2019 Volume 60 Issue 12 Pages 2530-2536
2019 Volume 60 Issue 12 Pages 2530-2536
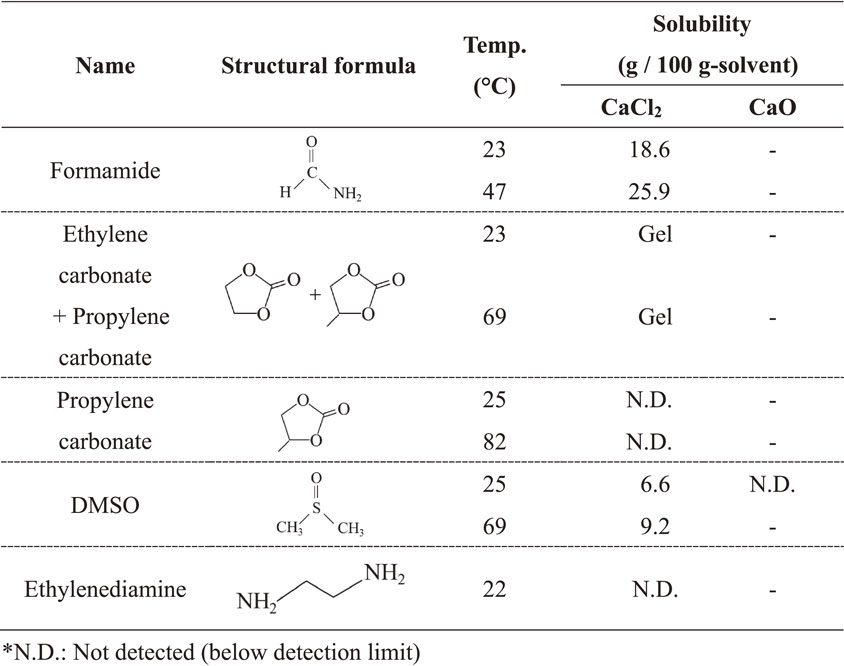
Deoxidation of titanium by a calcium reductant is a promising process for the recycling of titanium scrap. Molten calcium chloride (CaCl2) is typically used as a flux for calcium oxide (CaO) formed as a by-product of deoxidation. At present, removal of CaCl2–CaO can only be achieved by aqueous leaching. However, when CaCl2 hydrate is heated, a pyrohydrolysis reaction occurs, making it difficult to reuse the CaCl2. Therefore, in this study, we examined organic solvents as alternatives to aqueous leaching. Formamide, ethylene carbonate, propylene carbonate, dimethyl sulfoxide (DMSO), and ethylenediamine were selected as candidate organic solvents. The solubility of CaCl2 in DMSO was found to be 9.2 g per 100 g-solvent at 69°C and that in formamide was 25.9 g per 100 g-solvent at 47°C.
Vacuum distillation and crystallization separation were examined as separation methods for the solvent and solute after leaching. A low temperature vacuum distillation, i.e., less than around 200°C for DMSO, is required to prevent thermal decomposition. However, the rate of the vacuum distillation at low temperatures was slow. We therefore devised a process combining a nonpolar solvent-induced precipitation with vacuum distillation to reduce the amount of solvent requiring distillation. Benzene was selected as a nonpolar solvent to induce precipitation from DMSO. After the precipitation, DMSO-solvated CaCl2 was obtained and distilled under vacuum.
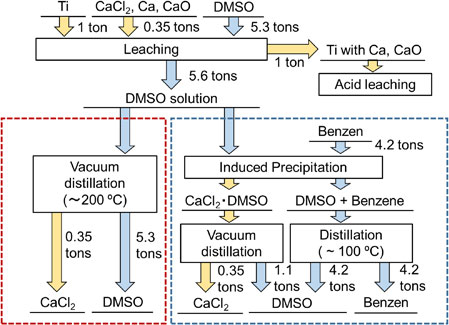
Fig. 9 Process flow of CaCl2-leaching by DMSO.
