2023 Volume 64 Issue 9 Pages 2077-2081
2023 Volume 64 Issue 9 Pages 2077-2081
A novel oxide YCu3Rh4O12 has been obtained using high-pressure and high-temperature conditions of 12 GPa and 1573 K. Electron diffraction and synchrotron X-ray powder diffraction data demonstrates that YCu3Rh4O12 crystallizes in a cubic AA′3B4O12-type quadruple perovskite structure. The valence state is estimated to be Y3+Cu3+3Rh3+4O12 by X-ray absorption spectroscopy. The electric resistivity and magnetization data prove that YCu3Rh4O12 is a diamagnetic insulator, which is expected from the electron configurations of Cu3+ (3d8, low spin, S = 0) and Rh3+ (4d6, low spin, S = 0) ions. The first-principle calculation displays the insulating band structure for YCu3Rh4O12. The valence state transition from Ca2+Cu2.8+3Rh3.4+4O12 to Y3+Cu3+3Rh3+4O12 indicates that the doped electrons by the substitution of Y3+ for Ca2+ are not simply injected to Cu and/or Rh ions, realizing unusual charge redistributions consisting of the simultaneous Cu oxidation (Cu2.8+ → Cu3+) and Rh reduction (Rh3.4+ → Rh3+).
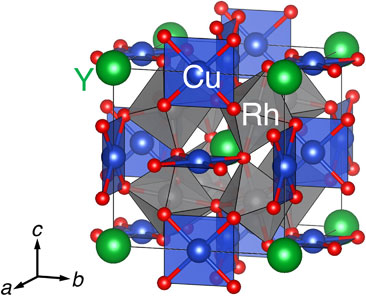
Quadruple perovskite oxides AA′3B4O12, a derivative of perovskite-related structures, have been extensively investigated in recent years.1–3) The properties of the quadruple perovskite oxides widely range between large magnetoresistance,4) ferrimagnetic half-metallicity,5) intersite charge-transfer,6,7) negative thermal expansion,8–10) thermal storage,11) multiferroics,12,13) metamagnetism,14) and electrocatalysis.15–18) Most of the reported properties are derived from the multiple transition metal ions at distinct crystallographic sites of pseudosquare-planar A′-site and octahedral B-site. Since the charge distributions in A′- and B-sites are not unambiguously determined,19–21) various electromagnetic properties are expected even in the nominally isoelectronic compounds: e.g., ferrimagnetism for YCu3Fe4O12 but antiferromagnetism for LaCu3Fe4O12.6,22)
The Rh-containing quadruple perovskite CaCu3Rh4O12 possesses a mixed valence state of Ca2+Cu2.8+3Rh3.4+4O12,23) which is located at intermediate between Ca2+Cu3+3Co3.25+4O12 and Ca2+Cu2+3Ir4+4O12 along the group 9 element column. In the Co-containing quadruple perovskite ACu3Co4O12 series consisting of the divalent, trivalent, and tetravalent A-site ions (Ca2+, Y3+, and Ce4+), the valence states sequentially change from Ca2+Cu3+3Co3.25+4O12 to Y3+Cu3+3Co3+4O12 to Ce4+Cu2.67+3Co3+4O12.19) The Ir-containing ACu3Ir4O12 series exhibits a simple valence state transition between just two examples of Ca2+Cu2+3Ir4+4O12 and La3+Cu2+3Ir3.75+4O12.24,25) ACu3Rh4O12 series has not been well investigated, although Ca2+Cu2.8+3Rh3.4+4O12 is expected to undergo intriguing valence state transition by hole- or electron-doping.
In this paper, we investigate the valence states and physical properties of the novel Rh-containing quadruple perovskite YCu3Rh4O12. YCu3Rh4O12 was successfully synthesized under high-pressure and high-temperature conditions of 12 GPa and 1573 K, crystallizing in a cubic quadruple perovskite structure. X-ray absorption spectroscopy revealed the valence state of Y3+Cu3+3Rh3+4O12, where simultaneous valence changes in Cu (Cu2.8+ → Cu3+) and Rh (Rh3.4+ → Rh3+) from Ca2+Cu2.8+3Rh3.4+4O12 were induced. YCu3Rh4O12 was a diamagnetic insulator, which can be interpreted by the electron configurations of nonmagnetic Cu3+ (3d8, low spin, S = 0) and Rh3+ (4d6, low spin, S = 0) ions. The insulating band structure was corroborated by the first-principle calculation.
