2023 Volume 64 Issue 9 Pages 2302-2308
2023 Volume 64 Issue 9 Pages 2302-2308
A technique, which can explain the behavior of fires by using microstructural and oxide examinations of melted marks on copper wire, has been required for fire investigators. However, a study on the microstructural and oxide evolution after a fire is insufficient due to various and complicated fire conditions. In this research, changes in surface morphology, oxide layer thickness, oxidation kinetics, and microstructure were investigated at various annealing temperatures and times on the melted mark on the copper wire. The results show that the copper dendrites surrounded by (Cu+Cu2O) eutectic structure under Cu2O and CuO surface is the fingerprint of melted mark on copper wire annealed between 220°C and 600°C. At an annealing temperature of 800°C to 1000°C, the characteristic microstructure of the melted mark is Cu2O precipitates without dendrites in grains under a single layer of Cu2O. Moreover, the diffusion processes contributed to Cu2O growth could be as follows: lattice diffusion at 220°C to 400°C and grain boundary diffusion at 400°C to 1000°C.
A commercial 99.95% pure copper wire has been extensively used for building wirings and electrical application because of their excellent electrical conductivity.1,2) The copper wire in electrical application is made by wire drawing process of solidified pure copper. Occasionally, the use of power exceeding the specified limit causes copper wires to overheat and come into contact with each other, resulting in a fire accident. At this time, the microstructure of a “melted mark” on the copper wire after a fire caused by arcing of electrical current in a fire site can be considered to be an indicator of explaining fire behaviors and determining the cause of the fire accident. The vital evidence used to determine the source of fire for the fire investigator is the melted mark on the copper wire because it is not destroyed and left after the fire.3) In order to use solidification and heat treatment structures as indicators, it is necessary to investigate structures obtained from actual fire accidents. On the other hand, it is also necessary to perform the model experiment to compare the conditions and structures after melting, solidification and heat treatment. In our previous work, the original microstructure of the melted mark on the copper wire, which comprises Cu dendrite and (Cu+Cu2O) eutectic structure, has been reported.4) Moreover, the different cooling rates of melted marks affect the solidification microstructure in terms of secondary dendrite arm spacing (SDAS) and Cu2O crystallite size. Nevertheless, the effects of holding temperatures and times on the microstructure of melted marks to simulate the fire environments have not been examined. These parameters were investigated and clarified in this paper. The various heat treatment conditions of copper wire before arcing were conducted by Mei et al.5,6) The original structure of copper wire before melting is a fibrous structure due to the wire drawing process, and it becomes an equiaxed crystal after annealing. After arcing by electrical current, Liu et al.7) presented that the Cu-dendrites and (Cu+Cu2O) eutectic structure under the cuprous oxide (Cu2O) surface layer is the fingerprint of original melted mark (without fire). Nevertheless, the melted mark on copper wire generated from arcing after a fire have received little attention. Moreover, the evolution of the melted mark on copper wire under various fire environments is not only in the microstructure but also the oxide layer. The oxidation kinetics of copper have been widely studied. Park et al.8) reported the temperature dependence between 350°C and 1000°C of the oxidation rates whereby the oxidation of copper can be identified in three different processes. The bulk diffusion, grain boundary diffusion, and surface diffusion with whisker growth occur at high, intermediate, and low temperatures, respectively. The whisker forms on the oxide surface at temperatures lower than 750°C. Moreover, R.A. Rapp9) reported that surface cold work, low temperatures, and the presence of water vapor all promote whisker formation. Additionally, Zhu et al.10–12) suggested that the oxidation kinetics at 350°C to 1050°C obey the parabolic oxidation rate law.
Because various fire behaviours are found in fire accidents, both the microstructure and oxide layer evolutions on the melted mark of copper wire under various fire environments play a role in fire investigation. In this study, typical microstructure and oxide layer of the melted mark on copper wire were examined under different annealing temperatures and annealing times.