Deoxidation of titanium (Ti) is difficult because of the strong affinity between titanium and oxygen. Scrap titanium with a high oxygen content is a by-product of machining and/or powder metallurgical processes in the manufacture of titanium products,1) and the development of reduction technologies to recycle this oxygen-contaminated titanium scrap would be desirable. Deoxidation of Ti by calcium (Ca) reduction has been investigated as a recycling method. To enhance the ability to remove oxygen, molten calcium chloride (CaCl2) is used as a flux because CaO is highly soluble in CaCl2. This process was first reported by Okabe et al.,1–3) whose experiments showed that the oxygen concentration in titanium can be reduced to the 10-ppm level. However, to realize an industrial process, a cost-effective method for removing the CaCl2 flux from titanium must be established. Because the vapor pressure of CaCl2 is not sufficiently high for distillation,4,5) aqueous leaching is used to remove CaCl2. However, it is very hard to extract anhydrous CaCl2 from an aqueous solution containing CaCl2 because a part of the CaCl2 hydrate decomposes to CaO and hydrogen chloride (HCl) gas on heating.6–8) To prevent this pyrohydrolysis reactions and complete conversion of CaCl2 hydrate to anhydrous CaCl2, requires heating in a dry HCl gas flow. We therefore propose a leaching process for CaCl2 with the use of a nonaqueous solvent for easy re-generation of anhydrous CaCl2.
In this study, we measured the solubilities of CaCl2 in various polar organic solvents having high specific dielectric constants at room temperature.9) Formamide, ethylene carbonate, propylene carbonate, dimethyl sulfoxide (DMSO), and ethylenediamine were examined. The polar solvent methanol was expected to have high solubility for CaCl2.10) However, methanol was excluded from consideration because it also reacts with Ca.11) To recover CaCl2 from the organic solution, vacuum distillation of the organic and nonpolar solvent-induced precipitates was examined. DMSO has potential to be reused; however it is also important that the organic solvent does not react with Ca. Therefore, we also confirmed whether Ca reacts with DMSO.
The solid samples obtained in this study were analyzed by X-ray diffraction (XRD). All treatments except XRD measurements were conducted in a glove box filled with argon (Ar). The samples for XRD were covered by polyimide tape (Kapton tape, t = 0.03 mm) in the Ar glove box because calcium chloride easily becomes hydrated in air.
2.2 Solubility measurementCommercial anhydrous CaCl2 (95%, Wako Pure Chemical Industries, Ltd.) was dried under a vacuum at a temperature higher than 200°C and at 5 Pa or less for more than 48 h. Within the Ar glove box, up to 20 g of CaCl2 was placed in an Erlenmeyer flask, and 100 mL of various organic solvents was added. Each sample was stirred for 2 h with a magnetic stirrer bar at room temperature or at around 80°C by hot-plate stirrer. The flask was embedded in an aluminum shot to ensure that the solution temperature uniform. In this study, we considered that the sample reached saturation solubility after 2 h of stirring. After stirring, the solution was filtered to separate the solution and the residue. The calcium concentration in the obtained solution was analyzed by inductively coupled plasma-atomic emission spectrometry (ICP-AES).
2.3 Vacuum distillationA glass container was charged with formamide or DMSO solution after the filtration, and vacuum distillation was conducted under the conditions in Table 1. The distillation temperature was selected to be close to the thermal decomposition temperature (T.D.temp.),12–14) or lower or higher than T.D.temp. A rotary evaporator, which was connected to a diaphragm vacuum pump was used for distillation experiments at 80°C. An electric furnace, connected to a diaphragm vacuum or rotary pump was used for distillation experiments at temperatures greater than 100°C. The evaporated solvent was condensed by cooling.

