2022 Volume 63 Issue 1 Pages 12-25
2022 Volume 63 Issue 1 Pages 12-25
We describe a new species of Gerhardtia from Japan based on basidiomata morphology, live culture characteristics, and molecular phylogenetic analyses. Gerhardtia venosolamellata is found on broad-leaf litter, and is characterized by tricholomatoid to marasmioid basidiomata, an off-white to pale salmon-pink pileus surface with faint marginal striae, subdistant lamellae with lateral veins, a tomentose to strigose stipe base with hyphal strands generating arthroconidia measuring 4-7 × 2-3 µm, cyanophilic, elongate-ellipsoid to cylindrical, slightly verrucose or undulate basidiospores measuring 4.5-6 × 2.5-3 µm, and cyanophilic basidia measuring 25-35 × 5-6 µm and containing siderophilous granules. Phylogenetic analyses based on the internal transcribed spacer and large subunit regions of the fungal nrDNA indicates that G. venosolamellata is related to G. sinensis and G. highlandensis, but differs from the former with respect to basidiomata color, basidiospore shape, and habitat. An isotype specimen of G. highlandensis exhibited relatively close lamellae without veins, and slightly larger basidiospores (4.5-6.5 × 2.5-3 µm). Cultured mycelia of G. venosolamellata produced arthroconidia measuring 4.5-8.5 × 2.5-3 µm with both schizolytic and rhexolytic secession on MA and PDA media, and chlamydospores occasionally covered with crystals on MA and MYG media.
The genus Gerhardtia is a saprotrophic fungal taxon in the family Lyophyllaceae, in the order Agaricales and the phylum Basidiomycota. Bon (1994) established the genus Gerhardtia based on Lyophyllum subgenus Lyophyllopsis (Gerhardt, 1982) and designated G. incarnatobrunnea (Ew. Gerhardt) Bon as the type species. The genus was initially characterized by the presence of verrucose basidiospores, basidia with siderophilous granules, a pileipellis with cutis, trichodermium, or hymeniderm organization, and an absence of clamp connections (Bon, 1994). Following the discovery of G. pseudosaponacea J.A. Cooper & P. Leonard, which has smooth basidiospores (Cooper, 2014), Vizzini, Consiglio, Setti, and Ercole (2015) partly amended the genus concept as having basidiospores that are smooth or verrucose. Based on phylogenetic relationships, Vizzini et al. (2015) also clarified that G. incarnatobrunnea is synonymous with G. borealis (Fr.) Contu & A. Ortega, which was described based on specimens from Sweden. Thus, G. borealis is currently recognized as the type species of the genus. Vizzini, Angelini, and Ercole (2017) subsequently observed that the basidiospores of the genus tend to be irregular and undulate to nodulose, rather than verrucose.
According to the Index Fungorum fungal database (http://www.indexfungorum.org/names/names.asp, 01 Feb 2021) and recent publications (Li, Li, Wang, Deng, & Song, 2017; Vizzini et al., 2017; Mu, Huang, Huang, Yang, & Tang, 2021), eleven species are currently recognized in Gerhardtia: G. borealis (= G. incarnatobrunnea); G. cibaria (Singer) Matheny, Sánchez-García & T.J. Baroni [= Pleurocollybia cibaria Singer]; G. citrinolobata Angelini & Vizzini; G. foliicola (Har. Takah.) N. Endo, S. Ushijima, Nagas., Sotome, Nakagiri, & N. Maek. (= Tricholoma foliicola Har. Takah.); G. highlandensis (Hesler & A.H. Sm.) Consiglio & Contu (= Clitocybe highlandensis Hesler & A.H. Sm.); G. leucopaxilloides (H.E. Bigelow & A.H. Sm.) Consiglio & Contu (= Clitocybe leucopaxilloides H.E. Bigelow & A.H. Sm.); G. piperata (A.H. Sm.) Bon (= Clitocybe piperata A.H. Sm.); G. pseudosaponacea; G. sinensis T.H. Li, T. Li, & W.Q. Deng; G. suburens (Clémençon) Consiglio & Contu (= Lyophyllum suburens Clémençon); and G. yunnanensis M. Mu & L.P. Tang. Gerhardtia marasmioides (Singer) Consiglio & Contu [= Clitopilus marasmioides (Singer) Noordel. & Co-David] and G. pudica (Bon & Contu) Vizzini, Consiglio & Setti [= Calocybella pudica (Bon & Contu) Vizzini, Consiglio & Setti] are currently excluded from the genus (Co-David, Langeveld, & Noordeloos, 2009; Vizzini et al., 2015). Of the eleven species with valid names, the nrDNA sequences and molecular phylogenetic positions of eight species (G. borealis, G. cibaria, G. citrinolobata, G. foliicola, G. highlandensis, G. pseudosaponacea, G. sinensis, and G. yunnanensis) have been elucidated (Vizzini et al., 2015, 2017; Li et al., 2017; Matheny et al., 2017; Endo et al., 2019; Mu et al., 2021). The monophyly of the genus is strongly supported by these studies.
