2020 Volume 61 Issue 1 Pages 181-187
2020 Volume 61 Issue 1 Pages 181-187
The effect of antimony (Sb) additions (0, 0.2 mass%) and cooling rates (0.56°C·s−1, 2.03°C·s−1, 7.42°C·s−1) on the Al–Si eutectic of hypoeutectic Al–Si alloys were investigated. The results show that Sb combines with Mg to form a compound Mg3Sb2 which poisoned the nucleation particle AlP, resulting in the nucleation undercooling of the eutectic silicon increased. In unmodified alloy, the sensitivity of eutectic temperatures to cooling rate decreases with the increase of cooling rate. The reason is that the growth rate of eutectic silicon increases and the latent heat of crystallization is released in large quantities. After adding 0.2%Sb, there was a good linear relationship between eutectic temperatures and cooling rates. Due to the inhibition of silicon growth by Sb and Mg3Sb2 particles, the growth of the eutectic silicon was slower and the latent heat of crystallization was released slowly. Hence, Sb increased the sensitivity between 2.03°C·s−1 and 7.42°C·s−1. In unmodified alloy, the eutectic nucleated on the primary dendrites and interdendritic liquid, after adding 0.2%Sb, the eutectic only nucleated independently in the interdendritic regions.

Fig. 7 EBSD images of (a)(c)(e) Al–7Si–0.6Mg alloy, (b)(d)(f) Al–7Si–0.6Mg–0.2Sb alloy at different cooling rates ((a)(b) correspond to 0.56°C·s−1, (c)(d) correspond to 2.03°C·s−1 and (e)(f) correspond to7.42°C·s−1).
Hypoeutectic Al–Si alloys are widely applied in the aerospace and automotive industries owing to their excellent castability, high specific strength, low density and good wear resistance.1,2) Besides of primary Al phase, Al–Si eutectic phases are also crucial in improving the mechanical properties of hypoeutectic Al–Si alloys. The hard and brittle silicon particles increase the yield and ultimate tensile strength of softer aluminium matrix, but platelet-like silicon causes poor ductility.3–5) Therefore, modification melt treatment becomes an important way to overcome this drawback. Microalloying elements such as sodium (Na) and strontium (Sr) usually serving as eutectic silicon modifier are added to improve alloy properties.6–10) These modifiers convert very efficiently the coarse eutectic into fine and fibrous form. However, they have many drawbacks, such as their gassing tendency, their volatility and rapid rate of burn-out from the melt, reaction with and resulting damage to the crucible and so on. In contrast, Sb-treated alloy has the advantages of low susceptibility to gassing, and due to excellent casting properties it produces very sound cast components.11,12) In addition to that, the refining action of Sb is hardly affected by holding time, degassing and remelting, which is different with sodium (Na) and strontium (Sr).13)
However, it still has inevitable limitations in practical production. Sb modification possess good modification effect on metal casting and thinner-walled sand casting but not clear influence on the thicker-walled sand casting.12) It means antimony is a kind of modifier sensitive to the cooling rate, which leads to the microstructures various from different areas of wall when it is used in some complex castings. To make better understand of the modification mechanism of Sb, many researchers have made great efforts. Bian has measure the structure factors of the Al–12.5% Si alloy with various additions of Sb, it is found that Sb in liquid Al–Si alloys promotes the aggregation of Si atoms, decreases the supercooling of solidification, and finally modifies the size and shape of the Si phases in the solid state.14) KHAN believed that Sb refines the eutectic structure by reducing the interflake spacing rather than acting as a modifier.13) Uzun found that in the rapidly solidified Al–12Si alloy modified by Sb, the larger Sb (1.0 mass%) content resulted in crystallization of Si atoms solved in Al matrix and led to poor mechanical properties.15)
It has been reported that Sb results in fine lamellar morphology of eutectic silicon. And faster cooling rate can heighten the modification effect significantly.16–21) However, the mechanism responsible for such behavior has not yet been well reported. Hence present work is an attempt to explore the effects of Sb addition and cooling rate on modification mechanism of hypoeutectic Al–Si alloys and provide guidance for the application of hypoeutectic Al–Si alloys with Sb modification in practical production, particularly in producing large and complex wall thickness castings.
The material used was standard Al–7Si–0.6Mg (commercial A357 alloy), Al–4 mass% Sb (mass% was used throughout the paper unless otherwise noted) master alloys was added to it to obtain Al–7Si–0.6Mg–0.2Sb alloy. The actual chemical compositions of the alloys were measured by a spectroscopic methodology, detailed in Table 1. In each experiment, 50 kg of raw materials made samples were used to ensuring the reliability and authenticity of the experimental results, and about 10 kg of pot bottom material was not used to prevent the influence of some sediments on experiments. The geometry of the casting is shown in Fig. 1, which is in a range of thicknesses changing from 10 to 30 mm. K-type thermocouples were connected with FLUKE2638A data acquisition equipment to record real-time data, and the position of them has been marked in Fig. 1.