To reduce the distillation time, precipitation of compounds containing CaCl2 from DMSO solution were collected and distilled. Precipitation was induced by the addition of a second organic solvent (in which CaCl2 has low solubility). This requirement for a low solubility of CaCl2 requires a nonpolar solvent. However, the solvent should also be miscible with DMSO, which is a polar solvent. In addition, to separate DMSO and the nonpolar solvent by distillation, the boiling point of the nonpolar solvent should be low. On the basis of these criteria, benzene, hexane, naphthalene, and bromobenzene were selected as the nonpolar solvents. A nonpolar organic solvent was added to a CaCl2-saturated DMSO solution after the solubility measurements, and the solution was stirred for 1 h. A precipitate in powder form was obtained and separated by filtration. All procedures were conducted under an Ar atmosphere. The obtained solid sample was analyzed by thermogravimetry (TG) under a vacuum and XRD.
2.5 Separation of CaO and CaCl2 by leachingFigure 1 shows a flowchart of the separation experiment. A solid mixture of CaCl2–3 mol% CaO, as a model sample without Ti and Ca, was prepared to confirm separation of CaO and CaCl2 by dissolution. The solid mixture was synthesized by melting CaCl2 and CaO (98%, Wako Pure Chemical Industries, Ltd.) together at 900°C and quenching the melt in an Ar atmosphere at room temperature. DMSO was poured into an Erlenmeyer flask containing the model sample, and the mixture was stirred for 2 h. The residue was separated by filtration. Benzene was then added and the resulting solution was stirred for 1 h. A precipitate formed as a powder, which was separated by filtration and subjected to vacuum distillation under conditions of 300°C and 10 Pa for 24 h.
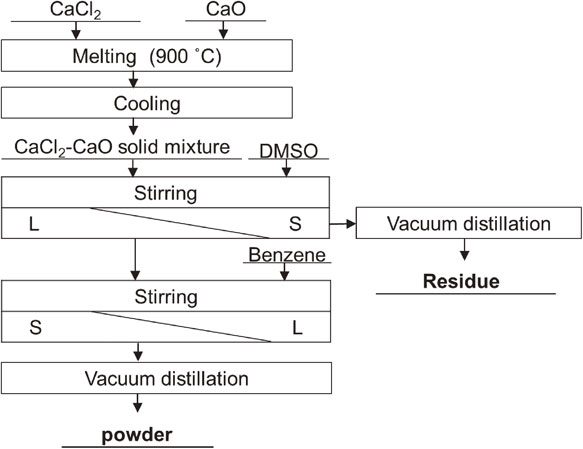
Flow chart of the CaCl2 and CaO separation experiment.
Calcium metal is a strong reductant and is reactive even at room temperature. Furthermore, Ca metal dissolves into molten CaCl2 at high temperature.15) This mixture might be kinetically more reactive than bulk calcium metal when cooled to room temperature suggesting the possibility of reactions between the organic solvents and Ca metal. We confirmed this possibility. The solid mixture of CaCl2–5 mol% Ca was synthesized by melting CaCl2 and Ca (99.5%, 1–3 mm, The Nilaco Co., Ltd.) at 900°C and quenching the melt in an Ar atmosphere at room temperature. DMSO was poured into an Erlenmeyer flask containing the quenched sample, and stirred for 24 h at room temperature. The residue was separated by filtration.
The results of solubility measurements are given in Table 2. Formamide and DMSO showed high solubilities. Regarding the solubility in formamide, the measure value was almost equivalent to the literature value at room temperature.16) In addition, our study showed that the solubility of CaCl2 in formamide increased at 47°C. There have been many previous reports of CaCl2 solubility in DMSO, which determined values in the range of 0–10 mol%17–19) at room temperature. We attribute the discrepancy between these reported values to the slow dissolution rate. One report claimed 10–25 days to reach saturation solubility. However, our values were obtained based on stirring for just 2 h. These conditions are suitable for the present purpose of demonstrating the practical utility of this separation process. There were no signs of dissolution in propylene carbonate or ethylenediamine, which is consistent with previous reports.20) When CaCl2 was mixed with a mixture of ethylene carbonate and propylene carbonate, a gel-like solid was formed. These results indicate that formamide and DMSO have potential for use as leaching solvents. In addition, we examined the solubility of CaO in DMSO. The CaO was immersed in DMSO for 2 h. However, Ca was not detected by ICP-AES analysis.
