2018 Volume 59 Issue 12 Pages 1915-1919
2018 Volume 59 Issue 12 Pages 1915-1919
The synthesis of a new hybrid polymer derived from glycerol citrate polymer (GCP) and yttrium oxide nanoparticles was performed without catalyst and using an equimolar concentration. The biocompatibility and antimicrobial capacity of citrate-based polymers suggest it to be involved in biomedical applications since citric acid is a natural molecule. Glycerol citrate polymer and yttrium oxide (Y2O3) nanoparticles were synthesized by autocatalytic polymerization and sol-gel methods respectively. Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), transmission electron microscopy (TEM), differential scanning calorimetry (DSC), antibacterial effect and cell viability assay were performed to characterize the material. The cubic crystalline Y2O3 nanoparticles dispersed in glycerol citrate polymer could be the cause of the increased antibacterial effect presented in the pure glycerol citrate polymer; supporting the potential of the hybrid polymer as a biotechnological material. The cytotoxicity was evaluated trough human neuroblastoma SH-SY5Y cell.
Biodegradable polymeric materials play an important role in the medical field, such as drug delivery systems, the manufacturing of prosthesis or devices that contribute in the human health. However, in the medical area there is a potential using of the polymer against microbial infections. In dairy products, Bacillus licheniformis is a common contaminant, it has been associated with different clinical conditions, food spoilage such as ropy bread, and incidents of food-borne gastroenteritis. Bacillus licheniformis has also been associated with septicemia, peritonitis, ophthalmitis, and food poisoning in humans, as well as with bovine toxemia and abortions.1) A baby milk powder containing toxigenic Bacillus licheniformis involved in fatal illness of an infant was recently described.2) An outbreak of food-poisoning after a meals-on-wheels lunch affected 49 persons, 1 of whom died. Bacillus cereus serotype 10 and Bacillus licheniformis were isolated in large numbers from many of the patients including the deceased and from the remains of the meal.3) Bacillus licheniformis can cause foodborne ill-ness, with nausea, vomiting, diarrhea and stomach cramps occurring 5–12 h after consumption of a variety of foods that have included ice cream, deserts, meat pies and sandwiches; counts, where made, ranged from 3 × 105 to 1 × 108 CFU g−1 of the implicated food but ingested doses are unknown.4) Likewise, Shigella bacteria’s group are commonly found in contaminated water with human feces, the fecal-oral route is the primary route of infection. It is responsible for a worldwide health problem and a serious concern in the developing countries. It is estimated that Shigella spp. account for 1.1 million deaths and 165 million cases of dysentery annually worldwide.5) The annual number of Shigella episodes and deaths in Asia was estimated to be 91 million and 414,000, respectively. A recent review of the literature concluded that the annual number of cases of Shigella diarrhea worldwide was approximately 165 million, 99% of which occurred in developing countries. Some 1.1 million people were estimated to die from Shigella infection each year, 60% of whom being children under 5 years of age.6)
Shigella infection promotes impaired nutrition, recurring infection and retarded growth. Antibiotic resistant strains are continuously emerging thus treatment regiments become very difficult against shigellosis.5) In this study, we synthetized a glycerol citrate polyester based on the combination of citric acid and glycerol and demonstrated its antibacterial effect. This material could be employed in short-term applications where rapid biodegradability is necessary. A possible application is in bio-medical devices, where other polyester materials such as polylactic and polyglycolic acids are currently used.7,8) The main properties of glycerol citrate polyesters have been previously reported. Pramanick and T.T. Ray reported that all the cross-linked polymer samples were initially insoluble in both water and common organic solvents, however, if kept suspended in water, become soluble probably due to partial hydrolysis of the cross-links.9) Budhavaram and Barone found that glycerol citrate polymers could be obtained from incomplete reactions, reducing both the energy