Schematic diagram of step casting (unit: mm).
After solidification and removing the thermocouples, each specimen was cut out from the sections where the tips of these thermocouples were located. Microstructures of this alloy was analyzed by scanning electron microscope (SEM) equipped with energy dispersive X-ray spectrometer (EDS). The samples were prepared by standard metallographic techniques and then were deep-etched in an alkali solution (20 ml NaOH and 80 ml H2O) for 10 min to observe the micromorphology of eutectic silicon. Width and length of eutectic silicon phase of alloys were measured by the Image Pro Plus (IPP) software, more than ten fields of view were collected for each statistical data to ensure reliable statistics. Electron backscatter diffraction (EBSD) were used to better understand the nucleation mode of the eutectic silicon. Samples for EBSD analyzed were cut into small round pieces with diameter of 3 mm and thickness of 1 mm, and polished. Ion milling used the following condition: 1 h at a voltage of 8 kv and gun tilt angle of 10°, and followed by 2 h at a voltage of 6 kV and gun tilt angle of 8°. Morphologies of twins were analyzed by transmission electron microscope (TEM) on Tecnai G2 F20 S-TWIN and operating at 200 kV. Thin foils for TEM was prepared as follows: 3 mm diameter discs were punched from the samples, mechanically ground down to 50 µm and then thinned by precision ion polishing system.
The cooling rate was determined from the slope of the cooling curve between growth temperature of Al dendrite and nucleation temperature of the eutectic reaction according to the following equation:
| \begin{equation} \text{Cooling rate} = (T_{G}^{\text{${\alpha}$-Al}} - T_{N}^{\text{Al–Si}})(t_{N}^{\text{Al–Si}} - t_{G}^{\text{${\alpha}$-Al}})^{-1}\ ({{}^{\circ}\text{C}}{\cdot}\text{s}^{-1}) \end{equation} | (1) |

(a) The cooling curve of Al–7Si–0.6Mg alloy with its corresponding first and second derivative curves indicating characteristic parameters; and (b) a correlation between derivative curves in the region of Al–Si eutectic reaction.
The cooling curves of the Al–7Si–0.6Mg alloy and Al–7Si–0.6Mg–0.2Sb alloy in different rates (0.56, 2.03 and 7.42°C·s−1) are given in Fig. 3. It shows that the solidification behavior of the alloy was affected by both the solidification conditions and Sb addition. After adding 0.2%Sb, the eutectic reaction platform became longer and the undercooling increased. In both alloys, the increase of cooling rate leads to the decrease of eutectic temperature. The depression can be explained by the concept of the coupled zone.22) The coupled zone represent fields within the phase diagram where the two phases of the eutectic can grow with similar velocities. In the Al–Si system, silicon tends to grow anisotropically into faceted crystals since it is a non-metal with directed covalent bonds and hence it requires more undercooling for its growth than the isotropic aluminum phase. Consequently, at higher cooling rate in addition to shifting the eutectic composition to higher silicon content, the system behaves decreased the eutectic freezing temperature to a lower level.