A 25 g portion of formamide CaCl2 solution (10 mass%) and 32 g of the DMSO solution (6.2 mass%) were vacuum-distilled. Figures 2 and 3 show the XRD patterns and photographs of samples obtained after vacuum distillation of formamide solution and DMSO solution, respectively. For the formamide solution, the solvent was not completely volatilized by distillation at 80 or 157°C. At 157°C, a gray powder remained after vacuum distillation. The weight of this gray powder was 4.33 g, which was heavier than the CaCl2 added to the solution. We attributed this additional weight to residual formamide or its thermally decomposition products. In contrast, the diffraction pattern of CaCl2 was confirmed after vacuum distillation at 300°C, which was somewhat higher than the thermal decomposition temperature. However, the powder was also similarly colored. The residue was considered to be a mixture of CaCl2 and decomposition products of formamide.

X-ray diffraction patterns before and after vacuum distillation of formamide solution. (a) Polyimide tape, (b) before leaching (anhydrous CaCl2), (c) 157°C, 1000 Pa, 24 h, (d) 300°C, 1000 Pa, 24 h.
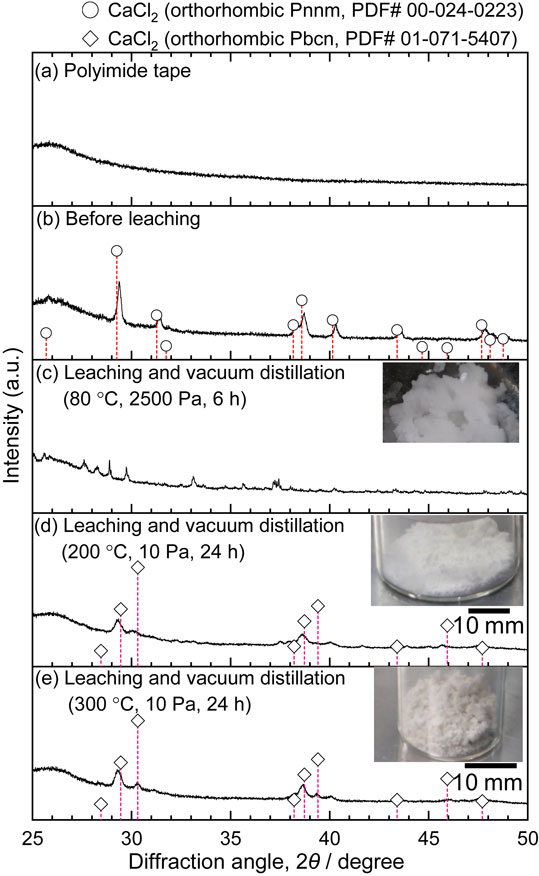
X-ray diffraction patterns before and after vacuum distillation of DMSO solution. (a) Polyimide tape, (b) before leaching (anhydrous CaCl2), (c) 80°C, 2500 Pa, 6 h, (d) 200°C, 10 Pa, 24 h, (e) 300°C, 10 Pa, 24 h. The diffraction pattern of orthorhombic Pnnm phase was measured before the experiment, however, that of orthorhombic Pbcn was confirmed after distillation. Orthorhombic Pbcn is a metastable phase from comment on PDF card.21,22)
Regarding the DMSO solution, it was not possible to completely separate the solvent by vacuum distillation at 80°C, and approximately 7.1% of the solvent was volatilized from CaCl2-saturated solution by distillation for 6 h. After distillation at this low temperature, white and/or transparent crystals were obtained in the solution, but these were not confirmed to be CaCl2 by XRD analysis. White powders were obtained by vacuum distillation at 200 and 300°C, which are temperatures higher than the thermal decomposition temperature of DMSO, 190°C. These powders were confirmed to be CaCl2 by XRD measurements. The weight of the powder obtained as a result of vacuum distillation at 200°C was 2.09 g, which was approximately equal to the weight of CaCl2 added to DMSO. However, both powders had a “sea-like” smell because DMSO partially decomposed to dimethyl sulfide.
