2021 Volume 62 Issue 4 Pages 492-497
2021 Volume 62 Issue 4 Pages 492-497
In this study, the effects of Fe3+ on corrosion behavior of high-purity aluminum (Al) were investigated in aqueous NaCl solutions containing Fe3+ using immersion tests, electrochemical analysis, and surface observations. For high-purity Al immersed in an Fe3+ solution, the open circuit potential was much lower and the corrosion rate was higher than that of high-purity Al in a solution without Fe3+. The corrosion morphology of high-purity Al after immersion in the test solutions containing Fe3+ was mainly general corrosion; this differed significantly from the morphology of the Al immersed in a NaCl solution without Fe3+. The effects of Fe3+ on the corrosion behavior of high-purity Al were due to a less-protective passive film composed of Al(OH)3 and Fe(OH)3 that formed on high-purity Al during immersion.
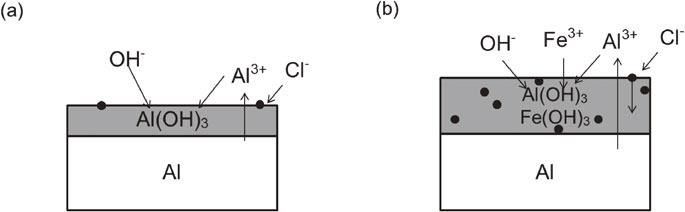
Fig. 11 Schematic diagram of passive film of Al formed after immersion in the test solutions: (a) without Fe3+ and (b) with Fe3+.
In recent years, demand for lighter, multifunctional industrial materials has increased to reduce environmental impact. Multi-materialization techniques can meet this need by combining structural members to form lighter alternatives. To date, steel materials have been widely used in various constructions. However, the use of lightweight aluminum (Al) alloys in combination with steel can reduce the overall weight of metallic structures and that is effective for energy saving, especially in transportation equipment such as automobiles.
When Al alloy and steel are used adjacent to each other, galvanic corrosion is likely to occur due to the potential difference between iron (Fe) and Al,1,2) causes the Al side to dissolve while the Fe is protected from corrosion. However, steel corrosion may also occur due to the weak sacrificial action of Al to steel, resulting in the generation of ferrous ions (Fe2+) in the corrosion environments. The Fe2+ ions can then be oxidized to ferric ions (Fe3+) by dissolved oxygen in the environment, which is transported to the Al surface. Thus, during galvanic corrosion of Al and Fe, Fe3+ can affect the corrosion of Al.
Some researchers have reported the effects of metallic cations in aqueous solutions on the corrosion behavior of Al.3,4) These effects and the corrosion resistance of Al and Al alloys have been classified using hard and soft acid-base (HSAB) theory.5,6) Sakairi et al. reported that hard-type metallic cations act as inhibitors against the corrosion of Al alloys. For example, Zn2+ inhibits the corrosion of Al–Mn alloys due to the enhanced passivity of Al with Zn2+ incorporation. However, few studies have examined the effects of Fe3+, a hard-type metallic ion, on the corrosion resistance of Al.7,8)
Therefore, in this study, the effects of Fe3+ on the corrosion behavior of high-purity Al were investigated based on the results obtained from electrochemical measurements, surface observation, and analysis. Further, we propose a corrosion mechanism for Al in the presence of Fe3+.
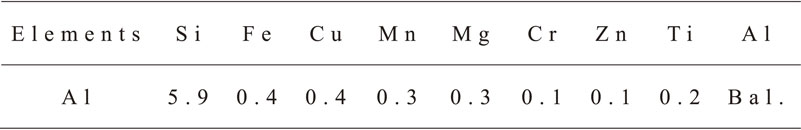
The test material used in this study was high-purity Al of 99.999 mass% purity; Table 1 lists its chemical composition. The material was machined into small pieces of samples with the dimensions of 1.3W × 1.3L × 1T mm3. The surface of each sample was wet-polished with water-resistant abrasive papers successively up to #800 grit. After being polished, sample surfaces were exposed to 5 mass% NaOH at 25°C for 30 s as a degreasing treatment, followed by rinsing with deionized water. Samples were immersed in 30 mass% HNO3 at 25°C for 60 s as a desmutting treatment, rinsed again with deionized water, and then dried in air. Next, the surface of each sample was masked with an insulating material to restrict the exposed surface to 1 cm2 for electrochemical tests.