consumption and process cost and concluded that temperature plays a key role in the polymer formation.10) Tisserat et al. studied the influence of various heating methods without catalysis to prepare this polymer.11) Mariano-Torres et al. studied the glycerol citrate polymer synthesis without catalysis, different concentrations and temperatures, in two steps poly-condensation: Pre-polymerization and polymerization.12) Shixin Liu et al. reported transparent thin films prepared using sulfuric acid.13) Halpern et al. used PTSA and ZnCl2 as catalyst, comparing the polymer formed without catalyst with them, finding different thermal properties in the polymer. About nanoparticles, Schubert et al. has reported that yttrium oxide particles protect nerve cells from oxidative stress and that the neuroprotection is independent of particle size. The yttrium oxide nanoparticles act as direct antioxidants to limit the amount of reactive oxygen species required to induce the cell death, suggesting that this group of nanoparticles could be used to modulate oxidative stress in biological systems.14,15) Polymer/inorganic nanoparticles hybrid have attracted attention recently because they combine the advantages of polymer as elasticity, transparence or dielectric properties and the inorganic particles have specific absorption of light, chemical activity or can be function as catalyzer.16,17) As hybrid materials are copolymers based on inorganic and organic comonomers, they display enhanced properties by bridging the property space between two dissimilar types of materials. Depending on the relative amounts of the two components, the properties of the resulting hybrid are intermediate between those of an inorganic and an organic polymer.18) Possess interesting optical, magnetic, and blending properties.19) It is important highlight that ultrasonic homogenization has been extensively employed for producing homogeneous composites. So far, many relevant researches have been conducted.20) It is well known that citric acid alone is effective for preventing bacterial growth or enhancing the antimicrobial properties of other antibiotics. Citric acid-based polymers also provide unique intrinsic antimicrobial properties, and can be easily manipulated physically and mechanically.21,22) According to the mechanism of antimicrobial activity, the activity of antimicrobial polymers can be categorized as either passive or active. Due to the mainly hydrophobic and negatively-charged properties of microbes, passive polymers should be either hydrophilic; negatively-charged; or have a low surface free energy. Active polymers actively kill bacteria that adhere to the polymer surface. Polymers functionalized with active agents, such as cationic biocides, antimicrobial peptides, or antibiotics, can kill bacteria on contact.23)
On the other hand, Y2O3 nanoparticles after penetration into the bacteria have inactivated their enzymes, generating hydrogen peroxide and caused bacterial cell death. Last studies show that Y2O3 nanoparticles can be used as effective growth inhibitors in various microorganisms, making them applicable to diverse medical medicines and antimicrobial control systems.24) Interestingly, glycerol citrate polymer with rare earth oxide nanoparticles as a hybrid material has not been reported. In this work, pure glycerol citrate polymer and its hybrid with yttrium oxide nanoparticles were synthetized and characterized to improve the efficacy to inhibit the bacterial growth and supporting the potential of these polymers as biotechnological materials. The glycerol citrate polymer and the hybrid formed by the dispersion of yttrium oxide nanoparticles were characterized chemical, thermal, structurally and morphological as well as its antibacterial effect.
Citric acid (CA) anhydrous, (A.C.S. reagent grade, Meyer) and glycerol (G), (A.C.S. reagent grade, Meyer) and methanol MeOH, (CH3OH 99.8%, Sigma-Aldrich) were used as received. Yttrium oxide was obtained from yttrium chloride (YCl3·6H2O 99.8%, Sigma-Aldrich). For the microbial culture was used nutrient agar for microbiology (Sigma-Aldrich) and calcium chloride dihydrate (CaCl2 2H2O ACS reagent, ≥99%; Sigma-Aldrich).
2.2 Yttrium oxide nanoparticles synthesisThe detail of sample preparation has been reported in the literature.25) Y2O3 was obtained by sol-gel method from hexa-hydrate yttrium chloride and methanol after 700°C for 1 h.