3.3 Nonpolar solvent-induced precipitationIn this study, an equal molar amount of nonpolar solvent to DMSO was added to the DMSO solution. Hexane did not mix with the DMSO solution. The mixture of DMSO solution and naphthalene was solid. Benzene and bromobenzene mixed well with CaCl2-saturated DMSO solution and white powders precipitated. Benzene was mixed with 50 g of DMSO–8 mass% CaCl2 solution. The weight of the powder precipitated by the addition of benzene was 12.6 g, and the weight of CaCl2 in the precipitated powder was determined to be 3.16 g by ICP-AES. From these results, the concentration of CaCl2 in the powder was approximately 25 mass%. These results suggest that the precipitated powder was CaCl2 solvated with DMSO, which is consistent with the previously reported crystal phase of CaCl2-DMSO.19)
The total amount of CaCl2 in the CaCl2-saturated DMSO solution was 4.0 g. Therefore, 79% of the original CaCl2 was recovered by the induced precipitation and filtration. The CaCl2 solubility in the DMSO-benzene solution was also investigated. Figure 4 shows the relationship between the concentration of benzene and CaCl2 solubility in DMSO-benzene solution at 40°C. As the concentration of benzene was increased, the CaCl2 solubility decreased. The solubility of CaCl2 was not directly proportional to the DMSO concentration in the solvent (dashed line in the figure) and the decreased more rapidly as the proportion of benzene was increased.

Solubility of CaCl2 in DMSO-benzene solution at room temperature. (Dashed line: value proportional to the DMSO concentration in the solvent.)
Figure 5 shows the results of the TG measurement in vacuum of 19.38 mg of the precipitated powder by mixing the DMSO solution and the same molar amount of benzene as DMSO. The weight of powder decreased in multiple stages on heating, and the decrease was completed at approximately 210°C. At this temperature, it is considered that a small part of the DMSO thermally decomposed because the temperature was higher than the reported thermal decomposition temperature. The amount of powder remaining was approximately 25 mass% of the initial amount, which was consistent with the initial CaCl2 amount. The XRD patterns are shown in Fig. 6. No diffraction peaks of CaCl2 appeared before the TG measurements; however, CaCl2 was formed during the TG measurements. These results suggest that the precipitate was CaCl2 solvated with DMSO and that DMSO was removed by vacuum distillation. The boiling point of DMSO was 189°C,23) which is lower than 210°C, suggesting a strong interaction between CaCl2 and DMSO.
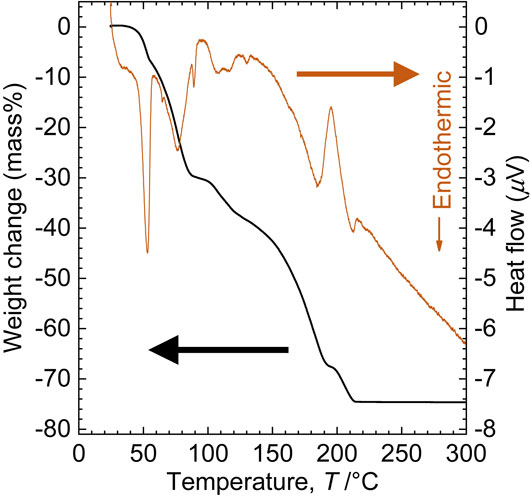
Thermogravimetric curve and heat flow of CaCl2·x DMSO on heating from room temperature to 300°C under vacuum (19.38 mg, 0.5°C min−1, <2500 Pa).