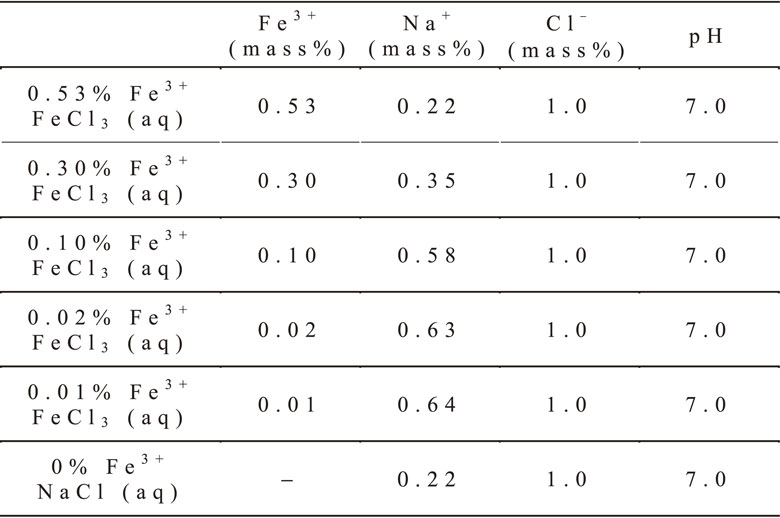
The test solutions used in this study were aqueous solutions of FeCl3 with various Fe3+ concentrations as shown in Table 2. An aqueous NaCl solution without Fe3+ was also used as a reference solution. NaCl and NaOH were added to the test solutions. The chloride ion concentration and the pH were adjusted to 1.0 mass% and 7, respectively.
Immersion tests were carried out in the test solutions at room temperature under open-to-air conditions. During the immersion tests, the open circuit potential (OCP) was measured in the test solutions against a commercial calomel electrode (SCE, +0.249 V vs. SHE at 25°C). Colloids of Fe(OH)3 can precipitate during pH adjustment because of the low solubility of Fe3+ in aqueous solutions. Thus the test solutions were stirred at 700 rpm by a magnetic stirrer to prevent the precipitation of colloidal Fe(OH)3 during the immersion tests.
After the immersion tests, we examined sample surfaces using an optical microscope (STM6, Olympus Corp., Japan), an optical three-dimensional measuring device (VR-3000, Keyence Corp., Japan), and a scanning electron microscope (SEM; SU5000, Hitachi Hi-Tech. Corp., Japan). We also determined the weight loss of each sample after the immersion test. To remove corrosion products from surfaces, samples were placed in a 30 mass% HNO3 solution for 60 s.
Additionally, elemental depth profile for the Al surface after the immersion tests was analyzed using a glow discharge optical emission spectrometer (GD-OES; GD-Profiler 2, HORIBA, Ltd., Japan).
2.4 Electrochemical measurementsWe conducted dynamic polarization and electrochemical impedance spectroscopy (EIS) measurements to investigate the electrochemical behavior of samples in the test solutions. The sweep rate for the dynamic polarization measurements was a constant of 10 mV/min. For EIS measurements, the amplitude of the applied alternating voltage was 10 mV, and the frequency range was from 100 kHz to 10 mHz. EIS data were obtained at OCPs for samples in the test solutions.
Figure 1 presents the OCP transients for high-purity Al measured during a 144 ks immersion in test solutions. In the test solution without Fe3+ (0% Fe3+, NaCl), the OCP shifted to a lower potential of ca. −1.25 V immediately after immersion, and increased gradually to ca. −1.11 V after 144 ks. In contrast, in the test solutions with Fe3+, the OCP decreased significantly immediately after immersion, and then reached a stable value. For example, in the case of 0.53% Fe3+, the stable OCP value was ca. −1.40 V, which was lower by ca. 0.3 V than that for the test solution without Fe3+ (0% Fe3+, NaCl).
