2023 Volume 64 Issue 9 Pages 2232-2236
2023 Volume 64 Issue 9 Pages 2232-2236
We performed 1.5 MeV electron-irradiation at 333 K and 533 K for Cu–4.2 at% Ti alloy with a single phase of super-saturated solid solution, and investigated the irradiation-induced changes in Vickers hardness and electrical conductivity. With increasing the electron fluence, both of the hardness and the electrical conductivity increase. Such phenomena can be ascribed to the formation of Ti-rich precipitates that are caused by irradiation-enhanced diffusion of Ti atoms. The increase in electrical conductivity is caused by the reduction of Ti content in Cu matrix because of the formation of Ti-rich precipitates. The increase in hardness is also caused by Ti-rich precipitates that are effective obstacles against the motions of dislocations. We found a clear correlation between the irradiation-induced change in the hardness, ΔHv and change in electrical resistivity, Δρ, or that in electrical conductivity, Δσ, as $\Delta H\text{v} \propto \sqrt{ - \Delta \rho } $, or $\Delta H\text{v} \propto \sqrt{\Delta \sigma /\sigma } $, irrespective of irradiation temperatures. This correlation suggests that the precipitate-cutting mechanism governs the irradiation-induced increase in hardness; that is, 1.5 MeV electron-irradiation at relatively low temperatures of 333 K to 533 K should promote the nucleation of fine Ti-rich precipitates preferentially rather than the growth of them. The present result shows that energetic electron irradiation is a good tool to improve the mechanical and electrical properties of Cu–Ti alloys.

Recently, Cu–Ti alloys with a Ti content around 4.2 at% have a great deal of attention as materials for electrical components, because of their good mechanical and electrical properties.1) Such good properties originate from fine metastable Cu4Ti precipitates which are formed by the isothermal aging at elevated temperatures around 723 K.2–4) The fine precipitates act as effective obstacles against dislocation motions, resulting in strength (hardness) increase. The formation of Ti-rich precipitates (Cu4Ti) reduces the content of Ti atoms in copper matrix. The reduction of Ti content in copper matrix leads to the decrease in electrical resistivity or the increase in electrical conductivity,5,6) because conductive electrons are less significantly scattered by the strain field in the matrix with a lower Ti content.
In supersaturated alloys which are usually produced by quenching dilute alloys from an elevated temperature to iced water, additive elements are forced to solve in matrix. Such a supersaturated state is a non thermal-equilibrium state. Energetic particle irradiation produces point defects such as vacancies and interstitial atoms. Point defects interact selectively with some solute atoms, and thermally diffuse with the solute atoms at relatively low temperatures. This phenomenon is called “radiation enhanced diffusion”.7) By the radiation enhanced diffusion, the alloy goes to the thermal-equilibrium state, i.e., solute atoms are precipitated in matrix. We have found that using this phenomenon, the hardening of supersaturated aluminum alloys can be realized by energetic ion irradiations even at room temperature.8–11) The hardening of the aluminum alloys can be explained as due to the radiation enhanced diffusion of Cu, Mg and Si atoms in aluminum matrix. We have also reported that the hardness of Cu–4.2 at% Ti alloy increases by 2 MeV electron irradiation at 473 K and 523 K,12) which was explained by the radiation enhanced diffusion of Ti atoms in copper matrix.
In the present study, the sheets of Cu–4.2 at% Ti alloy were irradiated with 1.5 MeV electrons at 333 K or 533 K in argon atmosphere by using a conventional electron accelerator which has mainly been used for the sterilization of medical materials, the modification of semiconductors and polymers and so on. After the irradiation, irradiation-induced changes in not only the hardness but also electrical conductivity were measured. The correlation between hardness change and the change in electrical resistivity or electrical conductivity is discussed.
Cu–4.2 at% Ti alloy was prepared by melting raw materials, i.e., pure Cu (99.99 mass%) and Ti (99.99 mass%) in vacuum. The alloy ingot was hot-rolled to thickness of 0.1 mm at approximately 1223 K. The phase diagram of Cu–Ti binary alloys13,14) shows that the solubility limit of Ti atoms in fcc-Cu matrix is larger than 4.2 at% at 1223 K. To obtain supersaturated Cu–4.2 at% Ti samples, therefore, the sheet samples were heat-treated at 1223 K for 1 hour in argon atmosphere, and then quenched into iced-water. The contamination layers at the sample surfaces were removed by a mechanical grinding. The sheets were cut into 50 × 5 × 0.1 mm3 in dimension for the electron irradiation and the measurements of Vickers hardness and electrical conductivity.