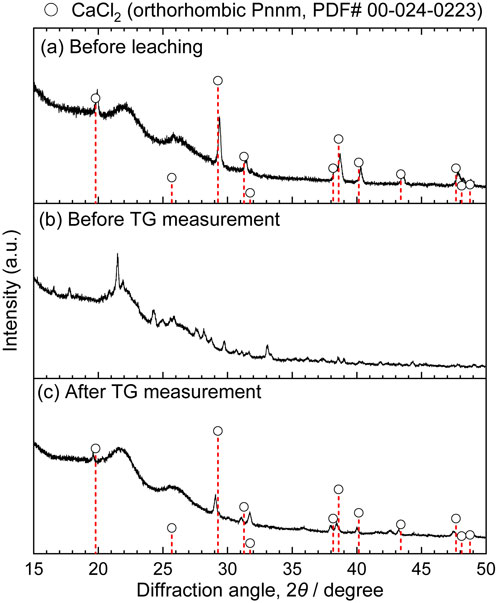
X-ray diffraction patterns before and after TG measurement of CaCl2·x DMSO (R.T. to 300°C, 0.5°C min−1, <2500 Pa). (a) Before leaching, (b) before TG measurement, (c) after TG measurement.
After leaching a solid mixture of 5 g of CaCl2–3 mol% CaO with 100 g of DMSO at room temperature, 0.57 g of a white residue was recovered by filtration. Then, 3.52 g of white powder was obtained from the filtrate by benzene-induced precipitation and vacuum distillation. Figure 7 shows XRD patterns and photographs of the CaCl2–3 mol% CaO solid mixture, the powder obtained from the DMSO solution by the induced precipitation and vacuum distillation, and the residue of leaching. The solid mixture of CaCl2–CaO consisted of CaCl2 and Ca4Cl6O. The residue of leaching was Ca4Cl6O. The powder obtained from the DMSO solution was CaCl2. From these results, CaO formed by Ca reduction can be separated from CaCl2 through this leaching process using DMSO.

X-ray diffraction patterns of samples in the selective dissolution experiment. (a) CaCl2–3 mol% CaO solid mixture, (b) the powder obtained from the DMSO solution, (c) residue.
After leaching 18 g of CaCl2–5 mol% Ca (CaCl2 17.7 g and Ca 0.34 g) with 500 g of DMSO at 45°C for 24 h, 0.46 g of residue was obtained. Figure 8 shows XRD patterns and photographs of CaCl2–5 mol% Ca and the residue. In the XRD pattern of the CaCl2–5 mol% Ca solid solution, only the diffraction peak of CaCl2 was confirmed. In contrast, the XRD pattern of the residue from leaching was that of metallic Ca. The amount of residue obtained was greater than the initial amount of Ca metal added. A small amount of DMSO might have reacted at the calcium surface to form a reaction product and/or residual DMSO might contribute to this mass. However, the extent of this contamination was not so large. We can say that Ca dissolved in CaCl2 was separated as a residue by leaching without a serious reaction with DMSO.
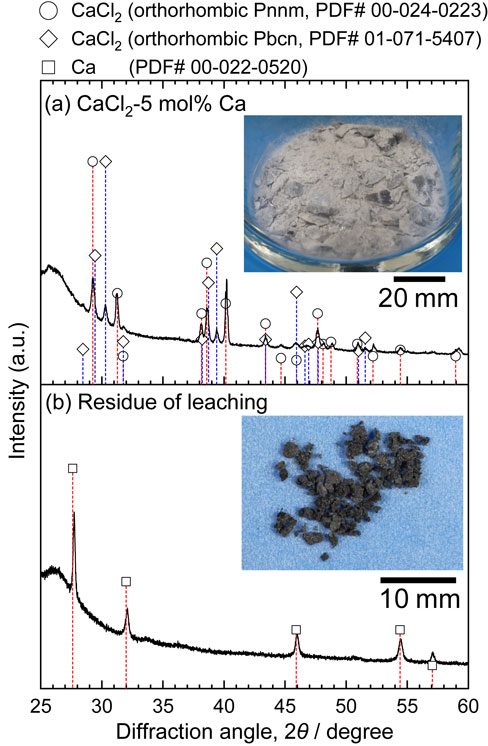
X-ray diffraction pattern of sample after leaching CaCl2–Ca solid solution with DMSO. (a) CaCl2–5 mol% Ca, (b) residue of leaching.
