2024 Volume 65 Issue 5 Pages 524-529
2024 Volume 65 Issue 5 Pages 524-529
In the hydrometallurgical process used for the recycling of platinum group metals (PGMs), a residue containing Cr2O3 and PGMs is generated. In this study, a pyrometallurgical process was applied, in which PGMs from the residue generated in the hydrometallurgical processes were concentrated in a molten Cu phase as a collector metal, and Cr2O3 was separated into a slag phase with SiO2 and CaO as the flux. To reduce the loss of PGMs into the slag, the dissolution of PGMs into the slag must be reduced. Therefore, the distribution ratio of Rh, as a representative PGM, between the liquid SiO2–CaO–Al2O3–CrOx or the liquid SiO2–CaO–CrOx slag and molten Cu were measured at 1773 K under an oxygen partial pressure of $p_{\text{O}_{2}} = 10^{ - 10}$. The experimental results revealed that the distribution of Rh in the slag increased with increasing CrOx concentration. At a constant Cr2O3 concentration in the slag, the solubility of Rh increased with increasing slag basicity, which is defined as B = (mass%CaO)/(mass%SiO2). Furthermore, compared with the distributions of Rh and Pt between the slag system and molten Cu, Rh was more easily lost to the slag, and the dependence of Rh on basicity was greater than that of Pt.

Fig. 2 Relationship between the distribution ratio of PGMs and the concentration of Cr2O3 in the slag (1773 K, $p_{\text{O}_{2}} = 10^{ - 10}$).
Rh is one of the platinum group metals (PGMs) and an important element in a wide range of fields owing to its unique catalytic properties, heat resistance, acid resistance, and chemical stability.1) In particular, Rh is used along with Pt and Pd as a catalyst for automotive exhaust gas purification, accounting for 88% of Rh demand.1) Rh is necessary for the treatment of NOx emissions from gasoline engines.2–4) Therefore, most automakers have sought to minimize their Rh consumption over the years without compromising catalyst performance.2) However, owing to the implementation of real-driving emission (RDE) test regulations in the EU since 2017, which require automobiles to meet strict NOx limits not only in the laboratory but also under a very wide range of real-driving conditions, Rh continues to be the most effective catalyst for reducing NOx emissions. As a result, it will be used in all gasoline aftertreatment systems.2) In addition to its use in automotive catalysts, Rh is in high demand as a catalyst in the chemical industry, high-grade glass crucibles, white-gold protection, thermocouples, and electronic components.3,5) Photocatalysis using Rh is expected to be in high demand in the future.6–9) Therefore, the price of Rh is now significantly dependent on the demand for automobiles in the larger market, as it is now used only for applications in which there are no economic or technological substitutes.2) Shortage of the Rh supply is a potential risk in the future, particularly, because of the possibility of a significant increase in automobile production.2)
Primary supplies of Rh are unevenly distributed among South Africa, Russia, and Zimbabwe, accounting for 85, 7, and 5% of the total supply, respectively. A few concerns are associated with the stable supply of resources.2) The secondary supply of Rh is reported to be 48% of the primary supply, and a maximum fraction of it is recycled from automobiles.2) In addition, a significant amount of PGMs is recycled among several closed-companies in a business alliance and are not reported to retain the ownership of the metal with the industrial consumer, who returns PGM-bearing materials to specialized companies for refining and reuse in the same application.2) Therefore, the importance of recycling PGMs, including Rh, is evident. Currently, a method for dissolving PGMs in hydrometallurgical processes are used to recycle PGMs. In the processes, Cr2O3 and PGMs are generated as residues, thereby resulting in a loss of PGMs present in these residues. To selectively recover PGMs from the residues, an additional pyrometallurgical process of residue treatment was considered in this study. PGMs were concentrated in the metal phase with liquid Cu as the collector and Cr was separated into a slag phase. The slag and metal were separated in a furnace; however, the chemical dissolution of Rh in the slag caused loss of Rh. Therefore, the distribution of Rh between slag and molten Cu was examined to investigate the operating conditions of the pyrometallurgical process for removal of Cr with minimal loss of PGMs. Slag used in this study was the SiO2–CaO–CrOx system because of its low flux cost. Based on thermodynamic data, $p_{\text{O}_{2}}$ was set to 10−10, which oxidized Cr; however, barely oxidized the PGMs. $p_{\text{O}_{2}}$ denotes a dimensionless number obtained by dividing the partial pressure of oxygen by the pressure of 1.01325 × 105 Pa.