Y2O3 (99.9%), CuO (99.5%), and Rh2O3 (99.9%) at a molar ratio of 1:6:4 were mixed using a mortar to obtain the nominal composition of YCu3Rh4O12−δ (δ = 1.5). An oxidizing agent KClO4 was added to the mixture to compensate for the oxygen deficiency, preparing the starting mixture. The starting mixture was pelletized, put into an aluminum tube, and covered with Pt film to avoid a reaction between the sample and Pt. The capsule was placed in a (Mg, Co)O pressure-transmitting medium with an octahedral shape to compose a high-pressure cell. The high-pressure cell was surrounded by eight WC anvils. The high-pressure cell was compressed up to 12 GPa using a Walker-type high-pressure apparatus in a few hours. After the compression, the sample was heated to 1573 K for 15 min and held at this temperature for 45 min. The pressure was released in several hours after the heat treatment. The sample was separated by grinding the aluminum tube. A KCl byproduct was removed by washing the as-prepared sample with distilled water several times.
Synchrotron X-ray powder diffraction (SXRD) pattern at room temperature was collected at the BL02B2 beamline of SPring-8.26) The powder sample was filled into a Lindemann glass capillary with an inner diameter of 0.2 mm. The wavelength was determined to be 0.42034 Å using the NIST SRM 672b CeO2 reference. Structural parameters were refined by using the RIETAN-FP program,27) and the crystal structure model was illustrated using the VESTA-3 program.28) Electron diffraction (ED) patterns at room temperature were collected using a transmission electron microscope accelerated at 200 kV (JEM-2100F, JEOL Co. Ltd.). X-ray absorption spectra at K-edges of Cu and Rh were collected in the transmission mode at the BL14B2 beamline of SPring-8. Electric resistivity was measured by the standard four-probe method using a Physical Properties Measurement System (PPMS, Quantum Design Inc.). Magnetic susceptibility data were obtained in a temperature range between 5 and 300 K in an external field of 1 kOe on field-cooling mode with a superconducting quantum interference device (SQUID, MPMS3, Quantum Design Inc.). The isothermal magnetization curves were collected at 5 and 300 K in an external magnetic field between −50 and 50 kOe.
The electronic structure of YCu3Rh4O12 was calculated by the plane-wave basis projector augmented wave method as implemented in the VASP code.29–31) The spin-unpolarized calculations were performed with the Perdew-Burke-Ernzerhof (PBE) functional to describe the exchange-correlation interactions.32) The plane-wave cutoff energy was set to 550 eV. The strong on-site Coulombic interactions on the localized d electrons were treated with the GGA+U approach. The Ueff parameters were 4.0 and 3.3 eV for Cu 3d and Rh 4d, respectively.33) Geometry optimization was performed using the 4 × 4 × 4 Monkhorst-Pack grid to evaluate the integrals over the Brillouin zone. The lattice constants and internal coordinates were optimized until the total energy difference and residual forces converged to less than 105 eV and 10−2 eV Å−1, respectively. The density of states (DOS) was calculated using a finer k-point mesh with 12 × 12 × 12.
A single-phase YCu3Rh4O12 sample was obtained. Figure 1(b) shows the SXRD pattern at room temperature and the Rietveld refinement result. The Bragg reflections were indexed by the cubic AA′3B4O12-type quadruple perovskite structure with the $Im\bar{3}$ space group (No. 204). All the ED patterns along [001], $[1\bar{1}0]$, and [111] directions were indexed with the $Im\bar{3}$ space group in Fig. 1(c). The dhkl spacing values were calculated to be d200 ∼ 3.73 Å and d110 ∼ 5.25 Å, estimating the cubic lattice constant of a ∼ 7.4 Å. We adopted a stoichiometric structure model with Y at 2a site, Cu at 6b site, Rh at 8c site, and O at 24g site for the structure refinement. The structure parameters obtained from the final refinement are listed in Table 1. In addition to the small deviation between observed and calculated patterns in the Rietveld refinement result (Fig. 1(b)), the small reliability factors (Rwp = 3.229% and RB = 7.067%) demonstrated that the refinement result is reasonable. The refined lattice constant of a = 7.40774(8) Å for YCu3Rh4O12 was comparable to that for CaCu3Rh4O12 (7.39267 Å).34) The calculated Cu–O bond length (1.937 Å) for YCu3Rh4O12 was larger than that for CaCu3Rh4O12 (1.878 Å), whereas the Rh–O bond length (1.994 Å) for YCu3Rh4O12 was almost identical to that for CaCu3Rh4O12 (1.993 Å). Although the elongated Cu–O bond typically indicates the reduction in Cu valence, the X-ray absorption spectroscopy revealed the Cu oxidation, as discussed below. The reason why the refined structure did not reflect the actual valence states of transition metal ions for YCu3Rh4O12 is unclear at the present stage.