In a previous study, we reevaluated the taxonomy of T. foliicola and transferred the species to Gerhardtia; this represented the first documented occurrence of Gerhardtia in Japan (Endo et al., 2019). In addition to the taxonomic treatment, we described the live culture characteristics of G. foliicola: the species produces numerous thallic conidia (arthroconidia) with both schizolytic and rhexolytic secession modes. According to Clémençon (1968) and Nagasawa and Arita (1988), arthroconidia produced by G. suburens and G. leucopaxilloides also exhibit both schizolytic and rhexolytic secession, whereas those produced by species in the genus Hypsizygus (Lyophyllaceae) exhibit only schizolytic secession. Furthermore, we found that live cultures of G. foliicola produced cystidia covered with granules, which have not been reported in other Lyophyllaceae species. Based on these observations, we hypothesized that live culture characteristics might be critical for distinguishing Gerhardtia from other Lyophyllaceae genera.
Our ongoing field surveys in Japan recently yielded a distinct Gerhardtia species (“Gerhardtia cf. highlandensis”) from Tottori and Yamanashi prefectures that is morphologically similar to G. highlandensis and G. sinensis. Here, we examine the morphology and phylogeny of these specimens, and describe a novel species of Gerhardtia. In addition, we examined live cultures isolated from the basidiomata of the novel species for comparison with known conidium-producing Gerhardtia species.
Seven basidiomata specimens of G. cf. highlandensis were sampled from Japan in 2018-2020 (Table 1). An isotype specimen of Clitocybe highlandensis (= G. highlandensis; TENN-F-16020) loaned from the University of Tennessee and included in this study (Table 1). Mycelia were isolated from the inner tissue or basidiospores of three specimens using malt extract agar [MA; 15 g malt extract (Becton, Dickinson and Company, Franklin Lakes, NJ, USA), 15 g agar powder (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan), and 1,000 mL distilled water]. The external morphology of the basidiomata specimens was observed, and specimens were air-dried at 45 °C for 1-2 d and deposited in the Tottori University Mycological Herbarium (TUMH) at the Fungus/Mushroom Resource and Research Center (FMRC). Several specimens were also deposited at the Tottori Mycological Institute (TMI). Three established strains (TUFC 101584, TUFC 101840, and TUFC 101893), isolated from TUMH 63383, TUMH 63956, and TUMH 64253, were deposited at the FMRC as Tottori University Fungal Culture (TUFC) strains (Table 1). Two strains of G. foliicola (TUFC 100720 and TUFC 101392) stored at FMRC were also included for comparison (Table 1).
