for these applications should have a narrow bandgap, appropriate band positions to support the reactions, low recombination rate of electrons and holes and high surface activity. In recent years, the high-pressure torsion (HPT) method was used to generate active photocatalysts for various applications. This method applies very high strain and pressure to material and subsequently introduces nanocrystals, lattice defects, lattice strain and heterojunctions. Furthermore, HPT can synthesize high-pressure and high-entropy phases. One main reason for the successful application of HPT to develop highly active photocatalysts is that the method can simultaneously introduce a few structural/microstructural features as effective strategies to enhance the activity. It was shown that the HPT method improves photocatalytic activity by increasing light absorbance, decreasing the optical bandgap, optimizing the electronic band structure, accelerating the electron–hole separation and migration and reducing the recombination rate of electrons and holes. The HPT method also contributed to the introduction of high-entropy photocatalysts which show high activity for hydrogen production, oxygen evolution and CO2 conversion.
Despite the high activity of HPT-processed photocatalysts, the mechanism for their high efficiency needs to be investigated further by employing theoretical calculations. Moreover, the catalysts produced by HPT have usually low surface area and new methods are expected to be developed to enhance the surface area of these catalysts. Although the HPT method has opened a new path to employing high-pressure and high-entropy phases as new photocatalysts, other synthesis methods should be used in the future for the large-scale production of these new catalysts. It is expected that HPT will continue its contribution to the field of photocatalysis, particularly due to the significance of establishing a carbon-neutral society in recent years.
The authors S.A. and P.E. thank the Hosokawa Powder Technology Foundation, Japan for funding HPTF21511 and HPTF21512. The author K.E. was supported in part by the MEXT, Japan through Grants-in-Aid for Scientific Research (JP19H05176 & JP21H00150, JP22K18737).
Saeid Akrami
Saeid Akrami obtained a M.Sc. degree in Chemical Engineering, and he is currently a Ph.D. candidate in Applied Chemistry and Life Sciences at Nagoya Institute of Technology, Japan. His research interest is the development of highly-strained and high-entropy ceramics for CO2 conversion and he is the author of several papers in this field.
Parisa Edalati
Parisa Edalati is currently a Ph.D. candidate in the Advanced Ceramics Research Center, Nagoya Institute of Technology, Japan. She was a visiting researcher at Kyushu University in 2019 before starting her Ph.D. study. She works on the design and synthesis of functional high-entropy alloys and ceramics for various applications including photocatalysis, biomedical application, hydrogen storage, and batteries.
Masayoshi Fuji
Masayoshi Fuji received his Dr. Eng. in 1999 and joined the Tokyo Metropolitan University, as a research associate in 1991. He was a visiting researcher at the University of Florida, USA, from 2000 to 2001. He joined the Nagoya Institute of Technology as an Associate Professor in 2002 and became a Professor in 2007. He is the editor-in-chief of Advanced Power Technology since 2019. His representative awards include the award from the Minister of Education, Culture, Sports, Science and Technology Japan in 2013, CerSJ award for academic achievements in ceramic science and technology in 2014, and the science award of the Society of Inorganic Materials, Japan in 2017.
Kaveh Edalati
Kaveh Edalati received his Ph.D. degree in Materials Physics and Chemistry from Kyushu University, Japan, in 2010. He currently serves as an Associate Professor at the International Institute for Carbon-Neutral Energy Research, Kyushu University, Fukuoka, Japan. His interests are the development of functional materials for carbon-neutral energy and hydrogen-related applications such as hydrogen storage and photocatalytic hydrogen generation and CO2 conversion. He particularly utilizes the high-pressure torsion method to develop new functional materials. He is the author of over 150 journal papers and serves as the editor of several journals.