Solid solution-treated Cu–4.2 at% Ti samples were irradiated with 1.5 MeV electrons by using an electron accelerator at Takasaki Institute of Advanced Quantum Science, National Institutes for Quantum Science and Technology (QST-Takasaki). As the range of 1.5 MeV electron is much larger than the sample thickness of 0.1 mm, the effect of electron irradiation covers the whole of the samples.
Figure 1 shows the schematic illustration for sample irradiation with electrons. The electron beam passed through a titanium window from vacuum into atmosphere. The samples fixed to basal copper plates were set on a water-cooling board, and were covered with aluminum chassis and aluminum foil about 10 µm thick. A space inside the aluminum chassis and foil was filled with argon gas to prevent an oxidation of sample surfaces during the irradiation. Sample temperatures during the irradiation were controlled by electron beam heating and stainless plates as poor thermal-conduction materials, which were put between the copper basal plate and the cooling board. In Fig. 1, the right sample with a copper basal plate is placed directly on the water-cooling board. For the left sample, a stainless plate is placed between the copper basal plate and the water-cooling board. During the irradiation, the temperature of the left sample is higher than that of the right sample. By using the irradiation arrangement in Fig. 1, we can simultaneously irradiate several samples at different temperatures. The sample temperature during the irradiation was monitored by a K-type thermocouple which was embedded into each basal copper plate.

Schematic illustration of electron irradiation.
In our previous irradiation experiments with energetic electrons, we used another facility of irradiation (a single-ended accelerator, one of TIARA accelerator complex at QST-Takasaki).12,15) Samples were set into a vacuum chamber, and were irradiated with electrons which were transmitted through a vacuum duct from the accelerator. Although such an irradiation procedure is a common procedure for the irradiation of solid targets with energetic particles, only one small sample can be irradiated at one time, and a long time is needed for vacuuming an irradiation chamber. While for the present irradiation method, as the electron beam is widely scanned by the magnet, we can irradiate simultaneously several samples with relatively large dimension. Moreover, we can easily set and change samples in atmosphere. As the samples are heated by electron beam energy, we do not need any other beam heating apparatuses such as beam heaters. Therefore, the present irradiation method is much better for industrial applications than the previous one.
The electron-fluence was from 8.8 × 1016 e−/cm2 to 1.0 × 1018 e−/cm2. The sample temperature during the irradiation was 333 K or 533 K, which was much lower than the temperature of around 723 K for conventional isothermal-aging process.3,16) For comparison, only the isothermal-aging was also performed for some solution-treated samples at 533 K.
Electrical conductivities for irradiated samples and isothermally-aged ones were measured at room temperature by a conventional DC four-terminal method using a micro-ohm meter (34420A, Agilent, Santa Clara, USA). The micro Vickers hardness was measured by using a Vickers hardness tester (MH101, Akashi, Tokyo, Japan) for irradiated and isothermally-aged samples with a load of 0.49 N and holding for 10 s. The hardness values were obtained by averaging the values from ten indentations.
Figure 2 shows the Vickers hardness for Cu–4.2 at% Ti samples irradiated with 1.5 MeV electrons at 533 K as a function of electron fluence. In the figure, we also show the processing time which means the irradiation time. For comparison, the figure shows the Vickers hardness of the sample which was not irradiated but only aged at 533 K for 440 min. The hardness for the sample irradiated at 533 K increases with increasing electron fluence and tends to be saturated for higher fluences. This is a similar trend of the previous result.12) Figure 2 also shows the dependence of hardness change on irradiation temperature. The increase in hardness for 533 K irradiation is much larger than for 333 K irradiation even at the same electron fluence of 1.0 × 1018/cm2. Moreover, even for the same processing time (440 min) and temperature (553 K), the increase in hardness is much larger by the irradiation than only by the isothermal-aging. Therefore, we can say that the large increase in hardness for the sample irradiated at 533 K was achieved by the synergy of electron irradiation effect with thermal effect.

Vickers hardness as a function of electron fluence and processing time (irradiation time). Data point for isothermal aging at 533 K for 440 min is also shown.
