2017 Volume 6 Issue 3 Pages S0071
2017 Volume 6 Issue 3 Pages S0071
To provide safe and effective products to customers in the cosmetic industry, mass spectrometry (MS) is an indispensable analytical tool. In addition to its outstanding sensitivity and specificity, the method is applicable to a wide variety of compounds, which makes it irreplaceable for the development of cosmetics, which requires the analysis of complex systems. Because most cosmetic products are applied directly to the skin and function as they are designed, monitoring the molecular compositions of endogenous or exogenous compounds in or on the skin is crucial to ensure the safety and efficacy of a cosmetic product. Recent advancements in MS and ionization techniques, such as MS imaging and ambient ionization, now provide access to richer and deeper molecular information with less time and effort. This brief review discusses advanced ionization techniques that are currently used in the field of cosmetic science using two examples, namely, the use of desorption electrospray ionization and zero-volt paperspray ionization to detect trace molecules in or on human skin.
Cosmetic science is a practical science dedicated to producing safe and effective cosmetic products and makes extensive use of mass spectrometry (MS) for the analysis of product ingredients1) and biological samples2) (typically skin specimens). The former often involves quality control and stability testing in the product-development cycle, and the latter involves the study of skin biology and transdermal drug delivery. Because of space limitations, this review focuses only on biological applications of MS by describing the latest advanced MS techniques that are in use in our laboratory.
The term “cosmetics” refers to products designed to maintain a healthy and beautiful skin. Most cosmetic products are complex mixtures of chemical compounds that are directly applied to the skin. Unlike pharmaceutical products, cosmetics are not intended to cure diseases. However, modern cosmetics are often “functional.” Products for whitening, wrinkle care, moisturizing, and treating pores, spots, etc. are produced to meet the needs of today’s consumers. Thus, some cosmetic products contain quasi-drugs, although their effects on the body remain mild and gentle. Because cosmetics are freely used by consumers with no daily-exposure limits, the absorption of quasi-drugs (and other ingredients) through the skin needs to be carefully controlled, which makes monitoring transdermal drug absorption one of the important subjects in cosmetic science.3,4) As is the case for transdermal absorption studies of topical pharmaceuticals, gas or liquid chromatography (GC or LC) combined with MS or tandem MS (MS/MS) of skin extracts are standard analytical methods.5)
Skin biology is another important subject in cosmetic science. The skin, the target of cosmetics, is the largest organ of the human body and protects the body against the external environment.6) As shown in Fig. 1, human skin is anatomically classified into two distinctive layers: the dermis and epidermis. The dermis consists of “wet” tissue composed of extracellular matrices and blood vessels, whereas the epidermis consists of relatively “dry” tissue and is further divided from bottom to top into four layers called the stratum basale, stratum spinosum, stratum granulosum, and stratum corneum. The stratum corneum is the primary research target for cosmetic science because it is the outermost skin barrier and therefore the most likely to interact with exogenous molecules, and its condition is closely related to the appearance and texture of skin.7)
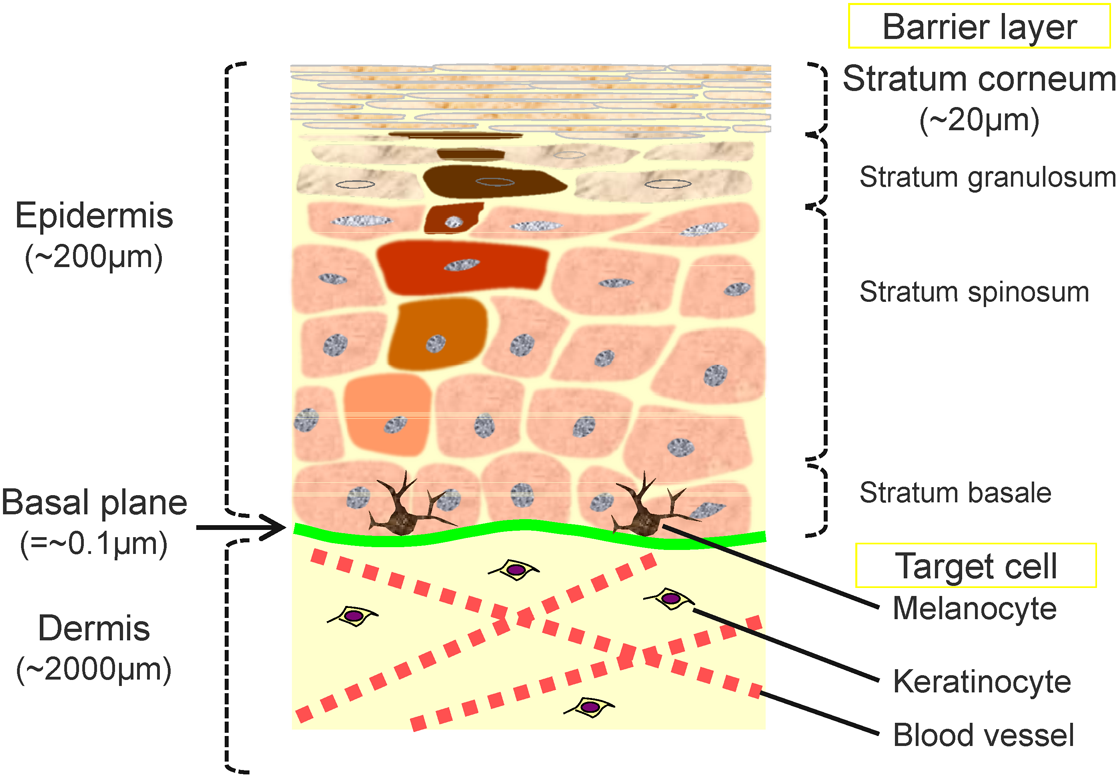
The stratum corneum is formed by the well-organized biological process called keratinization in which keratinocyte cells in the stratum basale proliferate during migration toward the surface to form 15 to 20 layers of dry, flattened cells that contain no nuclei or cell organelles. Keratinization is balanced by a process called desquamation (i.e., cell shedding), which refreshes the surface of the stratum corneum at the rate of approximately one layer per day. For healthy skin, the average thickness of the stratum-corneum layer remains constant at about 20 μm. The cycle of cell migration from the bottom of the epidermis to the skin surface, referred to as “turnover,” takes approximately 14 days and may be disrupted by various factors (e.g., ultraviolet irradiation, dryness, health status, etc.). During the turnover cycle, biological processes, such as the synthesis of intracellular lipids and the degradation of the filaggrin protein to amino acids, are driven by the harmonized activities of enzymes. Thus, the disruption of the turnover cycle changes the molecular composition of the stratum corneum, thereby degrading its health.