The commercial copper wire (99.95 mass% Cu) with a diameter of 2.6 mm and without PVC-insulating has been utilized in this study. The arc-welding machine was used to create the melted marks on copper to simulate arcing. The space between positive and negative electrodes was set at one centimeter, and all specimens were melted by a voltage of 100 Volt. Specimens were rapidly heated and melted by arcing then suddenly cooled in the atmosphere. The air-cooled specimens were then annealed in the furnace with varied heat treatment parameters as stated in Table 1 and finally cooled in the furnace to imitate the fire environments. In addition, arc melting and heat treatment processes must be performed consecutively to accurately reproduce a fire accident. However, since the arc melting machine could not be installed in the furnace, the melt and solidified processes were separated from the heat treatment process. The heating and cooling rates for each condition are shown in Table 1. The temperature ranges from 200°C to a given temperature were used to calculate the heating rate, while the cooling rate was calculated from the given temperature to 100°C. These rates in the annealing process at each temperature has subtle difference which may result in small change in the final microstructure.

To observe the microstructure and measure oxide layer thickness, the conductive resin (Technovit) has been utilized to mount the specimens before polishing. The longitudinal microstructure and oxide layer thickness were examined by first grinding with SiC paper from #500 to #4000 and then mirror-polishing with 0.1 µm diamond paste. The specimens were cleaned in an ultrasonic bath with alcohol after grinding and polishing. Then, the oxide layer thickness measurement was done before etching. Aqueous ferric chloride was used to etch the surface of melted marks for a few minutes before microstructural examination with an optical microscope.13–15) SEM (HITACHI SU3500) attached EDS was used to examine the surface morphology and chemical composition of specimens. The phase identification of the copper oxide on the melted mark surface was conducted by X-ray diffraction (XRD) RINT-2100 under 40 kV/4 mA and CuKα radiation at 0.1540 nm wavelength. Data were collected in the range 2θ of 20 to 80 degrees with 0.02 degree steps.
The effects of annealing temperature and time on the melted mark of copper wire are examined, which includes surface morphology observation, oxide phase identification and crystallite size calculation, parabolic rate constant and activation calculation, and microstructure characterization.
3.1 Surface morphology observationInitially, the effect of annealing temperature on the surface morphology evolution of melted marks on copper wire was examined. Figure 1 shows the surface micrographs of melted marks on copper wire annealed for two hours at temperatures of (a) 1000°C, (b) 800°C, (c) 600°C, (d) 400°C and (e) 220°C. At 1000°C, the middle of the oxide grains displayed a dark depression surrounded by strong ridges at their grain boundaries in the bright region. The elemental analysis by EDS was conducted both in the middle of the oxide grain at point 1 and at the grain boundary at point 2. The presence of copper and oxygen elements of 1:1, equivalent to the amount of copper and oxygen in CuO, is indicated by the EDS spectrum in the bright region at point 1. In the middle of the oxide grain (point 2), meanwhile, the amount of copper and oxygen at their grain boundary is about comparable to the theoretical copper and oxygen percentages in Cu2O. Accordingly, the presence of Cu2O grains enclosed by the CuO at the grain boundary is the typical morphology of melted marks after heat treatment at 1000°C.

Oxide surface evolution of melted mark after heat treatment for 2 h at (a) 1000°C, (b) 800°C, (c) 600°C, (d) 400°C, and (e) 220°C. Figures (f) and (g) show EDS spectrum of Fig. (a).