In Fig. 3(a), the dependence of electrical conductivity on electron fluence and irradiation time are plotted for 533 K irradiation. Figure 3(a) also shows the difference in electrical conductivity between 533 K irradiation and 333 K irradiation at the same irradiation fluence (1 × 1018/cm2) or the same processing time (440 min), and that between for only isothermal aging and electron irradiation at the same processing time (440 min) and temperature (553 K). The trend of change in electrical conductivity by the electron irradiation and the isothermal aging is just the same as that of the change in hardness (Fig. 2). For later discussion, we also show the change in electrical resistivity, which is merely the inverse of the electrical conductivity, in Fig. 3(b).

Electrical conductivity (a) and electrical resistivity (b) as a function of electron fluence and processing time (irradiation time). Data point for isothermal aging at 533 K is also shown.
Our previous paper has already reported the increase in Vickers hardness of Cu–4.2 at% Ti alloy by the electron irradiation at elevated temperatures.12) As mentioned in the introduction of this paper, the thermal diffusion of irradiation-produced point defects enhances the diffusion of solute atoms, resulting in the production of Ti-rich precipitates. Such precipitates surely cause the increase in hardness. However, irradiation-produced point defects and their aggregates would also act as obstacles against dislocation motions, and cause the hardening of the alloy. Therefore, only from the result of Vickers hardness, we cannot conclude that the hardening by electron irradiation originates from the radiation enhanced diffusion of solute atoms.
In the present study, we measured not only the hardness but also the electrical resistivity or conductivity. If the effect of electron irradiation was only the production of point defects and/or their aggregates, the electrical resistivity would also increase due to the scattering of conduction electrons by the lattice disordering. Figure 3(b), however, shows that the electrical resistivity decreases by the irradiation. The present result, therefore, demonstrates that the radiation enhanced diffusion produces Ti-rich precipitates and then the content of Ti atoms in Cu matrix decreases, causing the decrease in electrical resistivity or increase in electrical conductivity.
Next, we consider the relationship between the change in hardness and that in electrical resistivity or conductivity. It is well known that the precipitation hardening depends on the size of precipitates.17) When the size of precipitates is small enough, the hardening is governed by the particle-cutting mechanism.18,19) According to this mechanism, the hardness increase, ΔHv, is proportional to $\sqrt{fr} $;
| \begin{equation} \Delta H_{v} = A \cdot \sqrt{fr} = A \cdot \sqrt{\left(\frac{4}{3}\right)\pi r^{3}N \cdot r}, \end{equation} | (1) |
| \begin{equation} \Delta H\text{v} \propto \sqrt{N}. \end{equation} | (2) |
On the other hand, the change in electrical resistivity, Δρ, of the Cu–Ti alloy is provided by the following equation,5,6) if ignoring a small amount of precipitates.20)
| \begin{equation} \Delta \rho \propto \Delta C_{\textit{Ti}}, \end{equation} | (3) |
| \begin{equation} -\Delta C_{\textit{Ti}} \propto N. \end{equation} | (4) |
| \begin{equation} \Delta H\text{v} \propto \sqrt{-\Delta\rho}. \end{equation} | (5) |
Figure 4 shows the experimental data of ΔHv as a function of $\sqrt{ - \Delta \rho } $. The change in hardness induced by the irradiation at 333 K and 533 K, ΔHv, is proportional to $\sqrt{ - \Delta \rho } $. This is just what eq. (5) means. Equation (5) has been deduced under the assumptions that the particle-cutting mechanism governs the precipitation hardening and that the number of precipitates of N increases as the size of r remains constant during the electron-irradiation. Figure 4, therefore, implies that the hardening is dominated by the cutting of the irradiation-induced Ti-rich precipitates. As the previous result has shown that the size of the electron-irradiation induced Ti-rich precipitates is very smal,12) the particle-cutting mechanism should be reasonable to explain the present result. Figure 4 also indicates that the radius, r, of the Ti-rich precipitates is not changed and only the N increases during the electron irradiation irrespective of the irradiation temperature. The data point for the isothermal-aging at 533 K, however, significantly deviates from the straight line for the electron irradiation at 533 K and 333 K. This result means that the size of Ti-rich precipitates only by the isothermal aging is quite different from the case of electron irradiation even under the same temperature. We have found the similar correlation between ΔHv and Δρ in electron-irradiated Fe–Cu dilute alloys which are the simulation alloys for pressure vessels of nuclear power plants.15)
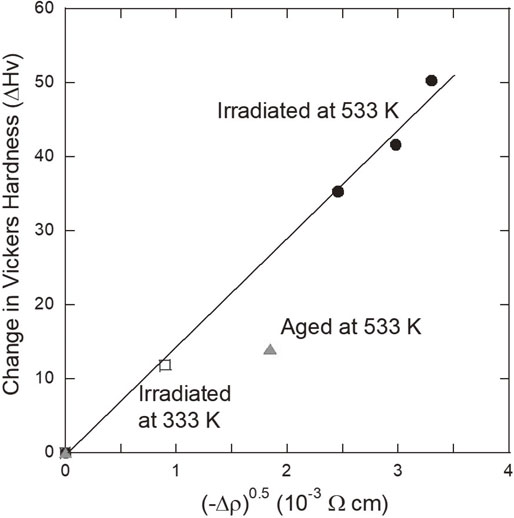
Correlation between ΔHv and Δρ.