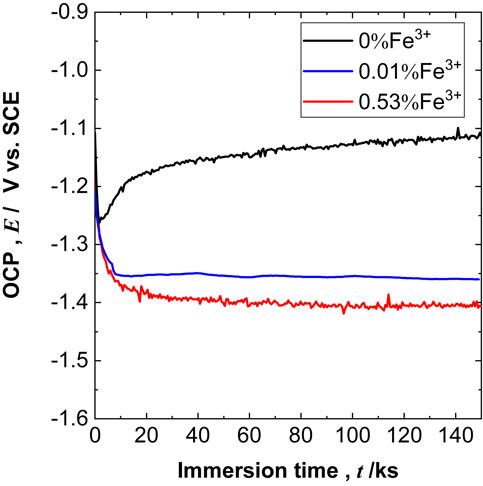
OCP transients of Al during the immersion tests in the test solutions with various Fe3+ concentrations.
Figure 2 presents the relationship between the OCP for Al after 144 ks immersion and Fe3+ concentration. The OCP values decreased with increasing Fe3+ concentration. In particular, the addition of a small amount of Fe3+ to the test solutions converted the OCPs to much less noble values. These results indicate that the corrosion behavior of Al differs between the test solutions containing Fe3+ compared with the solution without Fe3+.
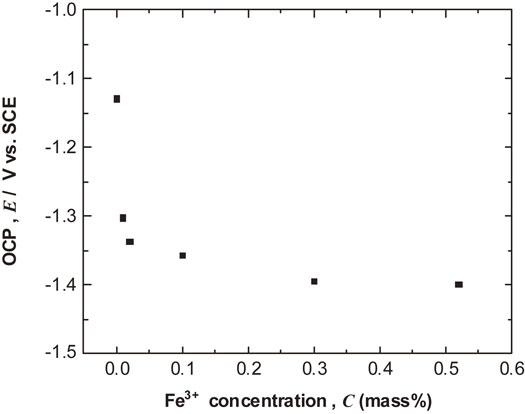
Change of OCPs after immersion for 144 ks as a function of Fe3+ concentration.
Figure 3 presents SEM images of samples before and after immersion testing. Without Fe3+ in the test solution, sample surfaces after immersion had scratches that had formed during the polishing step before the test, as shown in Figs. 3(b) and (b′). Pits was also observed; the pits were considered to be formed in the early stage of the immersion. In contrast, corrosion morphology changes were evident in samples immersed in the test solutions containing Fe3+. As shown in Fig. 3(c), for the 0.30% Fe3+ solution, general corrosion was observed on the whole surface, and the dissolution at the grain boundaries was also slightly discernible. At 0.53% Fe3+ immersion, as shown in Figs. 3(d) and (d′), the corrosion morphology was smoother with clear dissolution at grain boundaries as shown in Fig. 3(d′). Based on SEM observations, general corrosion was enhanced on high-purity Al samples in the test solutions containing Fe3+.