2.3 Glycerol citrate pre-polymer and polymer synthesisA relation CA/G 1:1 molar ratio was performed. Glycerol was heated at 80°C inside the reactor for 10 min before adding the CA to decrease the viscosity. CA was added slowly using a funnel ensuring a uniform mixture. According to the increasing of temperature the mixture became homogeneous and transparent. After 90 min at 140°C the reaction began; water was generated as steam and moved towards the condenser, achieving a steady drip of water. The water was separated from the main sample. The pre-polymer was placed in a silicon container and heat at 170°C in an oven for 1 h to obtain the complete polymerization of glycerol citrate polymer, it was kept inside to reach room temperature.
2.4 Glycerol citrate polymer/Y2O3 nanoparticlesThe preparation of hybrid polymer follows the same procedure of pure glycerol citrate polymer pre-polymerization. For this polymer, after 1 h of pre-polymerization, the distribution of Y2O3 nanoparticles (0.05%) by mechanical stirring (60 min) and ultrasonic (60 min) with a frequency of 40 kHz in water bath at 60°C was done. The polymerization step was performed using the same procedure of pure glycerol citrate polymer polymerization.
2.5 Characterizations and measurementsFourier transform infrared spectroscopy (FTIR) was performed in a Spectrum One equipment using Potassium Bromide (KBr) pelleting technique with 10 scan number, with a 4 cm−1 in the range of 450 to 4000 cm−1. Structural characterization was done by mean of X-Ray diffraction (XRD). This test was carried out in a Bruker D8 Advance Vantec, with Step size of 0.0685°, breakthrough time 1 second, 2θ range: 20–100° and scan time of 10 min. The microstructure of the samples was determined by Transmission electron microscopy (TEM) JEM 1400 Equipment (Tokyo, Japan). Thermograms were performed on a DSC instrument with a heating ramp of 10°C/min at the temperature range of −40°C to 180°C. The DSC was purged with nitrogen at 80 mL/min.
The bacteria growth inhibition by the hybrid polymer was evaluated in gram negative Shigella spp and gram-positive Bacillus licheniformis bacteria. These bacteria were routinely seed in nutrient agar + CaCl2 2H2O, at 45°C. Both GCP–Y2O3 films and paper-disks soaked in penicillin and distilled water as a positive and negative control respectively were cut and placed onto the bacteria an incubated for 24 h at 37°C. The antimicrobial effect was calculated by mean of percent decrease in microbial growth in comparison to the control sample. The experiment was performed in triplicates.
Cytotoxicity was measured by MTT Cell Proliferation Assay. Briefly, 5 × 104/well neuroblastoma SH-SY5Y cells were cultured in a 96-well microtiter plate in a final volume of 200 µl/well, 24 h prior the experiment. The cells were incubated with the polymers at concentration range 15, 30 and 60 µg/mL and for 24 h followed by MTT assay was performed according to the manufacturer’s instructions.26)
To demonstrate the polymer formation, we analyzed the reaction product by FTIR. The Fig. 1 shows the spectra of Y2O3 (a) GCP (b), and hybrid GCP–Y2O3 (c). In Fig. 1(a), a broad absorption band is observed around 500 cm−1 and 600 cm−1, corresponding to oxygen-metal as reported for Y2O3 when it is synthetized by sol-gel method.27–29) The esterification of GCP is shown in Fig. 1(b). The ester formation was accompanied by a decrease of intensity in the OH absorption band located at ∼3390 cm−1 from glycerol, a possible explanation is because for every ester function formed, one hydroxyl from glycerol is reacting with one proton from citric acid forming water. It could be also attributed to the loss of water from the reagents. After polymerization, a different C–O band at 1164 and 1711 cm−1 appears with a different frequency, which is characteristic of the ester groups. The spectra show a band at 789 cm−1 due to CH2 rock vibration corresponding to citric acid but with a lower intensity. The spectrum of hybrid GCP–Y2O3 (Fig. 1(c)) did not show a chemical interaction between them; the signal of yttrium oxide remained in the same wavenumber and the spectrum of GCP does not show variation in the transmittance signal.