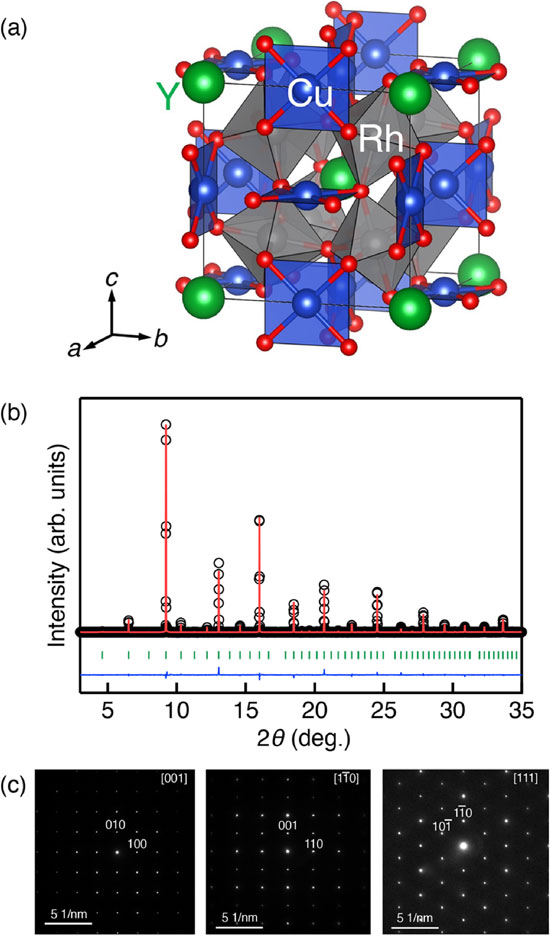
(a) Schematic of the crystal structure of YCu3Rh4O12. (b) Rietveld refinement result of the SXRD pattern of YCu3Rh4O12. The circles (black) and solid lines (red) represent the observed and calculated patterns, respectively. The difference between the observed and calculated patterns is shown at the bottom (blue). The above vertical marks (green) indicate the Bragg reflection positions. (c) Electron diffraction patterns along [001], $[1\bar{1}0]$, and [111] directions for YCu3Rh4O12.

The valences of Cu and Rh ions were examined by X-ray absorption spectroscopy. Figure 2(a) shows the Rh K-edge X-ray absorption spectrum of YCu3Rh4O12. The onset of the absorption of YCu3Rh4O12 (∼23210 eV) was almost identical to that of LaMn3+3Rh3+4O12, suggesting that the Rh ions are close to trivalent for YCu3Rh4O12. However, the slight difference at the higher energy side (∼23230 eV) disturbs the precise estimation of the Rh valence, thus we later discuss the total valence state of YCu3Rh4O12 consistent with the magnetic and electric resistivity data. Figure 2(b) displays the Cu K-edge X-ray absorption spectrum of YCu3Rh4O12. The absorption edge for YCu3Rh4O12 was shifted about 1.5 eV to higher energy compared to the Cu2+ reference of CaCu3Ru4O12. The substantial difference in Cu K-edge absorption indicates that the Cu ions in YCu3Rh4O12 are close to trivalent, but the precise estimation was difficult, as well as Rh valence. Therefore, we discuss the precise valence state based on the physical properties as shown below.

X-ray absorption spectra at (a) Cu and (b) Rh K-edges for YCu3Rh4O12.