Effect of cooling rate on solidification behavior for alloys studied.
Solidification parameters of Al–Si eutectic phases extracted from cooling curves at different cooling rates was showed in Table 2. Changes of $T_{N}^{\text{Al–Si}}$ and $T_{G}^{\text{Al–Si}}$ for Al–Si eutectic phase at different cooling rates was showed in Fig. 4. It can be see that at relatively low cooling rates (0.56°C·s−1 and 2.03°C·s−1), $T_{N}^{\text{Al–Si}}$ and $T_{G}^{\text{Al–Si}}$ are sensitive to the cooling rate in the unmodified alloy. When the cooling rate increased to 7.42, $T_{N}^{\text{Al–Si}}$ and $T_{G}^{\text{Al–Si}}$ decreases slowly, which means the sensitivity to the cooling rate decreases. After adding 0.2%Sb, there was a good linear relationship between eutectic temperature and cooling rate, indicating that the addition of Sb increased the sensitivity between 2.03°C·s−1 and 7.42°C·s−1. At the same time, the depression of $T_{N}^{\text{Al–Si}}$ and $T_{G}^{\text{Al–Si}}$ refers that Sb has an inhibitive effect on the nucleation and growth process of eutectic silicon.23,24)
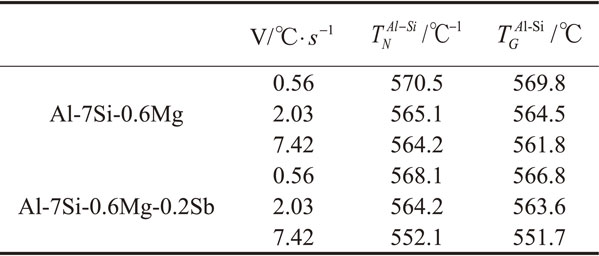

Changes of (a) $T_{N}^{\text{Al–Si}}$, (b) $T_{G}^{\text{Al–Si}}$ for Al–Si eutectic phase as a function of cooling rate for alloys studied.
The deep-etched morphology of eutectic silicon under SEM of as-cast alloys at different cooling rates are shown in Fig. 5. The corresponding quantitative analysis of eutectic silicon is shown in Fig. 6.

Silicon morphology for (a)(b)(c) Al–7Si–0.6Mg alloy, (d)(e)(f) Al–7Si–0.6Mg–0.2Sb alloy at different cooling rates ((a)(d) correspond to 0.56°C·s−1, (b)(e) correspond to 2.03°C·s−1 and (c)(f) correspond to7.42°C·s−1).