| Scientific name | Herbarium number of the specimen | Collection date | Locality | Habitat2 | Strain number of the culture3 | Isolation source of the culture3 |
| Gerhardtia cf. highlandensis | TUMH 63383 | 07 Sep 2018 | Narusawa Vil., Yamanashi Pref., Japan | Litter of BLT | TUFC 101584 | Tissue |
| TUMH 63956 | 02 Aug 2020 | Tottori City, Tottori Pref., Japan | Litter of BLT | TUFC 101840 | Basidiospores | |
| TUMH 64251 | 07 Sep 2018 | Narusawa Vil., Yamanashi Pref., Japan | Litter of BLT | N | N | |
| TUMH 64252 | 28 Sep 2020 | Tottori City, Tottori Pref., Japan | Litter of BLT | N | N | |
| TUMH 64253 | 03 Oct 2020 | Tottori City, Tottori Pref., Japan | Litter of BLT | TUFC 101893 | Tissue | |
| TUMH 64254 | 09 Oct 2020 | Tottori City, Tottori Pref., Japan | Litter of BLT | N | N | |
| TUMH 64255 | 23 Oct 2020 | Tottori City, Tottori Pref., Japan | Litter of BLT | N | N | |
| G. foliicola | TUMH 60927 | 07 Nov 2013 | Tottori City, Tottori Pref., Japan | Humus of BLT | TUFC 100780 | Tissue |
| TUMH 63019 | 01 Sep 2017 | Sendai City, Miyagi Pref., Japan | Humus of pine | TUFC 101392 | Tissue | |
| G. highlandensis1 | TENN-F-016020 (isotype) | 10 Sep 1937 | North Carolina, USA | Under pine | N | N |
1 Specimen information of Gerhardtia highlandensis was described with the permission from the University of Tennesee (TENN).
2 BLT: Broad-leaf tree
3 N: No culture strain
We photographed both fresh and dried basidiomata, and measured pileus diameter, lamella width, the length and width of the stipe, and the length of the striae on the pileus margin. Color names shown in double quotation marks (i.e., “oac”) indicate colors from the Online Auction Color Chart (Kramer, 2004). To characterize the micromorphology of the basidiomata, we measured the length range, width, and length:width ratio (Q value) of 30 basidiospores from each specimen using a differential interference contrast (DIC) microscope (Eclipse 80i; Nikon, Tokyo, Japan). Spore size ranges are shown as “(a)b-c(d)”, where (a) indicates the 5th percentile, (b) indicates the average - standard deviation (SD), (c) indicates the average + SD, and (d) indicates the 95th percentile. The cyanophilic and siderophilic reactions of basidia were observed under both DIC and light microscopes (Eclipse 80i; Nikon) after staining with cotton blue dissolved in lactic acid and acetocarmine with Fe3+ (Singer, 1986). To characterize arthroconidia on hyphal strands at the stipe base, we measured the length, width, and Q value of 30 conidia from each specimen under a DIC microscope (Eclipse 80i). Mean Q (Qm) values were calculated for both basidiospores and conidia. In addition, we observed the basidioles, hyphal system, pileipellis, stipitipellis, and hymenophoral trama. The surface structure of basidiospores was observed with a scanning electron microscope (SEM), using a small section of the lamellae. Procedures for fixing, dehydrating, and sputtering followed Endo et al. (2019).
2.3 Morphological observation of culturesWe sampled the mycelia of G. cf. highlandensis and G. foliicola precultured on MA plates using an autoclaved plastic straw (3 mm diam × 105 mm long; Shibase Kougyou, Okayama, Japan) and transferred them to the center of plates on either fresh MA, malt extract-yeast extract-glucose agar [MYG; 10 g malt extract (Becton, Dickinson and Company), 1 g yeast extract (Becton, Dickinson and Company), 10 g glucose (FUJIFILM Wako Pure Chemical Corporation), 15 g agar powder (FUJIFILM Wako Pure Chemical Corporation), and 1,000 mL distilled water], or potato-dextrose agar [PDA; 39 g Nissui PDA (Nissui Pharmaceutical Co. Ltd., Tokyo, Japan)]. Fungus-inoculated agar plates were incubated for 1 mo in an incubator (MIR-254-PJ; Panasonic Healthcare, Tokyo, Japan) at 25 °C in the dark. The growth rate of each isolate was evaluated by measuring colony diameter in two perpendicular directions, excluding the area of the inoculum. Five replicates were prepared from each isolate. The external morphology of the live cultures was photographed and observed under a dissecting microscope (SMZ-1500; Nikon). To characterize the micromorphology of live cultures, we measured the mean and range of the length, width, and Q values of 30 arthroconidia from each culture in each medium under a DIC microscope, and calculated Qm. Chlamydospores and hyphae were also observed. The surface structure of conidia was observed using an SEM, following the same procedure described above.