Several studies have been conducted on the dissolution of Rh in slags. Wiraseranee et al. and Morita et al. investigated the solubility of Rh in the Na2O–SiO2 slag at 1473–1573 K, the CaO–SiO2 slag at 1873 K and the Na2O–SiO2–Al2O3 slag at 1473 K.10–12) Borisov and Danyushevsky measured the solubility of Rh in the SiO2–Al2O3–MgO–CaO slag at 1723 K.13) Ertel et al. determined the solubility of Rh in the SiO2–CaO–MgO–Al2O3 slag at 1573 K.14) Dable et al. investigated the solubility of Rh in the CaO–Al2O3–SiO2 slag at 1700 K,15) Nishijima and Yamaguchi determined the distribution behavior of Rh between molten Cu and the the Al2O3–CaO–SiO2–Cu2O slag at 1723 K.16) Avarmaa et al. measured the distribution behavior of Rh between molten Ag and the Na2O–SiO2 slag at 1423 K.17) Murata and Yamaguchi investigated the distribution behavior of Rh between molten Cu and the Cu2O–SiO2 slag or the Cu2O–CaO slag, or molten Pb and the PbO–SiO2 slag or the PbO–CaO slag at 1523 K.18) In most of these studies, Rh was reported to dissolve into the slags at high concentrations of basic oxides and high oxygen partial pressures. We reported the distribution of Pt between the SiO2–CaO–CrOx slag and molten Cu.19) In the previous study, solubility of Pt in the slag increased with increasing Cr2O3 concentrations. However, to the best of our knowledge, no reports are available on the solubility of Rh in slag systems containing CrOx. Therefore, in this study, the distribution behavior of Rh was investigated in Al2O3 or SiO2 crucible by equilibrating the SiO2–CaO–Al2O3–CrOx and the SiO2–CaO–CrOx slag systems at 1773 K with Rh-containing Cu alloys at $p_{\text{O}_{2}} = 10^{ - 10}$ by changing the composition of the slags.
Two types of crucibles made of SiO2 (no purity statement) and Al2O3 (>99.6 mass% purity) were used for the measurements. Rh (0.2 g, >99.95 mass% purity) and Cu (1.8 g, >99.5 mass% purity) wires were placed in the SiO2 or Al2O3 crucible. Additionally, 8 g of a mixture of SiO2 (>99.5 mass% purity), CaO calcined from CaCO3 (>99.9 mass% purity), and Cr2O3 (>99.9 mass% purity) was added to the crucible to cover the Pt and Cu metals. In case of the Al2O3 crucible, excess Al2O3 powder (>99 mass% purity) was added to the crucible to prevent melting of the crucible. Thereafter, the sample was inserted into a reaction tube, maintained at 1773 K (±3 K) in a furnace, and heated using a SiC heating element. Oxygen partial pressure was maintained at $p_{\text{O}_{2}} = 10^{ - 10}$ using a mixture of CO and CO2 gas at a flow rate of 150 mL/min for 20 h. After attaining equilibrium, the samples were quenched with water. The slag composition was determined by chemical analysis similar previous study.19)
Subsequently, the slag and alloy phases in the crucible were carefully separated. Two types of dissolution methods (the alkali fusion method using Li2B4O7 and aqua regia) were employed to achieve total dissolution of the sample for complete and accurate characterization using instrumental methods. Si, Ca, Cr, and Al in the slag were dissolved through the alkali fusion method using Li2B4O7 as the flux. Cu and Rh in the slag, which have low concentrations and cannot be analyzed using the alkali fusion method, were dissolved in aqua regia. Furthermore, Cu, Rh, and Cr in the metal phase were dissolved in aqua regia. Details on the analytical method used in this study have been reported in a previous study, in which Pt was the metal of interest instead of Rh.19) Rh103 was monitored using inductively coupled plasma mass spectrometry (ICP-MS; Agilent Technologies, 7700x). Cr52 was monitored using ICP-MS for samples in which the Cr-concentration in the alloy phase was below the lower limit of quantification by performing inductively coupled plasma optical emission spectrometry (ICP-OES). Additionally, Rh wavelengths (343.488 nm) were used to determine concentration by employing ICP-OES (Agilent Technologies, 5100).