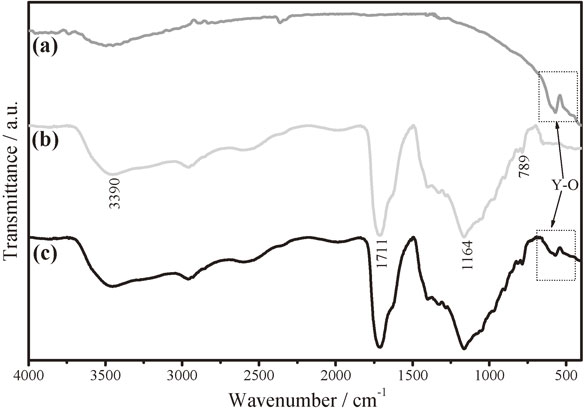
FT-IR spectra of Y2O3 (a), citrate glycerol polymer (b) and hybrid CGP–Y2O3 (c).
A conventional heat/cool/heat cycling test of the polymer and hybrid were performed. In Fig. 2 the thermogram of the second heat cycle of glycerol citrate polymers and hybrid are shown; no citric acid melting peak was observed in the DSC curve, indicating that citric acid was entirely reacted to produce this polymer. The Tgs and ΔCps of pure polymer (Fig. 2(a)) were measured at 53.53°C and 0.293 J/g*K respectively. The Tgs and ΔCps of hybrid polymer (Fig. 2(b)) were measured at 45.62°C and 0.23 J/g*K respectively. The Fig. 2 showed that the Tg value is reduced accordingly to dispersing nanoparticles, meaning that a lower amount of energy to be processed is required.
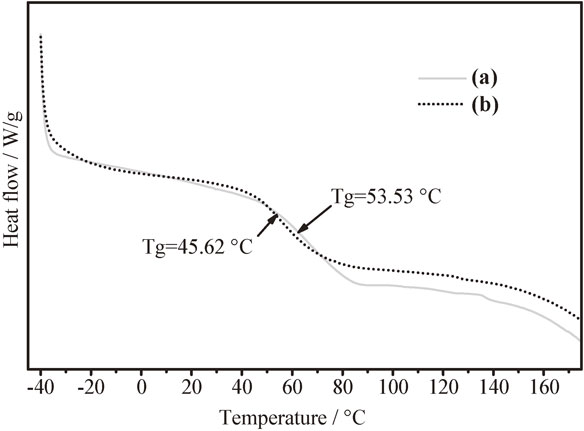
Comparison from DSC thermograms between citrate glycerol polymer (a) and hybrid CGP–Y2O3 (b).
To analyze if a new phase was generated when nanoparticles were dispersed into polymer matrix and proves the amorphous phase of the pure material, X-ray diffraction was performed. The Fig. 3 shows the comparative diffractograms between yttrium oxide particles (a) citrate glycerol polymer (b), and the hybrid CGP–Y2O3 (c). The Fig. 3(a) revealed that the cubic structure of Y2O3 is well formed at 700°C, no secondary phases were found within the detection limit of the XRD technique, the crystallographic phase is in accord with PDF Card #25-1200 with a spatial group Iā3 (lattice parameter 10.604 Å) as can be reported in other works.30,31) A typical formation of amorphous phase of citrate glycerol polymer is formed as shown in Fig. 3(b). The diffraction pattern in Fig. 3(c) shows the peaks match with the PDF card 251200 corresponding to yttrium oxide in cubic phase, indicating that there are no changes in the phases. The distribution of nanoparticles in the polymeric matrix is consequently a physical interaction. The main peaks of Y2O3 particles are overlapped over polymer matrix, it is known that the ultrasonic cavitation has very strong mixing and dispersion effect, much stronger than conventional stirring, as a consequence of that, nanoparticles are well dispersed in the polymer after ultrasonic irradiation, responsible for the low rate of agglomeration and precipitation.32)
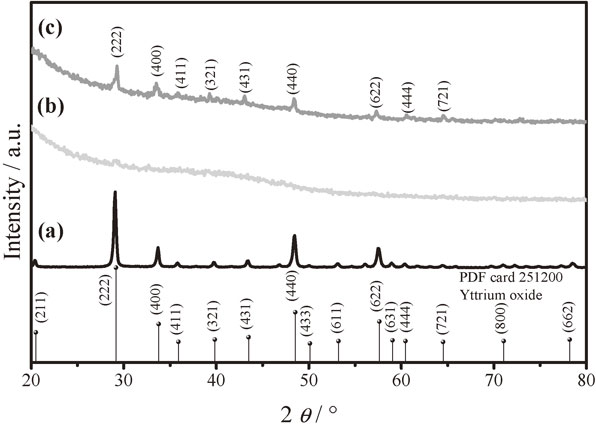
XRD diffraction patterns of yttrium oxide (a), glycerol citrate polymer (b), hybrid CGP–Y2O3 (c).