The stratum corneum is composed of ∼90 wt% proteins, ∼10 wt% intercellular lipids, and trace amounts of low molecular weight hygroscopic molecules and minerals called natural moisturizing factors (NMFs). A number of studies have shown that the condition and function of the stratum corneum is governed by the balance of these endogenous skin molecules.8,9) The composition of endogenous molecules in the stratum corneum is thus regarded as an important biomarker of the skin condition. For example, age-associated compositional changes in intercellular lipids affect cell packing and the resulting barrier properties of the stratum corneum.10) In addition, it has been shown that the amino acid content is related to the water content or transdermal water loss of the stratum corneum6) and that the amino acid composition varies depending on body location and season due to differences between enzymatic conversion activities11) and other factors. These facts clearly reveal the importance of the molecular composition and distribution of endogenous skin molecules.
The primary function of cosmetic products is to maintain the skin’s healthy balance by protecting it from the external environment and/or compensating for a decrease in the number of endogenous molecules. This means that the molecular composition on the surface and within the bulk of the skin becomes extremely complex, which makes MS an indispensable tool for use in the field of cosmetic science.
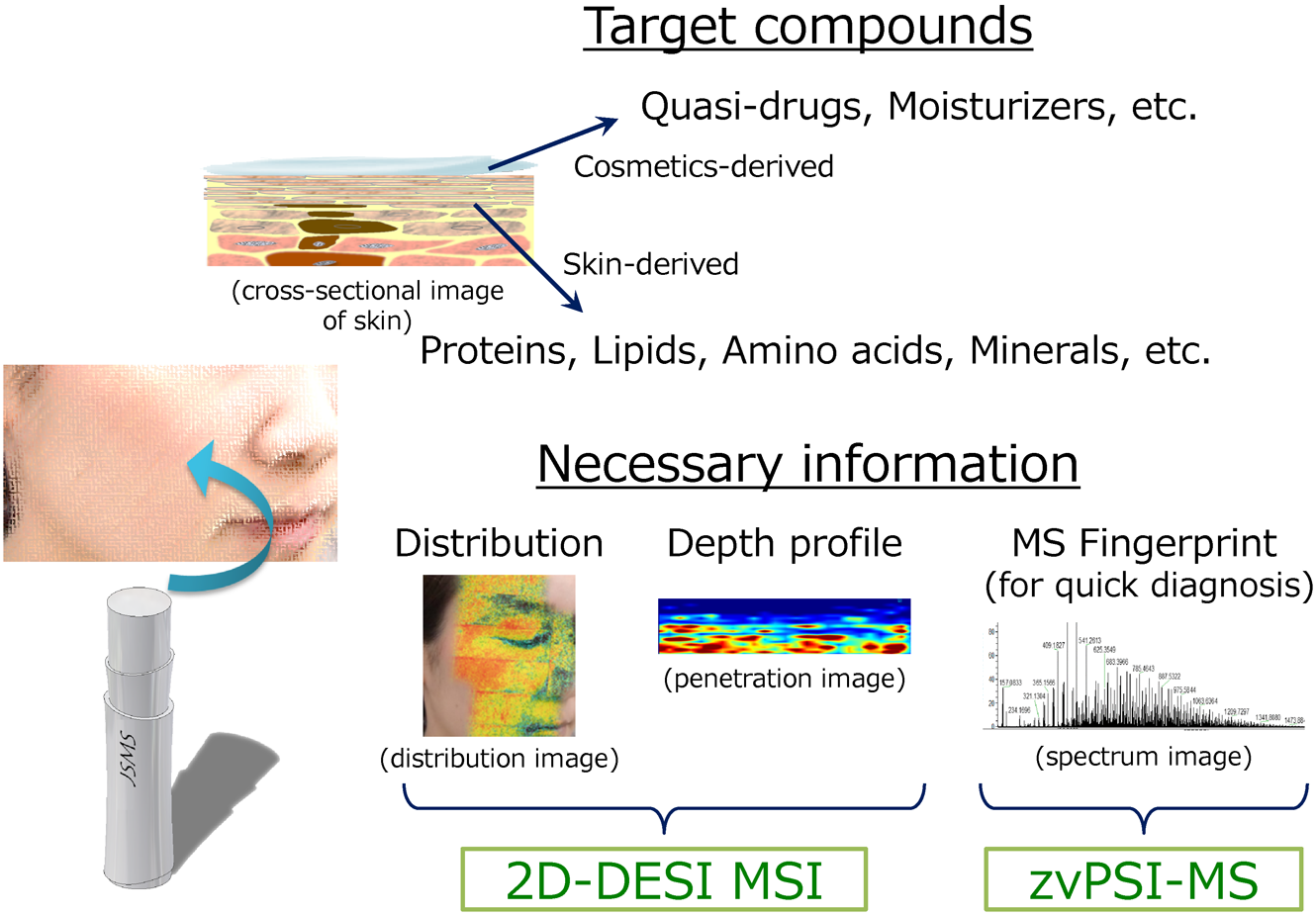
Figure 2 summarizes the advanced bioanalytical MS techniques used in our laboratory to assess the molecular composition of the skin. As mentioned above, the target molecules from skin specimens are cosmetic ingredients (quasi-drugs, moisturizers, etc.) and skin constituents (proteins, lipids, amino acids, minerals, etc.). In addition to conventional analytical methods, such as LC-MS, LC-MS/MS, GC-MS, GC-MS/MS, for bulk-sample analysis, two-dimensional (2D) desorption electrospray ionization12,13) MS imaging (DESI MSI) and zero-volt paperspray ionization MS (zvPSI-MS)14,15) have been introduced to obtain high-spatial-resolution molecular distributions and rapid profile information, respectively, with minimal sample pretreatment. As described in more detail in the following sections, 2D-DESI MSI, in conjunction with the tape-stripping stratum-corneum sampling technique,16,17) and zvPSI-MS are tools that promise to accelerate progress in the field of cosmetic science.
DESI, which was proposed by Takáts et al. in 2004,12) is the first ambient ionization method18) to allow the direct analysis of solid-state samples by mass spectrometry (MS) in an open environment. DESI generates gas-phase ions derived from analytes by using electrospray ionization. In addition to providing fast profiling with minimal or no sample pretreatment, DESI has already been used as a MSI method in a variety of applications.13)
To date, applications of MSI to skin samples have mostly used time-of-flight secondary-ion mass spectroscopy (TOF-SIMS) and matrix-assisted laser desorption-ionization MSI (MALDI-MSI), presumably because of the broader interests in applications that provide high spatial resolution.19–21) In fact, TOF-SIMS has been used for MSI analysis with submicron resolution, whereas, due primarily to the intrinsic characteristics of the ionization method used, the targets are limited to low-molecular-weight compounds.19) Conversely, MALDI-MSI offers relatively modest spatial resolution (~micron level) but permits the analysis of mid- to high-molecular-weight macromolecules (i.e., proteins and polymers) and gives more options for advanced analysis by MS such as MS/MS and/or high-resolution m/z measurements. To the best of our knowledge, only few studies have applied 2D-DESI MSI to the analysis of substances in skin samples,22) excluding the direct or indirect (offline) profiling (non-imaging) of the surface of skin in forensic,23) security,24) and healthcare studies.25) Note that most of the skin samples used in previous MSI studies consist of slice tissues or biopsies, which involve invasive procedures and are thus not suited for the daily evaluation of cosmetic products.