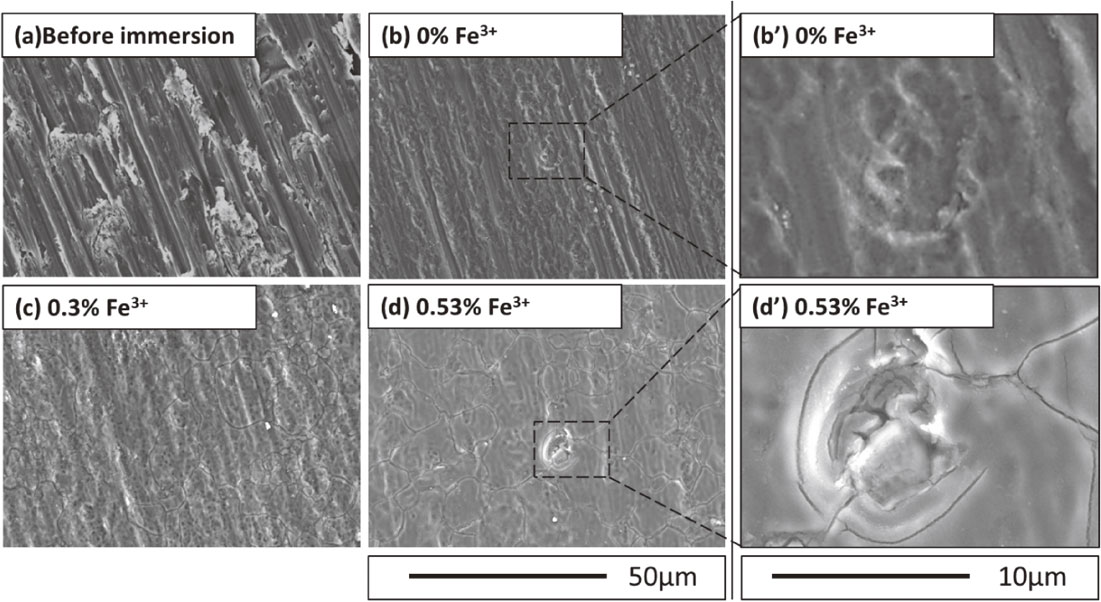
SEM micrographs of Al before and after the immersion in the test solutions with various Fe3+ concentrations: (a) as-polished Al, (b) and (b′) 0% Fe3+, (c) 0.30% Fe3+, and (d) and (d′) 0.53% Fe3+.
The relationship between weight loss and Fe3+ concentration is shown in Fig. 4. As noted above, almost no weight loss was evident in the case of 0% Fe3+, because only a few pits had formed during the immersion test. In contrast, weight loss increased linearly with Fe3+ concentration. This suggests that the dissolution is enhanced with increasing Fe3+ concentration.

Relationship between the corrosion weight loss and Fe3+ concentration in the immersion tests.
Figure 5 presents Bode plots for high-purity Al measured during immersion in the test solutions with and without Fe3+. All measured EIS behavior consisted of one time constant, which allowed the charge transfer resistance of Al in the test solutions to be estimated with a Randles-type electrical equivalent circuit.9–13) Figure 6 presents the change in the charge transfer resistance, Rct for Al during the immersion in the test solutions with various Fe3+ concentrations. As shown in Fig. 6, the Rct for Al in the case of 0% Fe3+ increased steadily with immersion time. For the test solutions containing Fe3+, Rct values did not change significantly over time during immersion. Additionally, Rct values decreased with increasing Fe3+ concentration. The reciprocal value of the Rct can be an index of the corrosion rate;14) therefore, the results in Fig. 6 indicate that the corrosion rate of Al increased with increasing Fe3+ concentration. This is in good agreement with the results shown in Fig. 4.
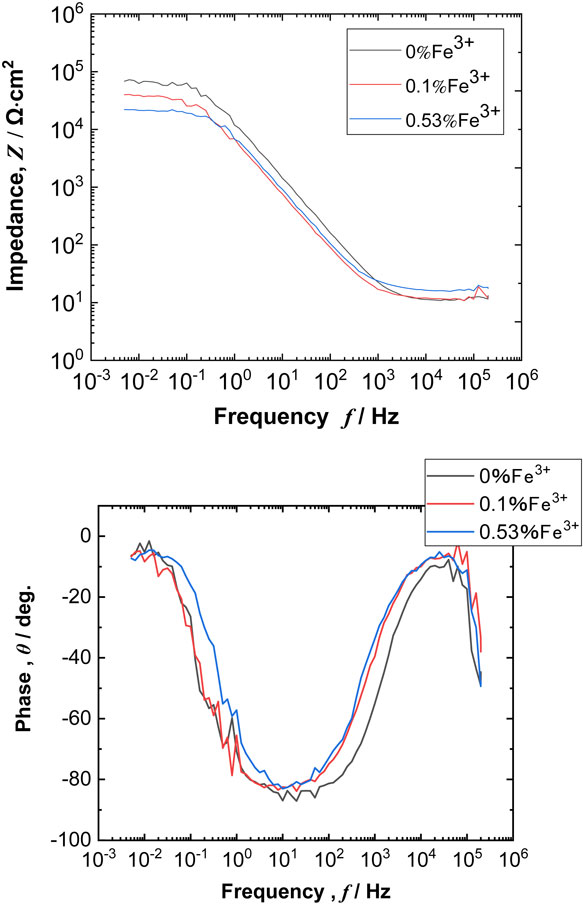
Bode plots for Al measured in the test solutions after the immersion for 144 ks.