2.4 Molecular phylogenetic analysesProcedures for DNA extraction from basidiomata specimens and cultured mycelia, polymerase chain reaction amplification, and cycle sequencing followed Endo et al. (2019). The internal transcribed spacer (ITS) and large subunit (LSU) regions of the fungal nrDNA were amplified and sequenced using the primers ITS1-F/LB-W and CTB6/LR5F (Gardes & Bruns, 1993; Tedersoo et al., 2008). The cycle sequencing reaction products (10 μL) were purified with ethanol and electrophoresed using the ABI Prism 3100 Genetic Analyzer (Thermo Fisher Scientific Inc., Waltham, MA, USA). The waveform of the chromatogram was verified using 4Peaks software (https://nucleobytes.com/4peaks/) and the nucleotide sequences obtained for each strand were assembled using ClustalW (Thompson, Higgins, & Gibson, 1994). The complementarity between strands was confirmed. Sequences were deposited in the DNA Data Bank of Japan (DDBJ; Table 2). Molecular phylogenetic analyses were performed using MEGA X software (Kumar, Stecher, Li, Knyaz, & Tamura, 2018). Both the ITS and LSU datasets, with sequence data obtained from GenBank shown in Table 2, were aligned using MUSCLE (Edgar, 2004) and manually refined using Seaview software (http://pbil.univ-lyon1.fr/software/seaview3). The refined alignments (LSU = 659 bp, ITS = 635 bp) were submitted to TreeBase (http://www.treebase.org; accession no. S28517 for LSU, S28518 for ITS). Maximum likelihood (ML) trees were constructed using MEGA X with a general time-reversible + gamma-distributed and invariant site model for both datasets. Model selection was conducted in MEGA X, based on Akaike information criterion values. The gaps/missing data treatment = “Use all sites” was applied. Maximum likelihood bootstrap support (MLBS) values were obtained using nonparametric bootstrapping with 1,000 replicates. The trees were rooted using Calocybella pudica (Bon & Contu) Vizzini, Consiglio & Setti as an outgroup because Calocybella is sister to Gerhardtia (Vizzini et al., 2015, 2017; Endo et al., 2019). Bootstrap support of the maximum parsimony tree (MPBS) was also obtained for both datasets using MEGA X. ML trees were visualized using MEGA X and edited in Microsoft PowerPoint (Microsoft Corp., Redmond, WA, USA) and Adobe Photoshop (Adobe, San Jose, CA, USA).
| Taxon name | Specimen/Isolate number | Accession Nos. | Locality | References | |
| ITS | LSU | ||||
| Calocybella pudica | AMB 15994 | KP858000 | KP858005 | Italy | Vizzini et al. (2015) |
| AMB 15997 | KP858003 | KP858008 | Italy | Vizzini et al. (2015) | |
| Gerhardtia borealis | LIP: PAM01072207 | KP192534 | France | Bellanger et al. (2015)1 | |
| AMB 15993 | KP858004 | KP858009 | Trentino-Alto Adige, Italy | Vizzini et al. (2015) | |
| U.Soderholm 1593 (H) | AM946449 | Finland | Vizzini et al. (2015) | ||
| G. cf. highlandensis | TUMH 63383 | LC637872 | LC637884 | Yamanashi, Japan | This study |
| TUMH 63956 | LC637874 | LC637880 | Tottori, Japan | This study | |
| TUMH 64251 | LC637873 | LC637885 | Yamanashi, Japan | This study | |
| TUMH 64252 | LC637876 | LC637879 | Tottori, Japan | This study | |
| TUMH 64253 | LC637875 | LC637881 | Tottori, Japan | This study | |
| TUMH 64254 | LC637878 | LC637882 | Tottori, Japan | This study | |
| TUMH 64255 | LC637877 | LC637883 | Tottori, Japan | This study | |
| G. cibaria | As. s.n. | KX981985 | Peru | Matheny et al. (2017) | |