The tape-stripping technique16,17) is also used in cosmetic science as a convenient method to rapidly and noninvasively collect stratum-corneum samples. The technique involves using an adhesive tape to collect a sheet of the stratum corneum from the skin surface. Repeating the process at the same site allows literally “layer-by-layer” sampling for rapid depth profiling. By combining 2D-DESI MSI with tape stripping, we developed a rapid method for analyzing the distribution of quasi-drugs26) and endogenous molecules27) in the stratum corneum.
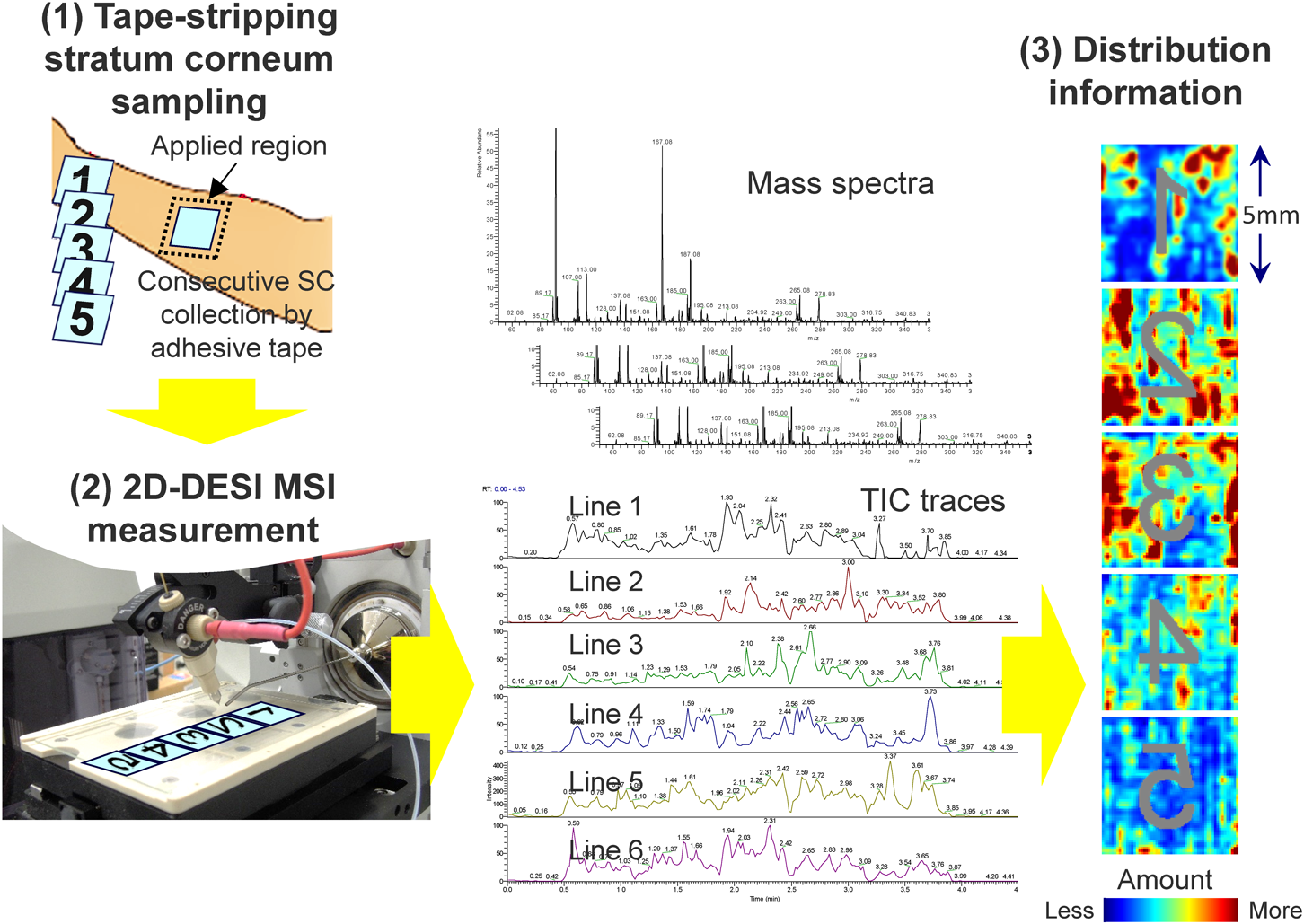
Figure 3 shows the general workflow for the proposed ex vivo 2D-DESI MSI method.26) Briefly, after applying a topical cream on the skin, the sampling site is gently washed with soap and water. After allowing the skin sample to equilibrate for 15 min at room temperature, the stratum-corneum samples are collected by tape stripping with an adhesive tape under an established protocol (gentle pressing at about 0.22 MPa followed by abrupt stripping, etc.). By repeating the process at the same site, deeper layers of the stratum corneum can be obtained. The collected stratum-corneum samples were directly analyzed by 2D-DESI MSI.
To validate and optimize the method, we visually inspected the stratum-corneum samples collected by tape stripping. Figure 4(a) shows microscopy images of stratum-corneum samples collected by repeated tape stripping from the inner forearm of a healthy male volunteer (aged mid 40s). As expected, the stratum corneum collected on the first strip consists of a “sheet” of flattened cells, whereas that collected from the fifth and tenth strips appear more scattered and less dense. The cells from the deeper samples have smaller diameters (10–20 μm) because the cells are immature (in terms of keratinization).