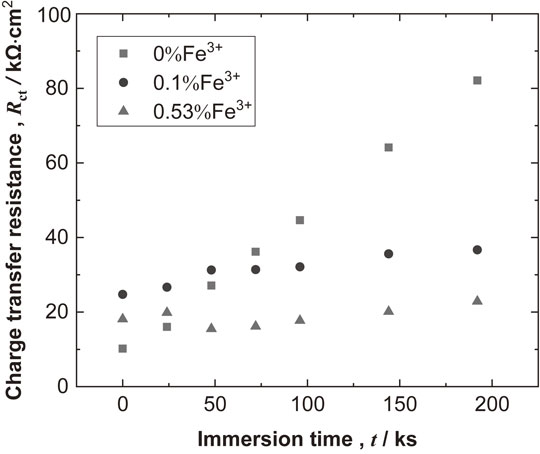
Comparison of the Rct transients in the test solutions with different Fe3+ concentrations.
From the results of immersion tests, it can be concluded that the presence of Fe3+ in the test solutions can change the corrosion morphology to a general one and accelerate general corrosion on the Al surface.
3.2 Cross-sectional compositional analysisFigure 7 presents cross-sectional backscattered electron (BE) images and energy dispersive X-ray spectroscopy (EDX) elemental mappings of oxygen (O) for the high-purity Al samples after the immersion tests. Oxygen was observed in the surface layer of the samples after immersion in the test solutions, both with and without Fe3+. Additionally, dissolution of the grain boundaries was enhanced significantly in the solution with 0.53% Fe3+ compared with 0% Fe3+. High-purity Al has a relatively lower corrosion resistance at the grain boundaries than in the matrix.15–17) General corrosion of Al mainly occurs during the immersion test with solution containing Fe3+. Additionally, it seems that Cl− promotes dissolution at grain boundaries.18)
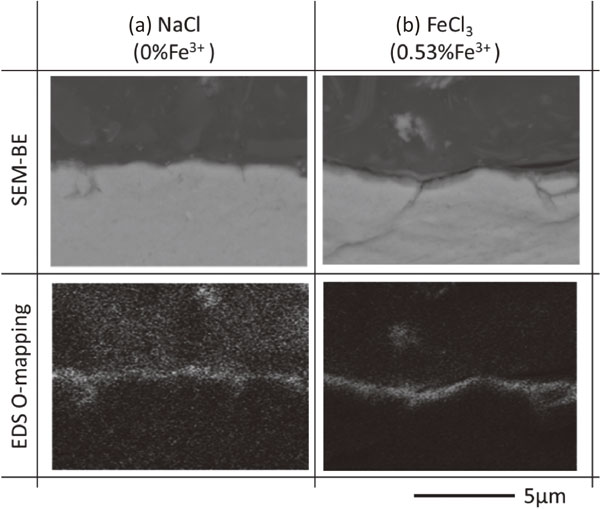
Cross-sectional BE images and EDS elemental mapping of O for Al after the immersion tests in (a) 0% Fe3+ and (b) 0.53% Fe3+.