The formation process of precipitates consists of their nucleation and subsequent growth around the nucleation sites. In the case of present electron irradiation, the supersaturated state rapidly transfers to the thermal equilibrium state by the radiation enhanced diffusion of Ti atoms in a short-distance. This short-distance diffusion makes nucleation sites for Ti-rich precipitates with less growth of them. In addition, irradiation-produced lattice defects which are distributed homogeneously in the sample may act as nucleation sites. As a result, the nucleation of precipitates occurs preferentially rather than the growth of them. Therefore, the number density of precipitates increases but their size remains small and almost constant during the electron irradiation.
Followed by the above discussion, the correlation of ΔHv with the change in electrical conductivity, Δσ, is easily obtained because the value of σ is merely the inverse of ρ,
| \begin{equation} \sigma = \frac{1}{\rho}, \end{equation} | (6) |
| \begin{equation} \Delta H\text{v} \propto \sqrt{-\Delta\rho} = \sqrt{\rho_{0} - \rho} = \sqrt{\frac{1}{\sigma_{0}}} \sqrt{\frac{\sigma - \sigma_{0}}{\sigma}} \propto \sqrt{\frac{\Delta\sigma}{\sigma}}, \end{equation} | (7) |
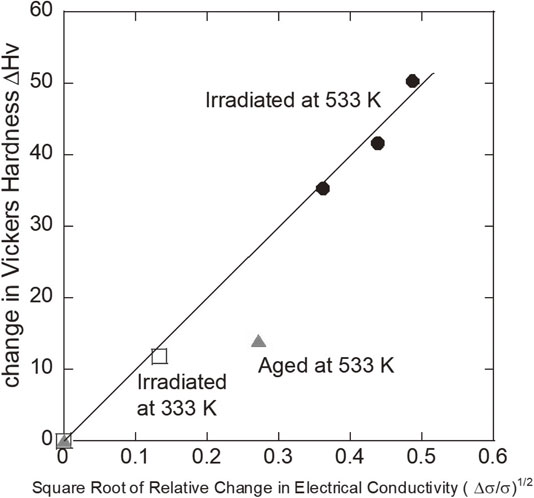
Correlation between ΔHv and Δσ/σ.
The present experimental result demonstrates that the energetic electron irradiation can improve the hardness and electrical conductivity of Cu–4.2 at% Ti alloy at a lower temperature of 533 K than conventional isothermal aging temperatures of around 723 K. The present result also suggests the possibility of the use of electron irradiation to industrial applications for the modification of Cu–Ti alloys as electronic devices.
Finally, we briefly mention the dependence of hardness and electrical conductivity on sample temperature during the electron irradiation. By analyzing the temperature dependence of hardness and conductivity changes, we could discuss more quantitatively the radiation-enhanced diffusion process such as the activation energy of diffusion. Up to the present, however, the experimental data have been obtained only for two temperatures. To discuss the temperature dependence of the phenomenon, experimental data for several other temperatures will be needed.
Cu–4.2 at% Ti alloy was irradiated with 1.5 MeV electrons at 333 K or 533 K, and the irradiation-induced changes in Vickers hardness and electrical conductivity were investigated. Their changes by the irradiation are due to the precipitation of solute Ti atoms through the radiation enhanced diffusion. The increase in hardness by the electron irradiation is much larger than only by the isothermal-aging at the same temperature and the same processing time. The increase in hardness, ΔHv, is proportional to the square root of the relative change in electrical conductivity, $\sqrt{\Delta \sigma /\sigma } $. The present result suggests that energetic electron irradiation can industrially be applied to the modification of mechanical and electrical properties of Cu–Ti alloys as electrical components.
The authors thank the technical staff at QST-Takasaki electron accelerator facility for their help. Two of the authors (A. Iwase and F. Hori) were supported by JSPS-KAKENHI Grant Number 20K12482.