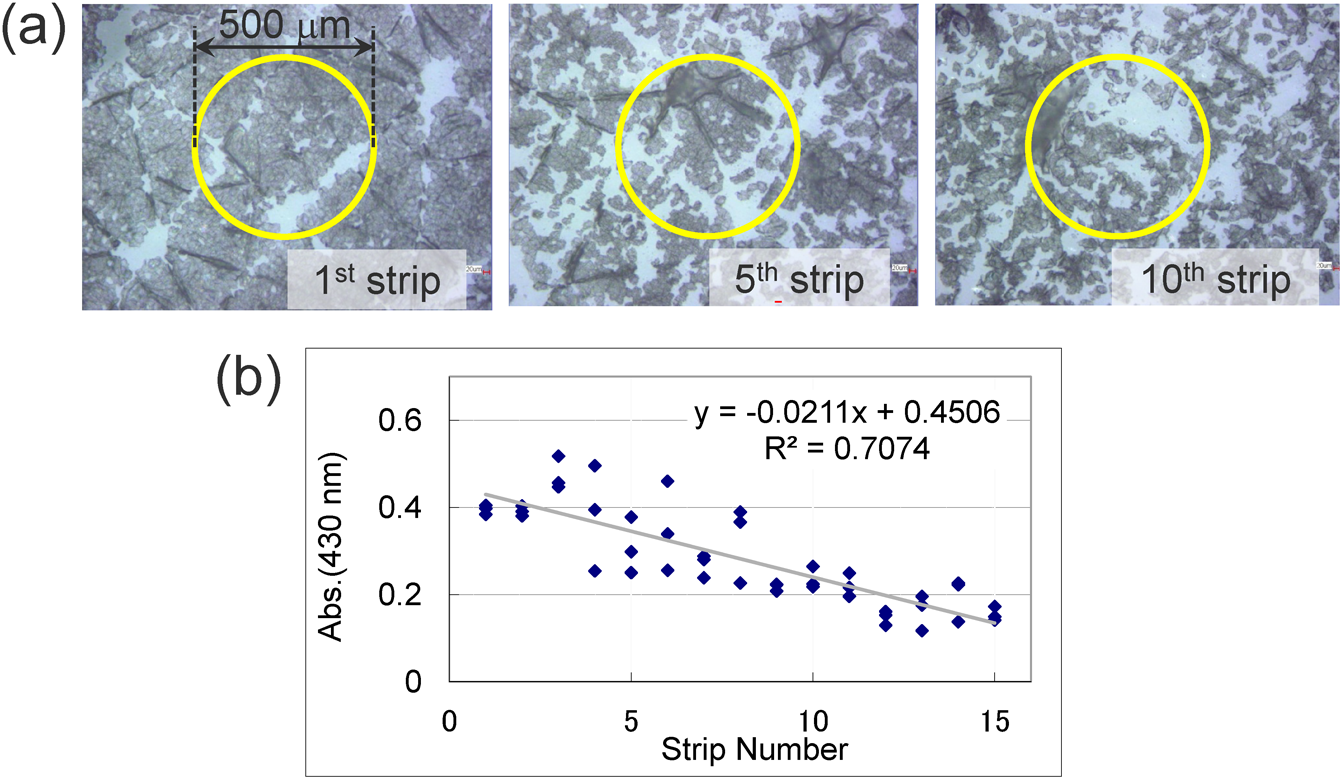
To compare the spatial resolution of 2D-DESI MSI to the size of stratum-corneum cells, in Fig. 4(a), yellow circles are drawn to show the typical resolution of 2D-DESI MSI (500-μm diameter). The results clearly show that the proposed 2D-DESI MSI method collects information on stratum-corneum cells averaged over the targeted area of the skin. TOF-SIMS or MALDI-MSI is clearly the preferred choice when a five- to ten-fold greater spatial resolution is required. We thus use 2D-DESI MSI as a complementary technique for the rapid acquisition of molecular distributions of larger areas (e.g., the human face).
For quantitative discussions, light absorbance is often used to estimate how much of the stratum-corneum protein is collected by tape stripping.28) Figure 4(b) shows the absorbance at 430 nm for tape-stripped stratum-corneum samples that were taken repeatedly from the three vicinal sites from the inner forearm of the male volunteer. The results reveal that the absorbance decreases for the deeper strips, which is consistent with the population of the cells, as determined by visual inspection [(Fig. 4(a)]. To convert absorbance into the quantity of protein, the samples are dispersed into a 0.1% RapiGest® (Waters, Inc.) aqueous detergent solution and the protein content quantified by using the Lawry method. The protein quantity thus obtained is used to correct quantitative data on target molecules from each strip.
Figure 5 shows an example of the evaluation of transdermal drug delivery by 2D-DESI MSI. Stratum-corneum samples were collected after the application of a topical cream containing 4-methoxy salicylic acid potassium salt [4MS(K)], which is a registered mild whitening drug, onto the inner forearm of a healthy male volunteer. For demonstration purposes, a test formulation containing 1% 4MS(K) was applied at a rate of ~50 mg/cm2, and the applied site was left exposed (i.e., not occluded by a cotton mask or a wrap film) for 1 h. After gently cleaning the skin with soap and warm water, the skin was allowed to equilibrate for 10 min at room temperature, the stratum-corneum samples were then collected by tape stripping. Small pieces of adhesive tape containing stratum corneum were directly analyzed by 2D-DESI MSI. The DESI experimental conditions were similar to the standard settings suggested by the manufacturer for samples on a glass surface, except that the distance between the DESI spray nozzle and the MS inlet was increased slightly (0.5–1 mm) in order to obtain a stable signal.
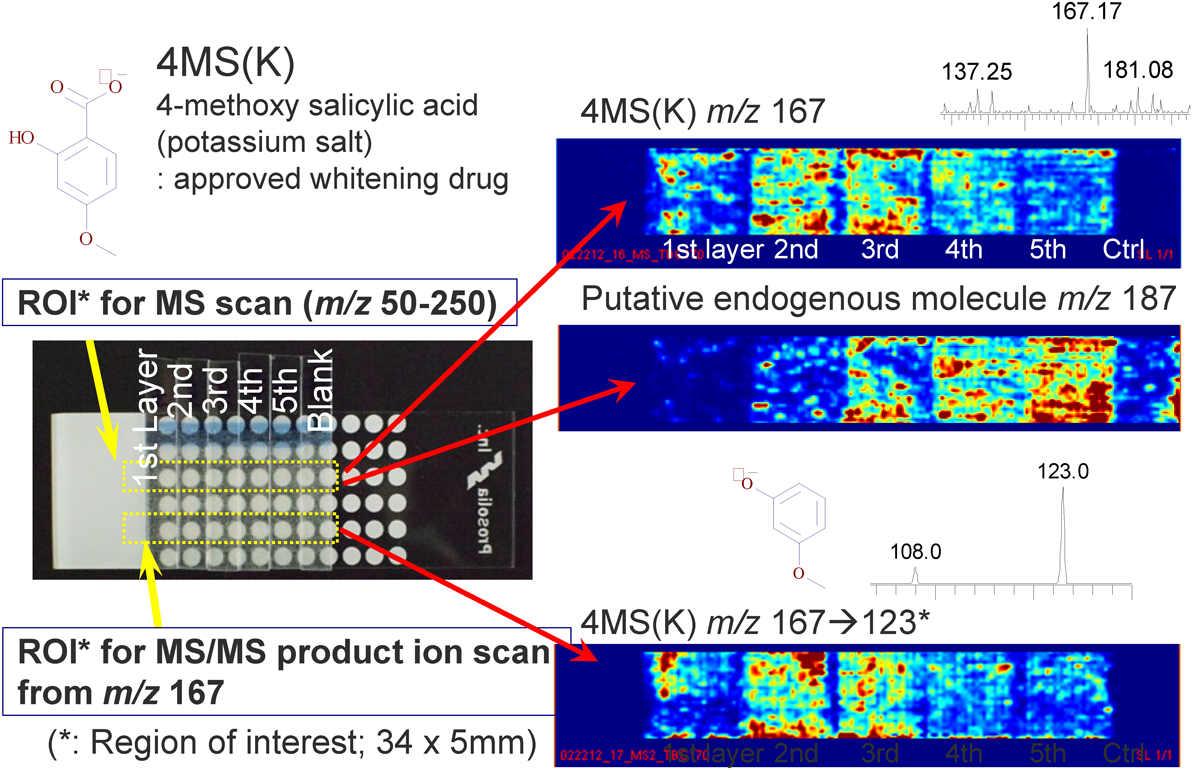
The data show that most of the 4MS(K) molecules accumulate on the second and third stratum-corneum layers, which is typical for compounds having moderate partition coefficients (P) [log P=2.10±0.26 for the free acid form of 4MS(K), calculated by ACD/Labs from Advanced Chemistry Development, Inc.]. The low concentration of 4MS(K) molecules in the first layer is attributed to the washing with soap, which removes any topical cream remaining on the surface. The identity of the analyte molecule is confirmed based on the presence of the major MS/MS product ion (m/z 123, CO2 eliminated ion). The middle panel on the right of Fig. 5 shows the depth profile of a putative endogenous molecule (m/z 187); the amount of this molecule decays gradually upon approaching the skin surface. Although its identity is currently unknown even after high-resolution MS and MS/MS measurements, circumstantial evidence suggests that the molecule originates from the body. We have been using this technology in our laboratory to evaluate the early performance of penetration enhancers, which play a crucial role in the transdermal delivery of hydrophilic (low log P) quasi-drugs. Note that the planar distribution is also very valuable for analyzing the penetration route, which will be discussed elsewhere.