Width and length of eutectic silicon phase of alloys with different cooling rates.
It is clearly visible that at low cooling rates, eutectic silicon shows a needle, long strip, or angle plate shape on Al–7Si–0.6Mg alloy (Fig. 5(a)), which is refined but the morphology is still needle or angle plate shape structure (Fig. 5(b), (c)) after increasing cooling rate. The typical Al–Si eutectic morphology like this is usually attributed to the relatively low interfacial energy between silicon and aluminum and to the strong anisotropy of growth of silicon.22) Comparing Fig. 5(a) and Fig. 5(d), it is not difficult to find that, for a given cooling rate (0.56°C·s−1), eutectic silicons in Al–7Si–0.6Mg–0.2Sb alloy are much thinner than that in unmodified alloy. From Fig. 6, it can be seen that the average length of the eutectic decreased from 51.57 µm to 17.28 µm after adding 0.2%Sb. With the increase of the cooling rate, both the average length and width of the eutectic silicon show a downward trend, regardless of Sb treatment. It is interested to note that when the cooling rate increased from 2.03°C·s−1 to 7.42°C·s−1, most of the eutectic Si in Al–7Si–0.6Mg–0.2Sb alloy were transformed into short rod-like structures. The average length of them from 15.43 µm shift to 5.76 µm, and the average width decrease from 3.65 µm to 1.13 µm, both down by about two-thirds.
3.3 Nucleation process of eutecticIt is well established that the mechanisms of eutectic solidification can be divided into three modes. Mode I: nucleation and growth opposite the thermal gradient; Mode II: nucleation on the dendrites; Mode III: heterogeneous nucleation of eutectic grains in the interdendritic liquid.25) From the previous work, it is point that the eutectic colonies growing out of the same nucleation core have the same orientation.26,27–29) Basing on these theories, the following views seem to have been generally preferred. Firstly, the crystallographic orientations of the aluminum in the eutectic and the surrounding primary dendrite arms are identical in Mode II. Secondly, in Mode III the eutectic nucleate on the heterogeneous nucleated cores, so there is no systematic orientation relationship between the aluminum in the eutectic and the primary aluminum dendrites.26)
In the present work, SEM equipped with EBSD detect was performed to investigate the effect of Sb addition on the eutectic silicon nucleation. When the difference in crystallographic orientation of adjacent regions is less than 5 deg, the color of the mapping pixels is identical, which means that identical color indicates identical orientation. It can be seen from Fig. 7 that primary phase occupies most of the field of view, and there are some small lumpy regions with different colors exist between the dendrites of primary aluminum or at the grain boundary, which correspond to eutectic silicon. Due to shading of the pattern or no pattern recorded, there are some black regions of the mapping image which represent areas undetected by electron back-scattering. In the unmodified alloy, the EBSD maps (Fig. 7(a)(c)(e)) show large variations in color and most colors are different from the primary dendrites. Only small part of regions in the eutectic with the same orientation as the dendrites, as indicated by the white rectangle. This result is different with the report by Nogita, who has stated that most of the eutectic grew from the primary phase in the unmodified alloy.26) Since AlP is believed to be the potent nucleation site for eutectic silicon,30–32) the difference may be due to different levels of P. Comparing Fig. 7(a) and (b), it is easy to find that eutectic silicon has been significantly refined. In Fig. 7(b), some refined eutectic silicon is connected to each other and shows the same color, which indicates that the eutectic silicon is grown from the same particle, as marked by the red circle. From Fig. 7(b)(d)(f) it can be observed that the eutectic regions are mainly different from the surrounding primary dendrites, as indicated by the white rectangle. This result indicates that the eutectic aluminium has not nucleated on the primary dendrites, but instead nucleated independently in the interdendritic regions. It is not difficult to conclude that there are two different modes of eutectic growth in the unmodified alloy, after adding 0.2%Sb, the nucleation of eutectic aluminum on primary aluminum was not observed, eutectic grows in only one mode.

EBSD images of (a)(c)(e) Al–7Si–0.6Mg alloy, (b)(d)(f) Al–7Si–0.6Mg–0.2Sb alloy at different cooling rates ((a)(b) correspond to 0.56°C·s−1, (c)(d) correspond to 2.03°C·s−1 and (e)(f) correspond to7.42°C·s−1).
Figure 8 was the SEM images of the Al–7Si–0.6Mg–0.2Sb alloy at different cooling rates. From Fig. 8(a) we can see a large number of white second phases clustered around the eutectic silicon. They appear as bars or blocks, and the energy spectrum (Fig. 8(e)(f)) indicates these phases are Mg3Sb2 phase. Comparing Fig. 8(a)(b) and (c), it is not difficult to find that as the cooling rate increases, the number of the coarse Mg3Sb2 phase decreases. From the phase diagram that the phase transition temperature of Mg3Sb2 is 656°C, which means that Mg3Sb2 phase exists in the alloy melt prior to the eutectic reaction.18) At low cooling rate (0.56°C·s−1), there was enough time for Mg3Sb2 particle to diffuse and enrich at the front of solid-liquid interface, so Mg3Sb2 phase is larger in size. When the cooling rate was increased to 7.42°C·s−1, there was not adequate time for Mg3Sb2 particle to diffuse and enrich owing to the high solidification rate, so Mg3Sb2 phases get smaller and smaller. Mg3Sb2 particles are too small to be observed by the SEM at high cooling rate.