From the results of solubility measurements, DMSO was found to be the most suitable solvent for leaching. The CaCl2 was also highly soluble in formamide but it was difficult to recover anhydrous CaCl2 by vacuum distillation without forming impurities. Figure 9 shows the process flow of CaCl2-leaching by DMSO. Titanium was deoxidized by Ca reduction, and we assume that, considering the density of titanium metal (4.5 g cm−3) and CaCl2 (2.0 g cm−3), the titanium will settle in the bottom of the reaction container. Hence, the molten salt containing Ca and CaO can be partially removed by tapping at high temperature, as is the case for the Kroll process. If we assume that the volume percentages of Ti and the molten salt are both 50%, that is, 350 kg of CaCl2 remains in one metric ton of titanium, 5.3 metric tons of DMSO will be required to dissolve 350 kg of CaCl2 at room temperature. Ca and CaO (Ca4Cl6O) do not dissolve in DMSO, and will therefore remain in the titanium. The remaining Ca and CaO can be removed by acid leaching. CaCl2 is removed from the DMSO solution by single-step distillation or two-step separation combining nonpolar solvent-induced precipitation and vacuum distillation. Single-step separation requires distillation of 5.3 metric tons of DMSO. Because the vacuum distillation needs to be performed at approximately 210°C, partial thermal decomposition of the DMSO may occur. Such decomposition may also increase costs, so a careful cost analysis with accurate determination of the decomposition ratio is needed. In two-step separation, only 1.1 metric tons of DMSO needs to be distilled from CaCl2, and it is expected that this route will reduce the amount of thermal decomposition of DMSO. The mixture of the remaining DMSO and benzene must be separated by distillation. The boiling point of benzene, 80.1°C, is much lower than that of DMSO, and unlike CaCl2 there is no strong interaction between benzene and DMSO. The separation of benzene and DMSO should therefore be much easier, which will reduce the processing time. In this scenario, 4.2 tons of benzene is required for 1 ton of titanium.
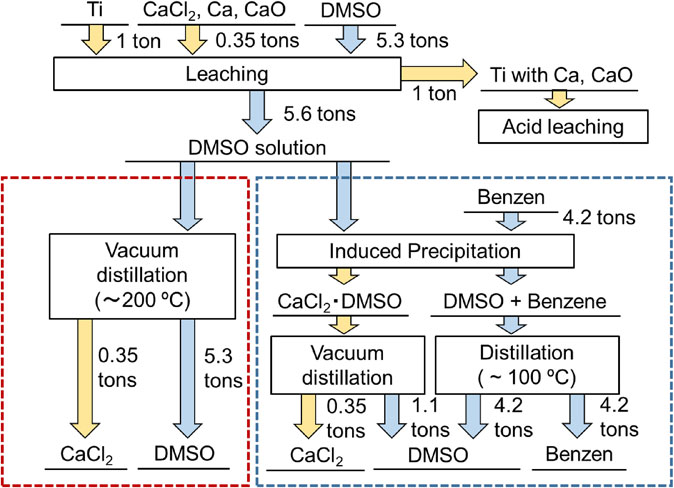
Process flow of CaCl2-leaching by DMSO.
This study investigated organic solvents for CaCl2 leaching. Among the solvents examined, DMSO was found to be the most suitable. The solubility of CaCl2 in DMSO was 9.2 g per 100 g-solvent at 69°C. Single-step distillation or two-step separation combining nonpolar solvent-induced precipitation and vacuum distillation can be applied for separation of the mixture of CaCl2 and DMSO. Two-step separation might be advantageous for productivity. In addition, because CaO does not dissolve into DMSO and Ca does not strongly react with DMSO, it is possible to easily separate CaO and Ca from CaCl2 by this process. This suggests that CaCl2 with a low oxygen concentration can be regenerated.
We thank Mr. Kazuhiro Kumamoto for scientific opinions and for carefully proofreading the manuscript.