| G. citrinolobata | JBSD 126508 (holotype) | KY363576 | KY363578 | Dominican Republic | Vizzini et al. (2017) |
| G. foliicola | TUMH 60927 | LC460455 | Tottori, Japan | Endo et al. (2019) | |
| TUMH 62882 | LC458827 | LC458838 | Tottori, Japan | Endo et al. (2019) | |
| TUMH 63019 | LC458828 | LC458835 | Miyagi, Japan | Endo et al. (2019) | |
| TUMH 63314 | LC458831 | LC458840 | Yamanashi, Japan | Endo et al. (2019) | |
| G. highlandensis | PBM 2806 | GU734744 | EF535275 | Massachusetts, US | Matheny et al. (2017) |
| TENN-F-070609 | KY777394 | Tennessee, US | Unpublished | ||
| TENN-F-016020 | FJ601808 | North Carolina, US | Matheny et al. (2017) | ||
| G. pseudosaponacea | PDD96650 (holotype) | KJ461911 | New Zealand | Cooper (2014) | |
| G. sinensis | GDGM 29981(holotype) | KU563151 | KU563152 | China | Li et al. (2017) |
| GDGM 46394 | KX882033 | KX882034 | China | Li et al. (2017) | |
| GDGM 42158 | KY465426 | KY465427 | China | Li et al. (2017) | |
| GDGM 45221 | KY465428 | KY465429 | China | Li et al. (2017) | |
| G. yunnanensis | MHKMU L.P. Tang2686 (holotype) | MT514924 | MT514921 | China | Mu et al. (2021) |
| MHKMU-HT 050 | MT514922 | MT514918 | China | Mu et al. (2021) | |
Gerhardtia venosolamellata N. Endo, Moe Takah., Nagamune, K. Oguchi, R. Sugaw., Sotome, Nakagiri, & N. Maek. sp. nov. Figs. 1, 2
MycoBank no.: MB 840523.


Diagnosis: This species is characterized by tricholomatoid basidiomata, off-white to pale orange or pale salmon-pink pilei with faint striae, subdistant lamellae with lateral veins, a strigose to tomentose stipe base, cyanophilic, elongate-ellipsoid to cylindrical, slightly verrucose or undulate basidiospores measuring 4.5-6 × 2.5-3 µm, cyanophilic basidia measuring 25-35 × 5-6 µm with siderophilous granules, arthroconidia production on hyphal strands at the stipe base, and an association with broad-leaf forests. The phylogenetically closest species, G. highlandensis, is distinguished by its slightly denser lamellae without lateral veins, and by its association with pine forests. Morphologically, G. sinensis is most similar to G. venosolamellata, but is distinguished by its whiter basidiomata and slightly broader basidiospores.
Type: JAPAN, Tottori Pref., Tottori City, Katsurami, Tottori Deainomori Park, on the leaf litter of broad-leaved trees, 03 Oct 2020, leg. K. Nagamune (holotype, TUMH 64253 [NaK20201003-07]; isotype, TMI 37395).
Culture ex-holotype, TUFC 101893, isolated from the inner tissue of basidioma.
Gene sequence ex-holotype: LC637875 (ITS), LC637881 (LSU)
Etymology: The epithet “venosolamellata” refers to the lamellae with lateral veins.
Japanese name: Myakuhida-shimeji
Macromorphology: Basidiomata medium-sized, tricholomatoid to somewhat marasmioid (Fig. 1A-G). Pileus 30-90 mm in diam., slightly hemispherical to convex when young, convex to plano-convex with age, margin sulcate-striate or mostly smooth, glabrous, not viscid, hygrophanous, surface off-white to pale orange (oac813) or pale salmon-pink (oac695 or 696) when moistened, slightly deeper (oac814) towards the center (Fig. 1A-G). Pileal context white (oac909), thin. Lamellae subdistant, adnate, lateral veins present, off-white (oac816) to pale, dull yellow (oac787) (Fig. 1H). Stipe 50-100 mm long, 6-12 mm in diam., smooth, surface white to off-white (oac909 or oac816), base somewhat enlarged, tomentose to strigose, with hyphal strands (Fig. 1A-G).