Figure 3(a) illustrates the electric resistivity data for YCu3Rh4O12. The measurement was conducted down to 55 K because the raw resistance exceeded the upper limit of the equipment below this temperature. The resistivity gradually increased on cooling, exhibiting the typical behavior of insulators. Figure 3(b) shows the temperature dependence of the magnetic susceptibility for YCu3Rh4O12. A diamagnetism was predominant at room temperature, as observed in the M–H curve at 300 K in Fig. 3(c), whereas a paramagnetic component emerges at low temperature. The magnetic susceptibility in the temperature range between 15 and 300 K was fitted by using a formula: χ(T) = C/(T − θ) + χdia, where C, θ, and χdia represent Curie constant, Weiss temperature, and temperature-independent diamagnetic component, respectively. The parameters obtained from the fitting were C = 3.617(18) × 10−3 emu K mol−1, θ = −10.60(16) K, and χdia = 1.7902(10) × 10−4 emu mol−1. The negative χdia value reflects diamagnetism. The obtained C value was assigned to the extrinsic paramagnetic component originating from the tiny amount of Cu2+ (S = 1/2) localized spins on the grain boundary because the C value corresponds to only 0.3% of the total Cu ions. The above electronic properties of YCu3Rh4O12 are reasonably interpreted by the Cu3+ and Rh3+ valence states, concluding that the valence state is represented as YCu3+3Rh3+4O12.

(a) Temperature dependence of the electric resistivity for YCu3Rh4O12. (b) Temperature dependence of the magnetic susceptibility for YCu3Rh4O12 measured on field cooling in an external field of 1000 Oe. The circles and the curve represent the observed data and the fitting result, respectively. (c) Isothermal magnetization curves at 5 and 300 K for YCu3Rh4O12.
The electron configuration of the pseudosquare-coordinatated Cu3+ ions is a nonmagnetic low-spin state (total spin quantum number, S = 0), as reported in the Cu3+-containing quadruple perovskite oxides such as LaCu3+3Fe3+4O12 and YCu3+3Co3+4O12.35–37) The Rh3+ ions in octahedral coordination possess a low-spin configuration (t2g6eg0) because of the large crystal-field splitting between t2g and eg orbitals in 4d electron system. The nonmagnetic states of constituent transition metal ions result in the absence of spin magnetic moments, as illustrated in Fig. 4(a). Substantial splitting energies between fully occupied and unoccupied orbitals are expected for both Cu3+ and Rh3+, resulting in the insulating band structure with an energy gap. Hence, the diamagnetic insulating properties are expected for YCu3Rh4O12.

(a) Schematic energy diagram of 3d orbitals of Cu3+ in square coordination and 4d orbitals of Rh3+ in octahedral coordination. (b) Calculated total DOS and partial DOS for YCu3Rh4O12.
Figure 4(b) displays the total and partial DOS of YCu3Rh4O12 obtained from the first-principle calculation. The valence band down to −7.5 eV consisted of the Cu, Rh, and O bands, whereas the band originating from Y was located at higher energy (>5.5 eV) above Fermi energy. The lowest conduction band was located at 0.3 eV above Fermi energy, exhibiting a substantial band gap.
The above experimental and theoretical study demonstrated that the novel quadruple perovskite oxide YCu3Rh4O12 is a diamagnetic insulator, which may be a limited example among several ten kinds of the reported quadruple perovskite oxides. We suggest that the valence-state transformation from CaCu3Rh4O12 to YCu3Rh4O12 by electron doping is not straightforward because the Cu valence increased from +2.8 to +3, whereas the Rh valence decreased from +3.4 to +3. This can be divided into the following sequence: (1) electron doping to Rh ions (4Rh3.4+ + e− → 4Rh3.15+) and (2) charge transfer between Cu and Rh (3Cu2.8+ + 4Rh3.15+ → 3Cu3+ + 4Rh3+). The unusual electronic state transition is not reported in the multielement transition metal oxides, suggesting the complex nature of the quadruple perovskite oxide system.
We synthesized the quadruple perovskite oxide YCu3Rh4O12 by using high-pressure and high-temperature conditions of 12 GPa and 1573 K. The X-ray absorption spectroscopy revealed the valence state of Y3+Cu3+3Rh3+4O12 with Cu3+ (3d8, low spin, S = 0) and Rh3+ (4d6, low spin, S = 0) electron configurations, explaining the diamagnetic insulating nature. The first-principle calculation unveiled the insulating band structure. The valence state transition from Ca2+Cu2.8+3Rh3.4+4O12 to Y3+Cu3+3Rh3+4O12 is an unusual example in the complex transition metal oxides.
The synchrotron X-ray experiment was performed at SPring-8 under the approval of the JASRI (proposal numbers 2022A1493 and 2022B1619). This work was supported by JSPS KAKENHI (grant numbers JP20H02825, JP22H04512), the Asahi Glass Foundation, and the Tanikawa Fund Promotion of Thermal Technology.