At 800°C, oxide grains are smaller than at 1000°C, with the CuO scale uniformly covered on the outer surface. With decreasing the temperature to 600°C, the EDS results of the cross-section of the melted mark in Fig. 2 reveal the existence of Cu2O at the inner surface (point 2) and CuO whiskers (point 1) randomly nucleated on the Cu2O surface. It has been reported that the CuO whiskers nucleated on Cu2O grow predominantly by surface diffusion resulting from the breakup of adsorbed oxygen due to the presence of water vapor.8–12) The surface at 220°C and 400°C is not flat with slight coverage by CuO, and whiskers cannot be observed at these temperatures. It can be seen that depending on the temperature, the morphology of copper oxide develops with distinct characteristics. The larger oxide grain structure is observed at surface of the melted mark annealed at 1000°C because the atoms transported at higher temperatures have enough diffusion activation energy to occupy the crystal lattice and induce the small grains by grain boundary diffusion, resulting in larger grains than when annealed at 800°C.16,17)
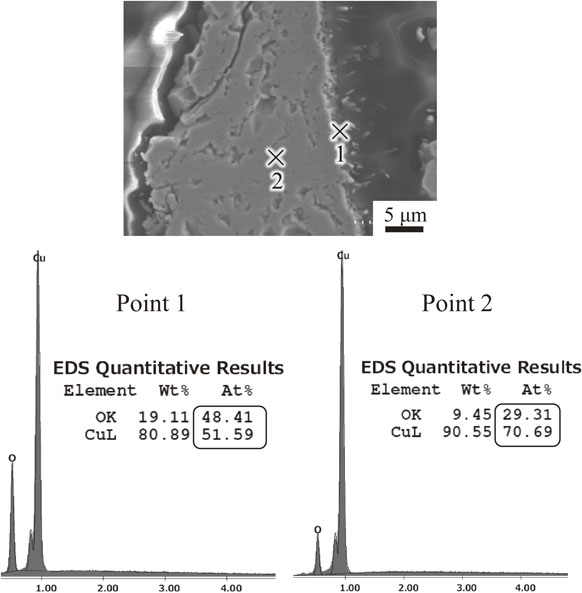
Elemental analysis by EDS on the surface of melted mark annealed at 600°C for 2 h.
CuO whiskers are observed on Cu2O surface of melted marks annealed at 600°C in Fig. 1(c). The growth of whiskers could be supported by surface diffusion through the overgrowth at their tips from screw dislocation emerging on the metal surface due to the breakup of the absorbed oxygen.8,9) Furthermore, the tiny oxides was investigated even at low temperature of 220°C and 400°C as shown in Figs. 1(d) and 1(e). Although the Cu2O and CuO were identified via EDS, which may not be sufficient to conclude their existence, XRD has been used to confirm the presence of Cu2O and CuO in Section 3.2.
3.2 Oxide phase identification and crystallite size calculationThe phase identification of the oxide layer by XRD can be utilized to validate the existence of the copper oxide. The XRD patterns of the copper oxide surface on melted marks of copper wire annealed under 1000°C, 800°C, 600°C, 400°C, and 220°C for 2 hours and cooled down in the furnace are demonstrated in Fig. 3. The oxide surface on the specimen or the oxide peeled off from the specimen was analyzed. The peak positions of 29.42, 36.29, 42.16, 61.27, 73.40, and 77.27 degrees correspond to the (110), (111), (200), (220), (311), and (220) planes of Cu2O. These peaks match Cu2O powder from JCPDS file no. 00-002-1067, indicating that Cu2O is present at the surface of the melted mark for all annealing temperatures from 1000°C to 220°C. The oxidation is described by the reaction of copper atoms with oxygen atoms to generate Cu2O, which is written as the following reaction: 2Cu + 1/2 O2 → Cu2O.16) At 220°C, the copper oxide formed on the surface of the melted mark has an extremely low amount due to the low temperature, and all peaks of oxide layers including a base copper melted mark were very slightly detected. From 600°C to 400°C, the peak positions at 35.44, 38.58, 48.74, 58.21, 66.36, and 68.08 degrees correspond to the (002), (111), (-202), (202), (022), and (113) CuO planes. Each peak position is in good agreement with CuO powder from JCPDS file no. 00-002-1040 confirming the presence of CuO. The reaction between copper and oxygen can be described as follows: 2Cu2O + O2 → 4CuO.16) The growth of CuO on the Cu2O layer is extremely small at high temperatures because of low atomic transport due to the absence of vacancies in CuO for fast atomic transport.8) As a result, a small amount of CuO compared to Cu2O results in unnoticeable CuO in the XRD patterns at 1000°C. A little CuO at 1000°C is also consistent with the appearance of CuO at the only grain boundary of the oxide grains examined by EDS in Fig. 1(a). With decreasing annealing temperature to 800°C, the CuO phase starts to appear and the XRD pattern presents an extra small peak near 2θ = 33° which agrees well with the reflection from the (110) plane of CuO. Based on the XRD patterns at 600°C and 400°C, the oxide surface of the melted marks consists of a mixture between the Cu2O and CuO phases. The phase diagram suggests that CuO is the stable phase at lower temperatures. Therefore, the Cu2O slowly and finally oxidizes into the CuO phase,8,12,16) but may not entirely oxidize due to the low temperature.