The 2D-DESI MSI method was also applied to an analysis of the distribution of NMF amino acids across the human face.27) As mentioned above, NMF amino acids are produced through the enzymatic degradation of filaggrin protein in the skin.29–31) In the process of keratinization, some amino acids are converted by heat, UV light, and/or enzymes, and act as natural humectants in the stratum corneum. We are interested in understanding the relationship between the molecular composition of NMF amino acids and skin conditions. The moderate spatial resolution of 2D-DESI MSI is advantageous for obtaining macroscopic molecular-distribution information from a human face within a reasonable time (∼8 h for half the face at 0.5 mm resolution).
Figure 6 shows a representative facial NMF distribution for a healthy female subject. Stratum-corneum samples were collected from eight sites over half of her face. Three samples were collected consecutively from each site by using the adhesive tape originally developed for gentle “layer-by-layer” sampling, and the results for the second sheets are shown. After estimating the protein quantity by near-infrared absorbance (850 nm), the stratum-corneum samples were directly analyzed by 2D-DESI MSI (i.e., without sample preparation).
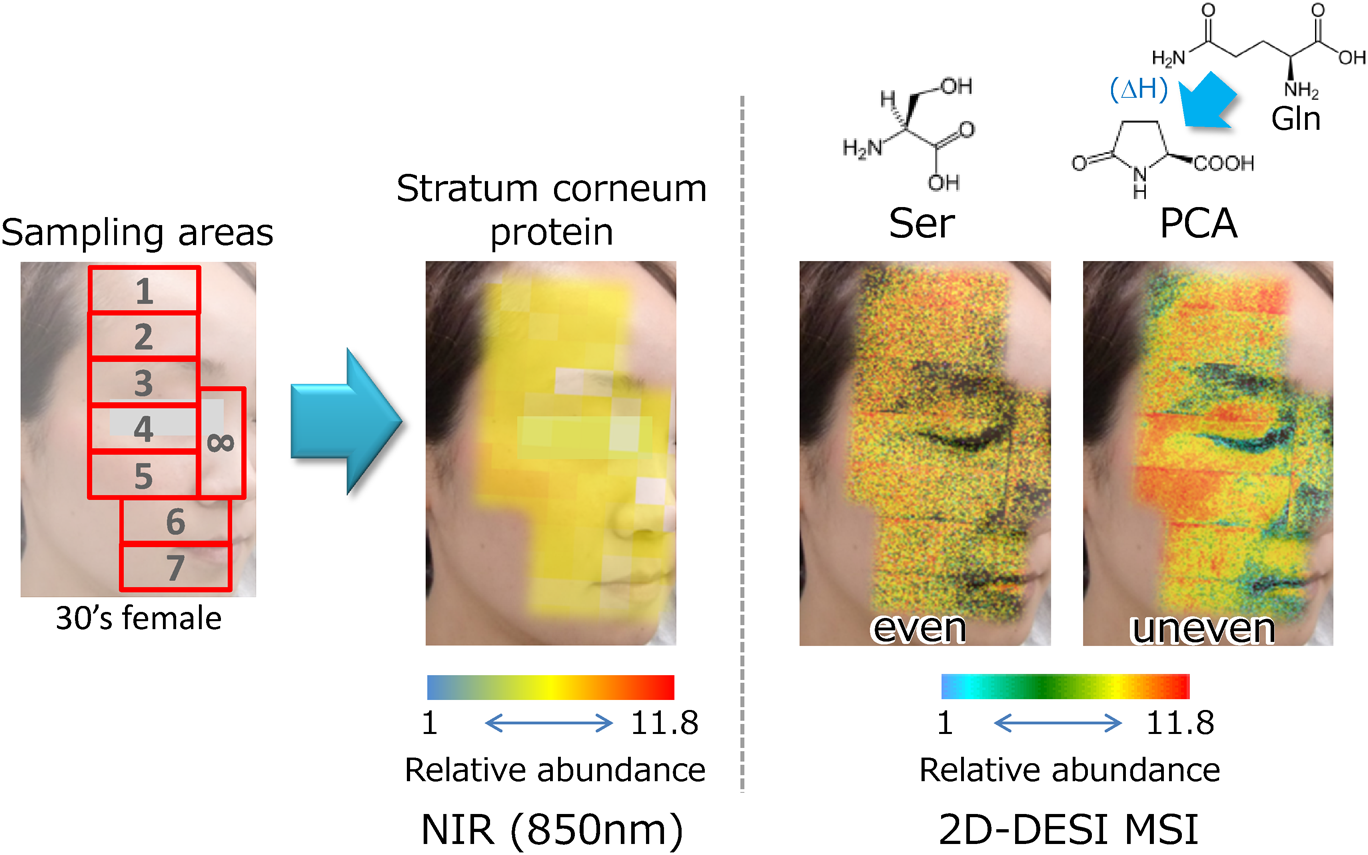
As an example, Fig. 6 shows the difference in distribution between serine (Ser) and pyrrolidone carboxylic acid (PCA). Serine is the most abundant NMF amino acid and is not converted in the stratum corneum during turnover. The distribution of serine is uniform across the (half-) face and similar to that of the stratum-corneum protein, which suggests that serine could serve as an index of the basal NMF-generation activities. Conversely, PCA, which is a conversion product of glutamine (Gln), exhibits a unique distribution pattern, indicating the differences in biological activity in the stratum corneum for different parts of the face. Unfortunately, the significance of these molecular conversions in the stratum corneum remains unknown. To find answers to this question, the relationship between NMF composition and skin parameters, such as bleomycin-hydrase activity,31) skin water content, and trans-epidermal water loss, are currently being investigated in detail.
