2019 Volume 60 Issue 9 Pages 2059-2068
2019 Volume 60 Issue 9 Pages 2059-2068
A new method for removing the dissolved oxygen (O) in titanium (Ti) is developed, wherein magnesium chloride–holmium chloride (MgCl2–HoCl3) and Mg are used as a flux and a reducing agent, respectively. Through the thermodynamic assessment using a 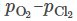 diagram as well as the experimental results, the deoxidation of Ti to a level below 1000 mass ppm O (and even 500 mass ppm O) via the formation reaction of holmium oxychloride (HoOCl), O (in Ti) + Mg + HoCl3 → HoOCl + MgCl2, was confirmed. The deoxidation limit decreases with the increase of the activity of HoCl3 in the MgCl2–HoCl3 flux. One advantage of this method is that the activity of the deoxidized product, aMgO, in the system can be effectively maintained at a low level by the formation of HoOCl. The E–pO2− diagram of the M–O–Cl system (M = Ho, Mg) constructed in this study indicates that the electrochemical deoxidation of Ti scraps in MgCl2–HoCl3 system will be more effective because the aMgO can be further decreased via the formation of HoOCl, and/or the electrochemical oxidation of oxide ions on the carbon anode. This new deoxidation technique using rare-earth-containing MgCl2 flux can be applied to the recycling of Ti scraps in the future.
diagram as well as the experimental results, the deoxidation of Ti to a level below 1000 mass ppm O (and even 500 mass ppm O) via the formation reaction of holmium oxychloride (HoOCl), O (in Ti) + Mg + HoCl3 → HoOCl + MgCl2, was confirmed. The deoxidation limit decreases with the increase of the activity of HoCl3 in the MgCl2–HoCl3 flux. One advantage of this method is that the activity of the deoxidized product, aMgO, in the system can be effectively maintained at a low level by the formation of HoOCl. The E–pO2− diagram of the M–O–Cl system (M = Ho, Mg) constructed in this study indicates that the electrochemical deoxidation of Ti scraps in MgCl2–HoCl3 system will be more effective because the aMgO can be further decreased via the formation of HoOCl, and/or the electrochemical oxidation of oxide ions on the carbon anode. This new deoxidation technique using rare-earth-containing MgCl2 flux can be applied to the recycling of Ti scraps in the future.