Table 1 lists the results of the analysis of the Cu–Rh alloy and slag phases. In this study, ( ) and [ ] represent the values of X in the slag and alloy phases, respectively. The oxidation state of Cr in the slag phase was not measured in the present work. Based on previous reports similar to the present experimental conditions, the oxidation state of Cr in the present slag was assumed to be trivalent.20–23)
3.1 Solubility of Al2O3 in the SiO2–CaO–Cr2O3 slagSolubility of Al2O3 in the SiO2–CaO–Cr2O3 slag as a function of basicity B, as expressed in eq. (1), is shown in Fig. 1.
| \begin{equation} B = \frac{(\text{mass%CaO})}{(\text{mass%SiO$_{2}$})} \end{equation} | (1) |
The solubility of Al2O3 in the SiO2–CaO–Cr2O3 slag ranged from 23.9 mass% to 33.6 mass%. The solubility of Al2O3 increases with increasing basicity B. This trend is consistent with the fact that the liquid phase line of Al2O3 saturation in the SiO2–CaO–Al2O3 slag approaches high Al2O3 concentration with increasing basicity (0.51 ≤ B ≤ 0.96).19,24) When the basicity is low owing to anorthosite, the solubility of Al2O3 is low.

Solubility of Al2O3 in the SiO2–CaO–CrOx system (1773 K, $p_{\text{O}_{2}} = 10^{ - 10}$).
In this study, the distribution ratios of Cu, Rh, and Cr between the slag and liquid Cu are expressed by eq. (2) as follows:
| \begin{equation} L_{\text{X}} = \frac{(\text{mass%X in Slag})}{[\text{mass%X in Alloy}]},\quad \text{X: Rh, Pt, or Cr} \end{equation} | (2) |
Figure 2 shows the relationship between the distribution ratios of Rh and Pt and the concentration of Cr2O3 in the slag. Figure 3 shows the relationship between the distribution ratios of Rh and Pt and the basicity of the slag under Al2O3 saturation. The distribution ratio of Rh between the SiO2–CaO–Al2O3–CrOx or the SiO2–CaO–CrOx slag and liquid Cu is 5.6 × 10−6–7.6 × 10−4. The distribution ratio of Rh is higher than that of Pt, indicating that Rh is lost to the slag. Figure 2 shows that the distribution ratio of Rh and Pt between the SiO2–CaO–Al2O3–CrOx or the SiO2–CaO–CrOx slag and liquid Cu increases with increasing CrOx concentration in the slags. Figure 3 shows that the distribution ratio of Pt between the SiO2–CaO–Al2O3–CrOx slag and liquid Cu is constant regardless of the basicity of the slag, whereas the distribution ratio of Rh increases with increasing basicity of the slag. Nakamura and Sano reported that the solubility of Pt in the slag increases with increasing concentration of basic oxide in the slag under high oxygen partial pressure unlike the trend observed in present study.25) In addition, the report suggested a smaller effect of CaO on increasing the solubility of Pt than those of BaO and Na2O.25) In particular, in slag systems with narrow liquid-phase regions, such as the SiO2–CaO–Cr2O3 system, the solubility of Pt is not expected to change significantly even if the basicity is increased. Therefore, the distribution ratio of Pt is constant regardless of the basicity level, which is nearly consistent with the trend reported by Nakamura and Sano.25) The distribution ratio of Rh increases with increasing basicity of the slag, which is consistent with the trend reported by Wiraseranee et al.10,11)

Relationship between the distribution ratio of PGMs and the concentration of Cr2O3 in the slag (1773 K, $p_{\text{O}_{2}} = 10^{ - 10}$).