The extractive nature of DESI is useful for analyzing skin samples because the selectivity of analytes can be easily controlled by changing the polarity of the spray solvent. In our experience, an acetonitrile–water (1 : 1, v/v) mixture containing 0.1% formic acid is the best balance for sensitivity and detection coverage for analyzing NMF amino acids. Seventeen out of twenty-one typical NMF amino acids were observed from the stratum corneum of healthy male and female subjects.27) For the analysis of skin lipids, such as fatty acids, glycerides, cholesterol, and ceramides, MeOH including 0.1% formic acid works best for the same reasons.27)
Absolute quantitation is a challenging task for most MSI techniques that desorb analytes from condensed-phase samples without the help of liquid dissolution or the use of chromatographic separation. In addition to the matrix effect, which alters signal intensity, the recovery of the analyte from the region of interest affects the quantitative accuracy. To estimate the recovery (or extraction efficiency) of NMF from stratum-corneum samples by using 2D-DESI MSI, we measured (data not shown) the residual amounts of serine in the samples that were formerly analyzed by 2D-DESI MSI. As a result, the recovery of serine by 2D-DESI MSI was approximately 50% in comparison with the recovery obtained by LC-MS/MS measurements of a solvent extract of the same sample. Briefly, the quantification by 2D-DESI MSI was based on an external calibration established for serine that was deposited onto a tape-stripped stratum corneum, and the peak volume obtained for m/z 104 under the negative-ion detection mode was plotted against the amounts that were spotted (11–280 pmol/mm2, the standard addition method). For LC-MS/MS quantification, serine was quantified in the form of a 3-aminopyridyl-N-hydroxysuccinimidyl carbamate derivative32) by reversed-phase liquid chromatography based on the internal standard method using serine-13C3, 15N as an internal standard. Because the extraction efficiency of 2D-DESI MSI depends on the chemical properties of the analytes, the sampling location, and DESI MSI parameters, quantitative data of 2D-DESI MSI need to be carefully considered, as is the case for other MSI methods.
The other important factor that may impact the quantitative performance of 2D-DESI MSI is possible variations in the quantity of stratum corneum collected after the application of a cosmetic.33,34) This is influenced by the degree of hydration and other ingredient-associated factors, which may influence the adhesive strength of stratum-corneum cells.34) The only possible countermeasure against this effect is to do a carefully controlled experiment each time. For example, to determine if the stratum corneum is completely removed under the given conditions, all stratum-corneum layers are removed by repeated tape stripping 20 to 30 times from the same site until all the stratum-corneum cells are collected. The number of strippings needed varies depending on the thickness of the stratum-corneum layer (body-site dependent) and on the adhesive strength of the stripping tape. By plotting the estimated amount of stratum-corneum protein versus strip number, the influence of the applied product can be estimated. Unfortunately, this process is invasive and tedious and so cannot be performed on a regular basis. In practice, our laboratory uses up to five strippings with a dedicated mildly adhesive tape (in-house product) to avoid potential skin troubles such as irritation and pigmentation. To ensure quantitative accuracy, the amount of protein on each strip is always measured by near-infrared or visible-light absorbance.
Thus, the proposed technique of applying 2D-DESI MSI to stratum-corneum samples obtained by tape stripping is a practical method that allows molecular events on human skin to be rapidly analyzed in a nearly noninvasive manner. Because DESI ionizes lipids, proteins, and even nonpolar compounds (by a technique known as Reactive-DESI35)), the technique is expected to gain widespread use in advancing skin research in cosmetic science.
Analysis by rapid profiling is also important for cosmetic science, which demands the fastest possible product-development cycles. Zero-volt paperspray ionization,14,15) originally developed by Cooks and colleagues,14) is a variant of paperspray ionization that requires no gas or high voltage to generate gas-phase ions of analytes. The additional benefit of zvPSI over paperspray ionization is that it uses a simpler and safer platform for sample loading and ionization because applying a high voltage (2–3 kV) to the paper slip is not needed. A known drawback, however, is the relatively low ionization efficiency, especially for non-surface-active compounds.14)
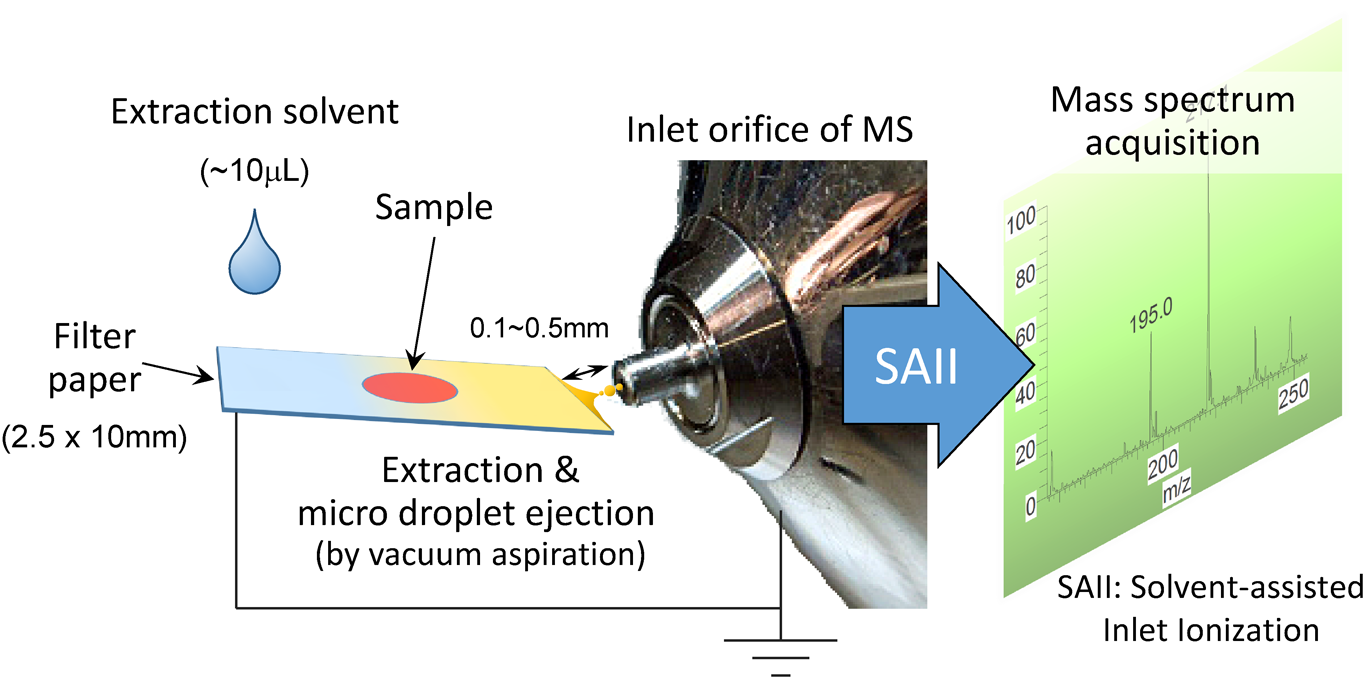
Figure 7 shows a flowsheet diagram for a zvPSI-MS measurement.15) A liquid or solid (condensed-phase) sample is loaded onto a piece of filter paper and extracted by a droplet of extraction solvent. The extract containing the analyte of interest is transferred almost simultaneously to the tip of the paper and drawn by aspiration force into the inlet orifice of the MS. Ionization is achieved by a mechanism similar to or the same as solvent-assisted inlet ionization.36) According to this principle, analyte ions (either pre- or post-formed) are ejected into the gas phase from the droplets that form inside the inlet capillary. A single run can be completed within a few seconds and an approximately pmol level of sensitivity is achieved when the instrument operates under optimal conditions (LTQ-Orbitrap, Thermo Fischer Scientific, Inc.).14,15) Note that the inlet orifice is grounded and the electric potential of all lens electrodes inside the vacuum chamber are set to zero by the control software. Based on the observation that negative and positive analyte ions are detected under the same conditions (except for the polarity of the MS detection mode), the influence of electrostatic forces on gas-phase ion generation was determined to be negligible.15) Likewise, the analytical performance was the same regardless of whether the sample-loading paper was grounded, suggesting that the contribution of “electric-field-driven charge separation” (as in electrospray ionization) is not significant for gas-phase analyte-ion generation.