SEM images of precipitated phases of Al–7Si–0.6Mg–0.2Sb alloy at different cooling rates, (a)(b)(c) correspond to 0.56, 2.03 and 7.42°C·s−1, respectively, (d) is a partial enlargement of (a), (e) EDX of p1 point and (f) EDX of p2 point.
In the previous studies, KHAN13) gives a point that in the Al–Si eutectic alloys, the Sb combines with Mg to form a compound Mg3Sb2 which dissolves phosphorus, resulting in the heterogeneous nucleation core AlP of eutectic silicon decreases. Hansen33) also hold the idea that Mg3Sb2 phase can react with or epitaxial growth on AlP, causing the AlP particles to decrease. So it is reasonable to speculate that the poisoning effect of Mg3Sb2 force the nucleation of silicon at large undercoolings, so the decrease of the $T_{N}^{\text{Al–Si}}$ was observed. Which explains the increase in undercooling caused by Sb.
3.4 Growth process of eutecticIn general, in addition to affecting the nucleation process of eutectic silicon, modifier such as Na and Sr may also affect its growth process. It appears to be universally accepted that, the Twin Plan Reentrant-Edge (TPRE) and the impurity induced twinning (IIT) are well known two kinds of mechanism.34–36) Lu and Hellawell35) have assumed a face centered cubic structure and calculated the ratio of impurity atom radius (ri) to matrix atom radius (r) that is required for elements induced twinning and found it to be ri · r−1 = 1.6457. The ratio of the Sb atom and Si atom is 1.23, which is far away from 1.65. Therefore, elemental Sb can not promote the emergence of twinning in theory.
Figure 9 shows the TEM image and the corresponding selected area diffraction pattern (SADP) of the alloys. As can be seen from the Fig. 9(b), at a given cooling rate (0.56°C·s−1), there are only a few twins distributed along the growth direction of the silicon phase after adding Sb in the alloy, which was not much different from the morphology without Sb modification (Fig. 9(a)). As seen in Fig. 5(f), even at the cooling rate of 7.42°C·s−1, there is still no obvious branching structure of eutectic silicon, which means that the refining mechanism of eutectic silicon is not changed by the increase of cooling rate. It can be concluded that Sb dose not bring an increase in the content of twins on the eutectic, which means Sb cannot change the growth procedure of Si by adsorbing directly into the interface step or twin plane reentrant. Similar results were found in Liu’s experiment,11) in his work, XPS and AES analyses were taken but failed to detect any adsorption of Sb on the (111)Si and (100)Si surfaces of the single crystal silicon seeds.

Twins (marked arrows) on eutectic Si at the cooling rate of 0.56°C·s−1: (a) TEM bright field image of Al–7Si–0.6Mg, (b) TEM bright field image of Al–7Si–0.6Mg–0.2Sb.
From Table 2, we know that the $T_{G}^{\text{Al–Si}}$ was reduced after adding 0.2%Sb, which means the growth process of eutectic was inhibited. Obviously, twin formation is not the cause of the growth temperature drop. Combined with Fig. 3, it is known that in unmodified alloy, the undercooling increases with the increase of the cooling rate. And the growth rate of the crystal increases, releasing a large amount of latent heat of crystallization, leading to a slower decline of $T_{G}^{\text{Al–Si}}$ than that of low cooling rate. Due to the distribution coefficient of Sb in Al is less than 0.01,37) Sb is prone to enriched at the front of the solid-liquid interface. Hence in Sb modified alloy, Sb and Mg3Sb2 particles enriched at the surface of the eutectic silicon (not into the lattice) inhibit its growth, resulting in the crystallization latent heat release slowly. In the cooling rate range of 2.03°C·s−1 to 7.42°C·s−1, $T_{G}^{\text{Al–Si}}$ decreases faster than that at low cooling rate due to the large undercooling and temperature gradient, which leads to a faster rate to remove the latent heat of crystallization. The refinement is also more pronounced.
The effect of Sb additions and cooling conditions on the Al–Si eutectic for the hypoeutectic Al–Si alloy were investigated and following conclusions were obtained.
The authors acknowledge the financial support of the National Natural Science Foundation of China (Nos. 51474101, 51271076, 51474195) and Aluminum Alloy Laboratory of Beijing Institute of Aeronautical Materials and Hunan university large precision precious instrument equipment.