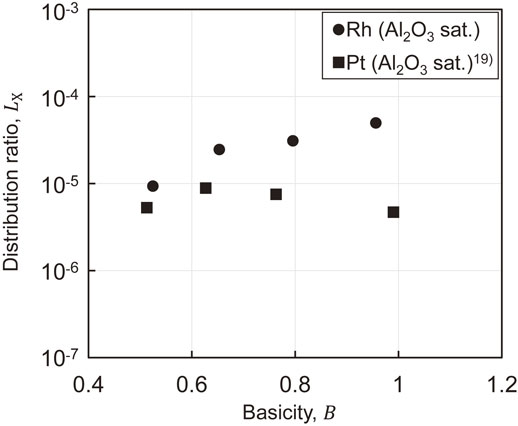
Relationship between the distribution ratio of PGMs and the basicity of the slag under Al2O3 sat. (1773 K, $p_{\text{O}_{2}} = 10^{ - 10}$).
Figure 4 shows the relationship between the distribution ratio of Cu and the concentration of Cr2O3 in the slag. Figure 5 depicts the relationship between the distribution ratio of Cu and basicity of the slag under Al2O3 saturation. Figure 4 displays that the distribution ratio of Cu was constant regardless of the CrOx concentration in the SiO2–CaO–Al2O3–CrOx slag. However, the distribution ratio of Cu decreases with increasing with increasing CrOx concentration in the SiO2–CaO–CrOx slag. Figure 5 shows that the distribution ratio of Cu decreases with increasing basicity of the slag.

Relationship between the distribution ratio of Cu in the slag and concentration of Cr2O3 in the slag (1773 K, $p_{\text{O}_{2}} = 10^{ - 10}$).

Relationship between the distribution ratio of Cu in the slag and the basicity of the slag under Al2O3 sat. (1773 K, $p_{\text{O}_{2}} = 10^{ - 10}$).
Figure 6 presents the relationship between the distribution ratio of Cr and concentration of Cr2O3 in the slag. Figure 7 shows the relationship between the distribution ratio of Cr and basicity of the slag under Al2O3 saturation. Figures 6 and 7 show that the distribution ratios of Cr between the the SiO2–CaO–Al2O3–CrOx slag and the Cu–Rh alloy is larger than that between the SiO2–CaO–Al2O3–CrOx slag and the Cu–Pt alloy. Furthermore, the distribution ratio of Cr is 9.8 × 10–1.2 × 104, which is sufficiently large compared to that of Rh, indicating that Cr is removed into the slag and separated from Rh.

Relationship between the distribution ratio of Cr and the concentration of Cr2O3 in the slag (1773 K, $p_{\text{O}_{2}} = 10^{ - 10}$).

Relationship between the distribution ratio of Cr and the basicity of the slag under Al2O3 sat. (1773 K, $p_{\text{O}_{2}} = 10^{ - 10}$).
Rh is believed to exist as Rh cations, or Rh ions in slag. The equilibrium conditions for the slag and alloy phases in which Rh exists in different oxidation states are given by eqs. (3)–(4). The solubility of Rh cations and ions are dependent on the partial pressure of oxygen.10)
| \begin{equation} [\text{Rh}] + \text{$m$O$_{2}$(g)} + \text{$n$(O$^{2-}$)} = (\text{RhO$_{(2m+n)}^{2n-}$}) \end{equation} | (3) |
| \begin{equation} [\text{Rh}] + \text{$m$O$_{2}$(g)} = (\text{Rh$^{4m+}$}) + \text{2$m$(O$^{2-}$)} \end{equation} | (4) |
In this study, both Rh cations and ions were considered as oxides without distinction. The activity coefficient of RhO1.5 in each slag composition was determined using eq. (5) for the oxidation reaction of Rh reported in the thermodynamic database of SGPS in FactSage,26) assuming that the oxidation state of Rh was RhO1.5, as reported by Wiraseranee et al.10)
| \begin{equation} \text{Rh(l)} + \frac{3}{4}\text{O$_{2}$(g)} = \text{RhO$_{1.5}$(s)}:\Delta G_{1773\,\text{K}}^{\circ} = 35623.5\ (\text{J}), \end{equation} | (5) |
where $\Delta G_{1773\,\text{K}}^{ \circ }$ denotes the standard Gibbs free energy change for the reaction at 1773 K. The equilibrium constant, K, for the reactions was determined from the activity, a, and the oxygen partial pressure, $p_{\text{O}_{2}}$, using eq. (6) as follows:
| \begin{equation} K = \frac{a_{\text{RhO${_{1.5}}$}}}{a_{\text{Rh}} \cdot p_{\text{O${_{2}}$}}^{\frac{3}{4}}}. \end{equation} | (6) |
The thermodynamic data for the dissolution of RhO1.5(s) into RhO1.5(l) were not available; therefore, the activity coefficient of the RhO1.5-solid standard $(\gamma_{\text{RhO}_{1.5}})$ was calculated using eq. (7):
| \begin{equation} (\gamma_{\text{RhO${_{1.5}}$}}) = \frac{[\gamma_{\text{Rh}}] \cdot p_{\text{O${_{2}}$}}^{\frac{3}{4}} \cdot (n_{\text{T}}) \cdot K}{[n_{\text{T}}] \cdot L_{\text{Rh}}}, \end{equation} | (7) |
where [γRh] and $(\gamma_{\text{RhO}_{1.5}})$ denote the activity coefficients of Rh in the alloy and RhO1.5(s) in the slag, respectively; and [nT] and (nT) are the total amounts of substances per 100 g in the alloy and slag phases, respectively. The concentration of Cr in the alloy phase was lower than those of Cu and Rh. The interaction of Cr with Cu and Rh is negligible; therefore, the alloy can be considered the Cu–Rh binary alloy. The activity coefficient of Rh, [γRh] in the alloy phase at 1773 K was determined from the results of the quantitative analysis of each element in the Cu–Rh alloy using SGnobl of the FactSage database.26) The equilibrium constant K was determined from the Gibbs free energy change ΔG° expressed in eq. (5). The values of [nT], (nT), LRh [γRh], and $(\gamma_{\text{RhO}_{1.5}})$ are listed in Table 2. Figure 8 shows the iso-activity coefficient curves of RhO1.5(s) obtained using the thermodynamic data. The curves were drawn on a phase-diagram projected onto the SiO2–CaO–CrOx system excluding Al2O3. The activity coefficient of RhO1.5(s), $(\gamma_{\text{RhO}_{1.5}})$ increases as the concentration of Cr2O3 in the slag decreases. The activity coefficient of RhO1.5(s) decreases with increasing slag basicity. When the slag composition has a low Cr2O3 concentration and basicity, the activity coefficient of RhO1.5(s) increases. The change in the concentration of Cr2O3 in the slag exhibits a more significant effect on the activity coefficient of RhO1.5(s) than that on the basicity of the slag. Therefore, a high Cr2O3 concentration in the slag allows a large amount of Cr2O3 to be processed; however, this increases the loss of Rh into the slag. Furthermore, the iso-activity coefficient curves for RhO1.5(s) indicates that RhO1.5 behaves similar to a weak acidic oxide in the SiO2–CaO–CrOx system.
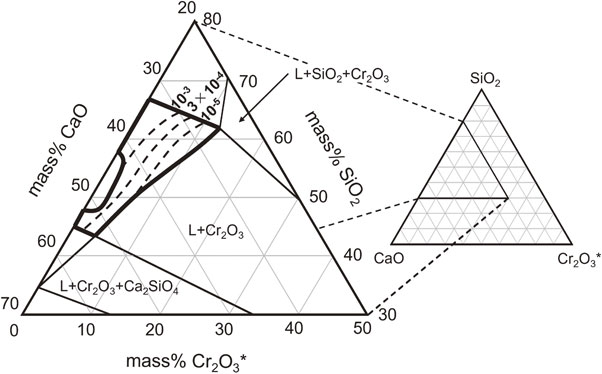
Iso-activity coefficient curves of RhO1.5(s) in the SiO2–CaO–CrOx system (1773 K, $p_{\text{O}_{2}} = 10^{ - 10}$).
The distribution of Rh between the SiO2–CaO–Al2O3–CrOx or the SiO2–CaO–CrOx slag systems and molten Cu was investigated. Rh and Cr were concentrated in the alloy and slag phases, respectively. The solubility of Rh in the slag increased with increasing Cr2O3 concentration. At a constant Cr2O3 concentration in the slag, the solubility of Rh increased with increasing basicity. Furthermore, compared to the distribution of Pt between the slag system and molten Cu, Rh was more easily lost into the slag, and the dependence of Rh on basicity was higher than that of Pt.
The authors would like to thank Tanaka Kikinzoku Kogyo K.K. for their helpful suggestions and discussions, the Environmental Safety Center of Waseda University for the use of ICP-OES and ICP-MS.