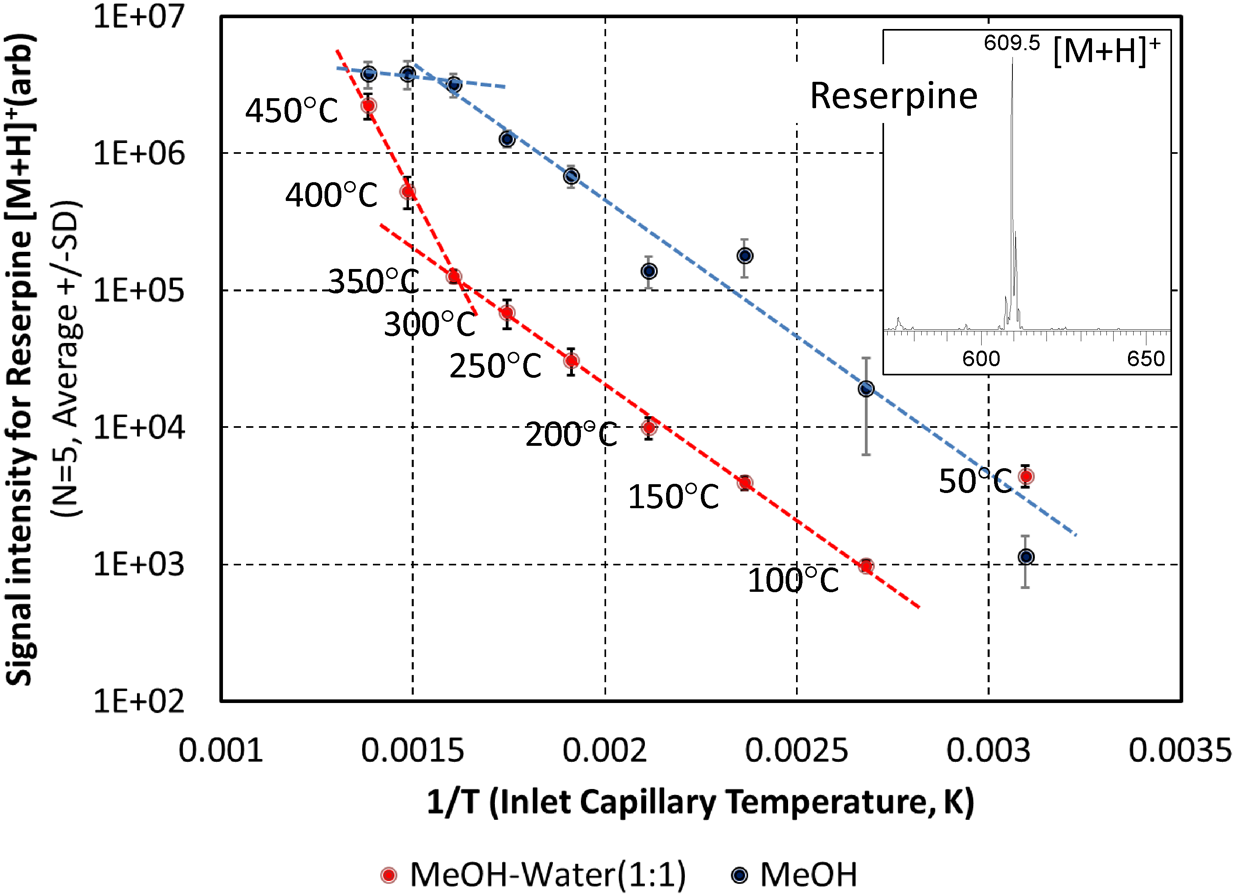
As assumed from its working principle, the sensitivity of zvPSI-MS depends on the temperature of the inlet capillary and the composition of the extraction solvent15) (see Fig. 8), suggesting that vaporization processes govern gas-phase ion generation in zvPSI. In general, higher capillary temperatures give rise to larger signals. Although the detailed mechanism responsible for this is unclear, the two-solvent system under test exhibits different profiles. The boiling points of MeOH and water at atmospheric pressure are about 65°C and 100°C, respectively, and that of a 1 : 1 v/v mixture is about 78°C. This difference may partly explain why, at temperatures below 100°C, the plot shows a completely different tendency. Because the solvent vapor pressure is known to increase with temperature, a rapid or even explosive evaporation of liquid droplets may influence the ionization efficiency in zvPSI-MS. Note that little or no fragmentation is detected for the analytes that were tested (reserpine, caffeine, and amino acids) even at inlet temperatures in excess of 300°C (see Fig. 8 inset for reserpine). To ensure the durability of insulating materials inside the ion source, however, 350°C is recommended as an upper limit for a long-term routine use.