Micromorphology: Basidiospores (4.1-)4.7-5.7(-6.9) × (2.0-)2.4-2.9(-3.6) µm, average 5.0-5.5 × 2.5-2.7 µm, Q = (1.55-)1.80-2.21(-2.57), Qm = 1.96-2.06 (210 spores from seven collections), ellipsoid to cylindrical, smooth or slightly verrucose, sometimes undulate, thin-walled, non-amyloid, cyanophilic, hyaline (Fig. 2A-D; Supplementary Table S1). Basidia (20-)25-35(-40) × (4.5-)5-6(-7) µm, subcylindrical to subclavate, smooth, thin-walled, non-amyloid, cyanophilic, containing siderophilous granules, hyaline to subhyaline, clampless at the basal septa, 4-spored (Fig. 2E-G). Sterigmata 1-4 µm in length, smooth, hyaline (Fig. 2E, F). Basidioles (20-)25-30(-35) × (3-)3.5-5 µm, subcylindrical to subclavate, smooth, thin-walled, hyaline to subhyaline, non-amyloid, cyanophilic, containing siderophilous granules; basal septa clampless (Fig. 2E-G). Hymenophoral trama regular, densely parallel; hyphae 2.5-10 µm in thickness, smooth, thin-walled to slightly thick-walled (up to 1 µm), non-amyloid, not cyanophilic or siderophilic, hyaline, clampless. Pileipellis 15-35 µm in thickness, cutis, parallel; hyphae 2-7 µm in diam., cylindrical, smooth, thin-walled, hyaline to subhyaline, clampless (Fig. 2H). Pileitrama parallel; hyphae 3-12.5 µm in diam., cylindric to inflated, smooth, thin- to thick-walled (up to 1 µm), hyaline, clampless (Fig. 2H). Stipitipellis 5.5-20 µm in thickness, cutis, parallel; hyphae 1-6 µm in diam., cylindrical, smooth, thin-walled, hyaline, clampless (Fig. 2I). Stipititrama longitudinally parallel; hyphae 2-6 µm in diam., cylindric to inflated, smooth, thin- to thick-walled (up to 1 µm), hyaline, clampless (Fig. 2I). Hyphal strands at the stipe base parallel; hyphae 1-5 µm in diam., cylindrical, smooth, thin- to thick-walled (up to 1.5 µm), hyaline to pale yellow, producing arthroconidia (Fig. 2J). Arthroconidia (2.8-)4.2-6.9(-10.3) × (1.9-)2.3-3.1(-4.4) µm, average 5.1-6.7 × 2.5-3.0 µm, Q = (1.15-)1.59-2.61(-3.97), Qm = 1.94-2.25 (180 conidia from seven collections), cylindrical to roundish, smooth, thin- to thick-walled (up to 0.7 µm), hyaline, secession mode mostly schizolytic, rarely rhexolytic (Fig. 2J; Supplementary Table S2).
Ecology: Cool- to warm-temperate regions of Japan, Aug to Nov, on the leaf litter of mixed broad-leaf forests, solitary to scattered, saprotrophic.
Additional specimens examined: JAPAN, Tottori Pref., Tottori City, Katsurami, Tottori Deainomori Park, on the leaf litter in a mixed forest of Ilex, Quercus, and Eurya, 02 Aug 2020, leg. K. Oguchi (TUMH 63956; pers. no. KO20200802-16); 28 Sep 2020, leg. K. Nagamune (TUMH 64252; pers. no. NaK20200928-001); 09 Oct 2020, leg. K. Nagamune (TUMH 64254; pers. no. NaK20201009-10); 23 Oct 2020, leg. K. Nagamune (TUMH 64255; pers. no. NaK20201023-02); Yamanashi Pref., Minamitsuru-gun, Narusawa Village, on the leaf litter in a mixed forest of Fagus, Quercus, Carpinus, and Betula, 07 Sep 2018, leg. N. Endo (TUMH 63383; pers. no. NaoE20180907-02); leg. M. Shishikura (TUMH 64251; MS20180907-15).