X-ray diffraction patterns of copper oxide on melted mark of copper wire after heat treatment for 2 h at different temperatures.
The crystallite size of Cu2O was also estimated using the Debye-Scherrer formula:18–20)
| \begin{equation} D = 0.9\lambda/\beta \cos\theta \end{equation} | (1) |

Influence of annealing temperature on Cu2O crystallite size after heat treatment for 2 h.
The oxide layers were observed on the surface of the melted marks annealed at various times and temperatures, as depicted in Fig. 5. The specimen was cut perpendicular to the oxide layer passing through the center of the specimen, as much as possible. As discussed in the previous section, CuO is slightly formed on the Cu2O surface, so it cannot be observed with an optical microscope in the cross-section after mounting and polishing. Thus, measuring the CuO layer for each specimen is impossible, whereas the Cu2O layer clearly appears on the copper melted mark. As a result, Cu2O layers were measured for all heat treatment conditions. The variation of Cu2O layer thickness with annealing time at constant temperature is displayed in Fig. 5. In addition, specimens with a holding time of 0 hours are shown as reference microstructures for each temperature. An increase in annealing time mainly increases the thickness of Cu2O due to the longer time promoting more reaction between copper and oxygen to form Cu2O. Along with the increase of annealing temperature, the thickness of the Cu2O is rapidly increasing with temperature. At 220°C and 400°C, the Cu2O layer is very small because the generation of Cu2O is restrained due to the low temperature, then Cu2O slowly grows and gradually oxidizes into CuO. At an intermediate temperature range, the thickness of Cu2O increases, as copper cannot transfer to combine with oxygen at the copper/oxygen interface. Then, Cu2O become unstable and finally, fully oxidizes into CuO as exhibited in the SEM and XRD results. At 1000°C, the Cu2O layer with some porosities grows predominantly because of its thermodynamic stability and hardly oxidizes into CuO.17)

Microstructures of oxide layer on the surface of the melted mark annealed at various times and temperatures.
Furthermore, the linear relationships are observed between the average oxide layer thickness and the square root of time in Fig. 6, indicating that the oxidations at 1000°C, 800°C, 600°C, 400°C, and 220°C are controlled by a diffusional process.21) Moreover, Cu2O growth at 220°C to 1000°C obeys the parabolic rate law, which is consistent with reporting by Zhu et al.11) The parabolic rate constant (kp) or the diffusion rate constant can be calculated from the slope of the parabolic plot. The values are 20.4 µm2/s at 1000°C, 5.8 µm2/s at 800°C, 0.13 µm2/s at 600°C, 6.12 × 10−4 µm2/s at 400°C and 1 × 10−4 µm2/s at 220°C. These rates are controlled by the ability of the copper and oxygen atoms to migrate through the oxide layer and encounter each other. As the thickness increases, it takes longer for the copper and oxygen atoms to diffuse through, and so the oxidation is limited by the diffusion rate. Additionally, the parabolic rate constant at the low temperature of 220°C is similar to the rate of 1 × 10−4 um2/s as reported by Lee et al.21)

The variation of Cu2O layer thickness with annealing time at constant temperature.
An increase in oxide layer thickness and faster oxidation rate is caused by increasing the processing temperature, as discussed previously. Therefore, the parabolic rate constant (kp) must be modifiable for temperature effects in order to describe oxidation using the parabolic approach. Accordingly, the Arrhenius expression in eq. (2) well describes the effects of temperature on the parabolic rate constant.22)
| \begin{equation} k_{p} = A\,\mathit{exp} (-Q/RT) \end{equation} | (2) |

Arrhenius plots of the parabolic rate constants at 220°C to 1000°C for Cu2O oxidation under atmosphere.