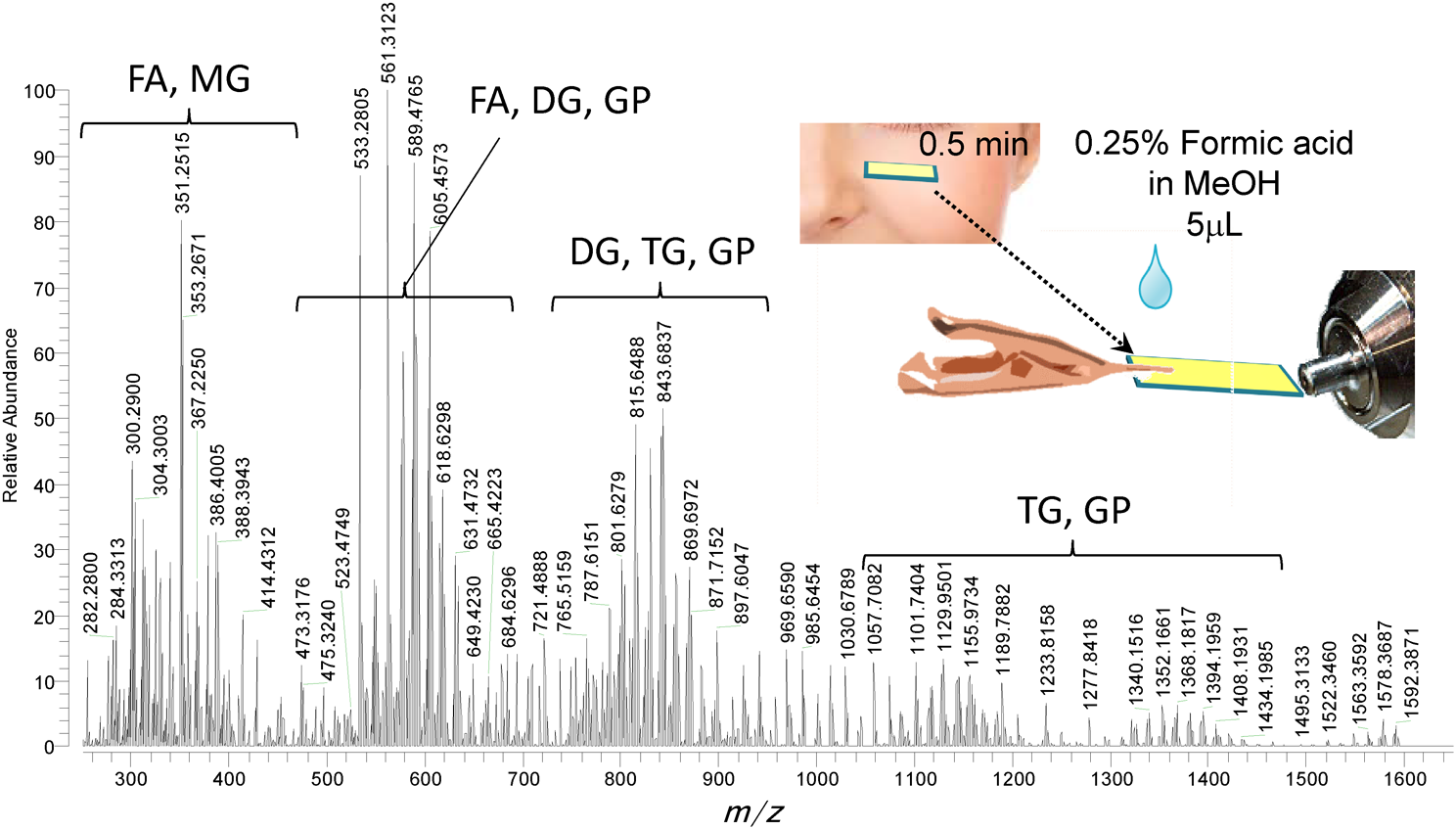
The most attractive feature of zvPSI-MS is the rapid profiling of (semi-)solid and liquid samples that can be loaded onto a filter paper slip. Figure 9 shows a representative result of an imprint sample collected noninvasively from a human face.15) After gently washing the face with soap and water and allowing the skin to equilibrate for 15 min at room temperature, lipids from the skin surface were collected from the cheek of a male volunteer onto a small piece of filter paper (2.5×10 mm2), which was gently pressed against his cheek for 30 s. A zvPSI-MS analysis of the imprint sample was done by applying 5 μL aliquot of 0.25% formic acid in MeOH onto the paper as an extraction solvent. As shown in Fig. 9, free fatty acids (FA), monoacyl glycerides (MG), diacyl glycerides (DG), and triacyl glycerides (TG) were detected in the positive-ion detection mode as protonated or cationated molecules.15) The lipids were putatively identified by comparing the measured accurate m/z with theoretical equivalents in the LIPID MAPS® database (www.lipidmaps.org) at up to ±0.005 m/z tolerance. In the negative-ion detection mode, sphingomyelins (SM), ceramide phosphoethanolamines (PE-Cer), ceramide 1-phosphates (Cer-P), cholesteryl esters, and wax esters (WE) were additionally detected from an identical imprint sample. Because the imprint samples allow easy remote sampling and one analysis requires only 5 s, zvPSI-MS is a promising tool for rapid diagnostics.
Although zvPSI-MS is suitable for rapid profiling (i.e., qualitative analysis), it is reasonably quantitative when an appropriate internal standard is added to the extraction solvent.15) As a demonstrative trial, calibration curves for 17 amino acids were generated for an equimolar mixture deposited onto filter strips (1, 5, 10, and 50 nmol each). Gly(d2), dissolved in the MeOH–water (1 : 1, v/v) extraction solvent, was used as an internal standard, and peak-height ratios of zvPSI-MS data were plotted against the deposited amounts. The R2 coefficients of determination fall in the range of 0.9694 (Met) and 0.9964 (Ser) and are linearly correlated.
The samples that may be analyzed by zvPSI-MS range from small polar compounds, such as amino acids, to mid-polar drugs, polyols, and polymers such as polyoxyethylene-based materials and peptides and small proteins.15) Because zvPSI-MS involves no chromatographic separation, combining it with high-resolution or high-selectivity MS is recommended.
Thus, zvPSI-MS is especially useful for rapid screening applications such as diagnostics. Because zvPSI requires no external peripheral equipment, such as nebulizing gas, heaters, or high-voltage, its combination with a compact MS instrument should also be beneficial for implementing on-site analytical platforms.
Recent innovations in MS have contributed to many scientific discoveries in a variety of fields, and the cosmetic industry is no exception. Because the cosmetic industry deals with very complex systems involving mixtures of chemical and complex interactions with the human body, it relies heavily on state-of-the-art MS techniques. Although this brief review has focused only on applications involving two advanced ionization and detection methods, technical innovations in MS are also crucial for finding new phenomena and for defining biological systems. One result of the technical improvements is the increased speed of data acquisition; for example, the data-acquisition speed of the latest MSI instrument has improved by 10 to 20 times. In addition, other vendors are now striving to develop methods for acquiring real-time visual information from MSI experiments. The combination of innovative ionization techniques and state-of-the-art instrumentation should thus continue to maximize the research outcome and accelerate science in many fields.
DESI, desorption electrospray ionization; DG, diacyl glycerides; FA, fatty acids; MG, monoacyl glycerides; GC, gas chromatography; LC, liquid chromatography; MS, mass spectrometry; NMF, natural moisturization factors; PCA, pyrrolidone carboxylic acid; SAII, solvent assisted ionization inlet; TG, triacyl glycerides; WE, wax esters
This brief review is a summary of the presentation at the satellite conference of the BMS symposium 2016 held in Kyoto, Japan. The authors wish to thank the organizer of the symposium, Dr. Tohru Yamagaki, Suntory Foundation for Life Science, for his cordial support. The authors also wish to thank the reviewer(s) and the editors of the journal MS Tokyo for their critical review and countless efforts. All of the research presented here are the results of a team effort of a number of researchers in our laboratory. We also wish to thank all those were not able to list because of space limitations. The authors would like to thank Enago (www.enago.jp) for the English language review.