The morphology and size distribution of Y2O3 particles was obtained by TEM. The micrograph of yttrium oxide is shown in Fig. 4(a) and the size frequency distribution is shown in Fig. 4(b). The average nanoparticle size was 9 nm, estimated from a 100 nanoparticles sample. To compare the pure polymeric matrix with the hybrid material, the micrograph of glycerol citrate polymer used as blank is shown in Fig. 4(c), this image shows a characteristic shape of amorphous polymer. In Fig. 4(d) the glycerol citrate polymer with yttrium oxide particles distributed by ultrasonic cavitation is presented. The yttrium oxide is capping in the polymer matrix. The homogenous dispersion of the nanoparticles in the polymeric matrix was observed, confirming that ultrasonic dispersion is able to distribute the nanoparticles in homogenously in the whole polymeric matrix avoiding agglomerates.
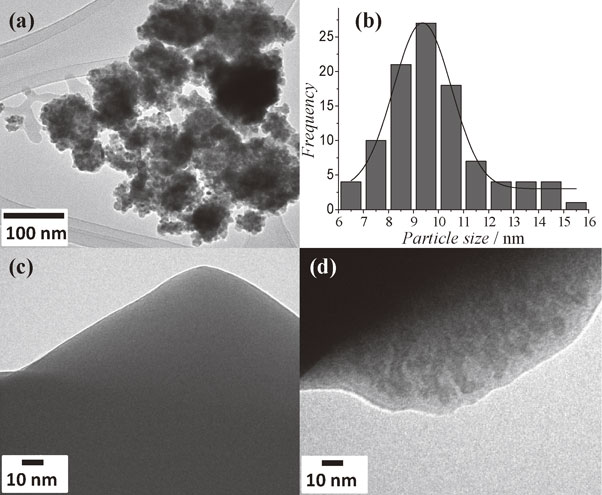
TEM micrographs of yttrium oxide nanoparticles (a) and their size frequency distribution (b); micrograph of citrate glycerol polymer used as blank (c) for the comparative effect of Y2O3 particles distribution into citrate glycerol polymer matrix (d).
Antibacterial effect tests basically measure the ability of an antibiotic or other antimicrobial agent to inhibit the in vitro bacterial growth. The Fig. 5 illustrates the halo of inhibition generated by the glycerol citrate polymer and the hybrid material with both Shigella spp (a) and Bacillus licheniformis (b). For pure and hybrid polymers, the range of bacterial growth inhibition in the presence of Shigella spp is higher compared to the antibiotic, moreover; between them GCP–Y2O3 polymer is more efficient, suggesting highly specificity and antibacterial effect for Gram negative bacteria. In ratio to Bacillus licheniformis (Fig. 5(b)), the GCP and GCP–Y2O3 polymer showed similar inhibition between them, but lower compared with control disc. These qualitative data confirm the antibacterial effect of the hybrid material in these specific strains.