Figure 8(a) shows GD-OES profiles that in the case of 0% Fe3+, Al, O, and H were detected in the surface layer, suggesting that the surface layer is composed of Al(OH)3.19,20) Cl have been detected on the outermost surface. In contrast, as shown Fig. 8(b), in the case of 0.53% Fe3+, Fe was detected in the surface layer, as well as Al, O and H. This indicates that the surface layer is composed mainly of Al(OH)3 containing Fe.7) Furthermore, the surface layer formed in the presence of Fe3+ was much thicker than that without Fe3+. These results indicate that the presence of Fe3+ can enhance dissolution of the Al surface with the formation of Al(OH)3 incorporating Fe. However, we are considering that further investigations on ionic permeability and composition of the surface film such as ionic permeability are needed to explain the mechanism of the enhancement of Al dissolution by Fe3+.
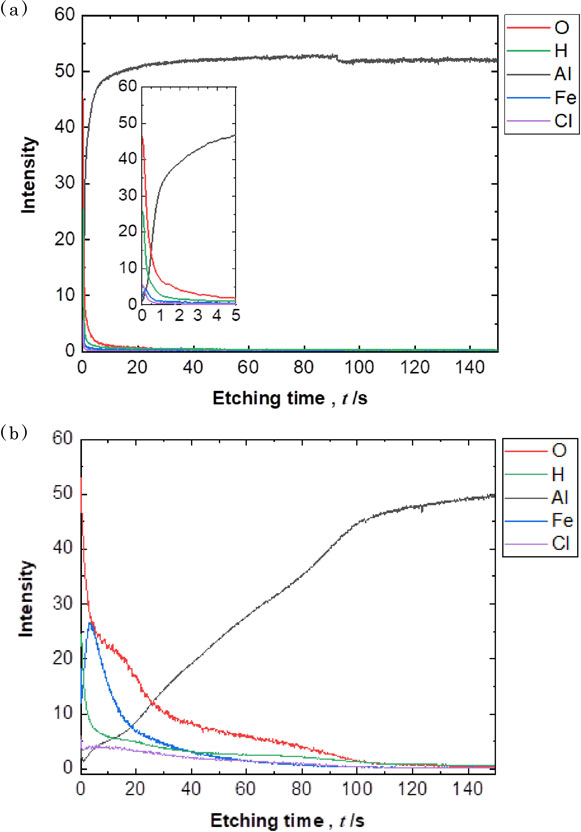
GD-OES concentration profiles for O, H, Al and Fe for the cross-section of the Al surface after the immersion tests for 580 ks in (a) 0% Fe3+ and (b) 0.53% Fe3+.
Polarization curve measurements were performed on high-purity Al after the immersion tests for 144 ks. Figure 9 presents the anodic and cathodic polarization behavior. From the results of the anodic polarization behavior, the pitting potentials for Al in the test solutions did not depend on the presence of Fe3+. Although pitting potential of Al depends on Cl− concentration,21–25) the presence of Fe3+ in the test solution had not impact on the pitting potential. However, the presence of Fe3+ had significant effects in the passive region of the anodic polarization behavior and the passive current densities clearly increased with increasing Fe3+ concentration as shown in Fig. 10.
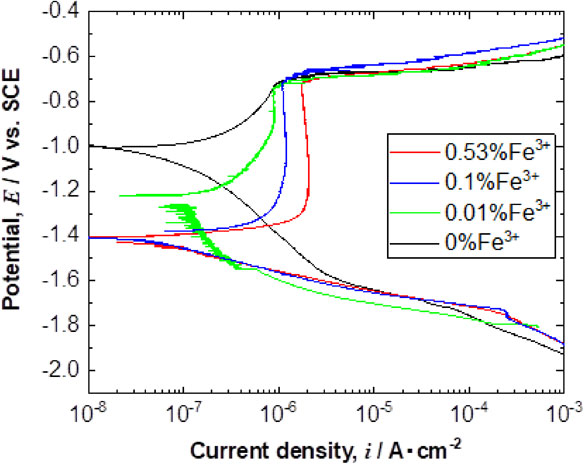
Anodic and cathodic polarization curves for Al after immersion for 144 ks in the test solutions with various Fe3+ concentrations.