In detail, the rate of lattice, grain boundary, and surface diffusion is characterized by the diffusion coefficients related to activation energy Ql, Qgb, Qs where Ql > Qgb > Qs.25) It was thought that the oxidation in Cu2O over the wider range from 400°C to 1000°C with higher activation energy might have contributions from lattice diffusion. Meanwhile, lower activation energy may result in grain boundary diffusion, which contributes to copper oxidation at low temperatures of 220°C to 400°C.8,12) Additionally, because the activation energy for surface diffusion is lower than that for lattice diffusion, growth associated with screw dislocation steps will result in whisker growth at 600°C by surface diffusion. In contrast, as temperature rises, lateral lattice diffusion becomes more favorable, and oxide growth could result in flatter morphologies such as pyramids.25)
3.5 Microstructure characterizationThe microstructural evolutions of melted marks on copper wire under various annealing temperatures and annealing times are demonstrated in Fig. 8. Due to the structure of the electric furnace, the heating rate varied from 0.4 to 0.9 K/s, so the specimen holding 0 hours was prepared as the reference structure for each holding temperature. It can be clearly seen that there is a difference in microstructural evolution from low temperatures to high temperatures. A dendritic structure in columnar grains can be seen at low temperatures (220°C to 600°C), but it gradually disappears at 800°C and 1000°C. The mechanism underlying the microstructural change is discussed as follows. Before heat treatment, the original microstructure of melted mark on copper wire comprises copper dendrites in columnar grains surrounded by the (Cu+Cu2O) eutectic structure.26,27) After heat treatment at 220°C to 400°C for different annealing times, the dendritic structures can be noticed for all annealing times due to low temperature, similar to the microstructure before heat treatment. However, the darker color on the copper dendrites gradually appears after annealing at 400°C for 4 and 8 hours. It was thought that small particles of Cu2O start to precipitate on the copper dendrites during the heat treatment process for a longer time.28) When increasing the temperature to 600°C, the dendritic structure is still observed for all annealing times. Additionally, more precipitated Cu2O particles on copper dendrites can be observed. It is interesting that the dendrites disappear after heat treatment for an annealing time of more than one hour at 800°C and for all annealing times at 1000°C. Dendrites can still be seen in unannealed specimens at 800°C, but they vanish after that. With the above discussion, the microstructure before heat treatment consists of the primary copper dendrites in a bright region inside columnar grains and the (Cu+Cu2O) eutectic phase in a dark region.

Microstructural evolution of melted marks on copper wire after various heat treatment conditions.
After heat treatment at high temperatures, copper dendrites in the bright region disappear completely because copper dendrites in the same crystallographic orientation must minimize their interfacial energy by dissolving and trying to connect to form a large grain over time. Meanwhile, the (Cu+Cu2O) eutectic phase is transformed into Cu2O particles that precipitate all over the copper matrix. Furthermore, the different colors separated by the grain boundary on the microstructure after annealing, for example, at 1000°C for 120 minutes, indicate different crystal orientations for each grain. Therefore, the columnar grain structure with precipitated Cu2O particles is the final microstructure of the melted mark after heat treatment at a high temperature range (800°C to 1000°C).
For that reason, the microstructural evolution under various heat treatment conditions might be used to describe the fire behaviors. In the case of high fire temperatures, the final structure should be the same as the microstructure after high-temperature annealing, with dendrites disappearing and columnar grains with precipitated Cu2O.
In summary, the characteristic feature after heat treatment at low temperatures (220°C to 600°C) is copper dendrites in columnar grains enclosed by the (Cu+Cu2O) eutectic under Cu2O layer and CuO. At high annealing temperatures (800°C to 1000°C), columnar grains with Cu2O precipitates under Cu2O layer become the typical microstructure.
In a real fire accident, oxygen gas or other gases may be released due to the combustion of materials in the fire. These gases may have an impact on the microstructure and copper oxide. Moreover, oxygen pressure in the real fire site may differ from this experiment. Because of various fire behaviors, this work could not comprehensively simulate all fire conditions. As a result, the oxygen effect on the melted mark of copper wire is expected to be studied in the future.
The oxide layer and microstructural evolution under various annealing temperatures and annealing times on the melted mark of copper were investigated. The following outcomes have been obtained:
This work was supported by the Royal Thai Government scholarship.