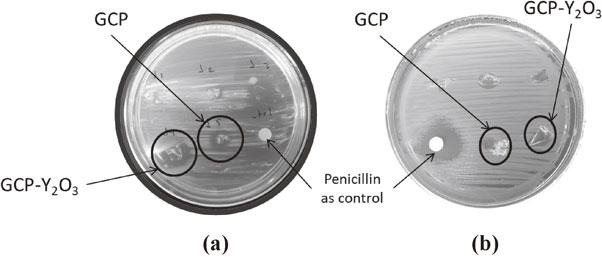
Representative image for the growth inhibition of Shigella spp as a Gram-negative (a) and Bacillus licheniformis as a Gram-positive (b) bacteria in the presence of the polymers.
Due to the high capacity to inhibit bacterial growth, as an independent experiment we tested in vitro the cytotoxicity of GCP, GCP–Y2O3 and Y2O3 to SH-SY5Y cells, as a cancer cell line, to determine its capacity to compromise the viability against a nonspecific tumor cell. To measure cytotoxicity, MTT assay was performed. SH-SY5Y were separately incubated in a plate with three different concentrations of the elaborated materials. For pure GCP it was found that the highest toxicity against these cells is in a concentration of 30 µg/mL, allowing a percent of proliferation of only a 30%. With the concentration of 15 µg/mL the proliferation obtained was 34%, however is lower than the report for other authors. For the hybrid material, the lowest percent of proliferation was obtained using a concentration of 60 µg/mL, however for a concentration of 15 µg/mL the percent was 44%, which continues within the permissible ranges as reported. For pure yttrium oxide, the behavior was similar than the hybrid, the lowest proliferation was with 60 µg/mL of concentration (24%), however a 35% was obtained using 15 µg/mL, as shown in Fig. 6. Other authors have reported hyperbranched polymers as anticancer materials with around 40% of proliferation against cancer cells; indicating that the lowest concentration reported in this study, accomplished the percent to be consider as anticancer material.33)
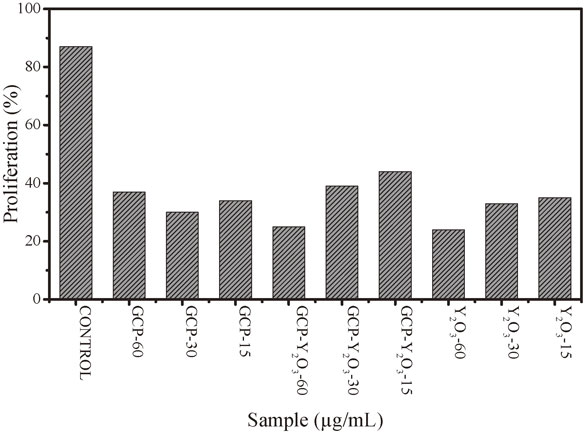
Proliferation of tumor cells SH-SY5Y of human neuroblastoma against glycerol citrate polymer, hybrid polymer and yttrium oxide at 15, 30 and 60 µg/mL as concentration.
It is possible to synthetized successfully glycerol citrate polymer and hybrid GCP–Y2O3 by the methodology reported in this work without using a catalyst. The yttrium oxide nanoparticles have a physical interaction with the polymer, without formation a new phase. The hybrid and pure polymer have not a Tm, which indicates the formation of an amorphous materials. A lower Tg was obtained after dispersion reducing the amount of energy necessary for processing. The glycerol citrate polymer and GCP–Y2O3 showed growth inhibition against the Gram-positive bacteria Bacillus licheniformis and even higher inhibition against the Gram-negative bacteria Shigella spp, Penicillin was used as reference. However, hybrid GCP–Y2O3 showed a stronger sensibly than pure glycerol citrate polymer, which supports the importance of using hybrid polymer as a potential biomaterial. Pure polymer and hybrid polymer are able to reduce the proliferation of tumor cells SH-SY5Y of human neuroblastoma.
The authors gratefully acknowledge the financial support of this work by SEP-CONACYT 178817 project and also by the institutional scholarship given to Jaime A. Mariano Torres and Convocatoria Institucional de Investigación 2018 from University of Guanajuato.