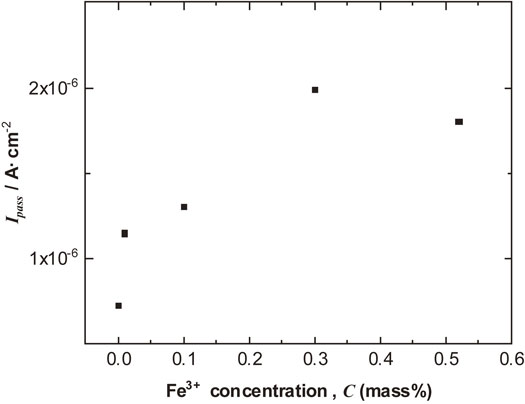
Relationship between Ipass and Fe3+ concentration after immersion for 144 ks.
On the other hand, the cathodic polarization behavior for Al depended on the presence of Fe3+ in the test solutions. In the cathodic polarization curves for Al for 0.53% and 0.1% Fe3+, the oxygen reduction reaction (ORR)26) activity on the Al surface was reduced compared with that in the 0% Fe3+ solution. This may be related to the protectiveness and thickness of the passive film formed on the Al in the test solutions with and without Fe3+. In the solution containing Fe3+, the thickness of the passive film was thicker compared with that without Fe3+. This may be attributed to the increase in the diffusion length of oxygen to the Al surface. This can cause the retardation of ORR rate.
Based on the results of the anodic and cathodic polarization behavior, immersion in the test solutions containing Fe3+ enhanced Al dissolution through the passive layer and suppressed ORR on the surfaces of Al samples.
The results of the cathodic polarization curves for the test solutions containing Fe3+ revealed no increase in the cathodic current compared with that in the solution without Fe3+, suggesting that the reduction of Fe3+ does not take place on the Al surface. This means that Fe3+ is present in the test solution without no change in its oxidation state.
In the test solution containing Fe3+, the OCPs for Al shifted to lower potentials and the dissolution of Al increased with increasing the Fe3+ concentration. Additionally, Al in the Fe3+-containing solutions exhibited general corrosion morphology. The results of surface observation and elemental mappings of the surface layer by EDS and GD-OES revealed that the surface layer was composed mainly of Al(OH)3, and Fe was incorporated into the surface layers formed in the test solutions with Fe3+. Surface layers became thicker than that formed in the solution without Fe3+.
Due to the low solubility of Fe3+ in the neutral aqueous solutions containing dissolved oxygen, Fe3+ likely oxidized to Fe(OH)3, thus resulting in its precipitation onto the Al surface. The low charge transfer resistance indicates that the passive film of Al(OH)3 formed in the test solutions with Fe3+ is less protective than that formed in NaCl.27,28) This finding is also confirmed in the Ipass results in the anodic polarization curves. Taken together, the formation of Al(OH)3 containing Fe(OH)3 likely deteriorates the passivity of Al, resulting in general corrosion during immersion.29–31)
Based on the effects of Fe3+ on the corrosion behavior of Al, Fig. 11 presents a dissolution model for high-purity Al in the test solutions containing Fe3+. Figure 11(a) shows a schematic representation of the Al surface after immersion in NaCl without Fe3+. The dissolution of Al promotes the formation of Al(OH)3 and makes passive film.29) Furthermore, Fig. 11(b) shows a schematic representation of the Al surface after immersion in FeCl3. In this case, a mixed hydroxide layer of Al(OH)3 and Fe(OH)3 formed over the entire surface of Al. This layer was thicker and anodic dissolution rate of Al was larger than in the test solution without Fe3+. Therefore, the passivity of Al due to the hydroxides became less protective, resulting in enhanced general corrosion. From the results, it can also be inferred that the dissolution at grain boundaries was enhanced in the test solutions containing Fe3+, given that the grain boundary dissolution rate was faster than that of the matrix.

Schematic diagram of passive film of Al formed after immersion in the test solutions: (a) without Fe3+ and (b) with Fe3+.
In this study, the effects of Fe3+ on the corrosion behavior of high-purity Al were investigated in the neutral test solutions using immersion tests, electrochemical measurements, and surface and cross-sectional observation and analysis. Our findings are summarized below.