Gerhardtia highlandensis (Hesler & A.H. Sm.) Consiglio & Contu. Micol. Veg. Medit., 19, 151-162, 2004.
≡ Clitocybe highlandensis Hesler & A.H. Sm. Lloydia, 6, 254, 1944.
Macromorphology: Basidiomata medium to large, tricholomatoid to somewhat marasmioid (Fig. 3A). Pileus glabrous, margin mostly smooth or sulcate-striate (Fig. 3A). Stipe glabrous, base enlarged, strigose, with hyphal strands (Fig. 3A). Lamellae close to subdistant, adnate to decurrent, lateral veins absent (Fig. 3B).

Micromorphology: Basidiospores (4.2-)4.6-6.2(-6.8) × (2.3-)2.5-2.9(-3.2) µm, average 5.4 × 2.7 µm, Q = (1.58-)1.79-2.18(-2.38), Qm = 1.99 (30 spores from one collection), oblong to cylindrical, rarely ellipsoid, smooth or slightly verrucose, sometimes undulate, non-amyloid, cyanophilic, hyaline (Fig. 3C-E; Supplementary Table S1). Basidia 20-30(-35) × (4-)5-6(-6.5) µm, clavate to subclavate, smooth, cyanophilic, containing siderophilous granules, hyaline to subhyaline, basal septa clampless, 4-spored (Fig. 3F-H). Sterigmata 1-3 µm in length, smooth, hyaline. Basidioles (15-)20-25(-35) × (2.5-)3.5-5.5(-6.5) µm, subcylindrical to subclavate, smooth, cyanophilic, containing siderophilous granules, hyaline to subhyaline, basal septa clampless (Fig. 3F, G). Hymenophoral trama regular, dense, parallel; hyphae 2-12 µm in diam., smooth, hyaline, clampless. Pileipellis cutis, parallel; hyphae 1-9 µm in diam., cylindrical, smooth, thin-walled to slightly thick-walled (up to 1.5 µm), non-amyloid, not cyanophilic or siderophilic, hyaline to subhyaline, clampless (Fig. 3I). Pileitrama parallel; hyphae 2.5-15 µm in diam., cylindric to inflated, smooth, thin- or thick-walled (up to 1.5 µm), non-amyloid, not cyanophilic or siderophilic, hyaline, clampless (Fig. 3I). Stipitipellis cutis, parallel; hyphae 1.5-4 µm in diam., cylindrical, smooth, thin-walled, non-amyloid, not cyanophilic or siderophilic, hyaline, clampless (Fig. 3J). Stipititrama longitudinally parallel; hyphae 2-6.5 µm in diam., cylindric to inflated, smooth, thin- to thick-walled (up to 1 µm), non-amyloid, not cyanophilic or siderophilic, hyaline, clampless (Fig. 3J). Hyphal strand at stipe base parallel; hyphae 1-5 µm in diam., cylindrical, smooth, thin- to thick-walled (up to 1 µm), non-amyloid, not cyanophilic or siderophilic, hyaline to pale yellow, producing arthroconidia (Fig. 3K). Arthroconidia (3.0-)3.8-5.8(-6.6) × (2.2-)2.6-3.2(-3.6) µm, average 4.8 × 2.9 µm, Q = (1.02-)1.32-1.97(-2.45), Qm = 1.64 (25 conidia from one collection), cylindrical to roundish, smooth, thin- to thick-walled (up to 0.7 µm), hyaline, secession mode schizolytic (Fig. 3K; Supplementary Table S2).
Specimen examined: UNITED STATES, North Carolina, Macon, Highlands, under pine, 10 Sep 1937, leg. L.R. Hesler and A.H. Smith (TENN-F-016020, isotype).
3.2. Descriptions of cultures 3.2.1. Gerhardtia venosolamellataMacromorphology: After 28 d in the dark, colonies on MA white (oac909) to pale pinkish-white (oac816), thin, dense or sometimes loose, downy to cottony with abundant aerial hyphae (Fig. 4A); colonies on MYG pale pinkish-brown (oac778 to 779), thin to slightly thick, dense, pellicular to downy with a few aerial hyphae (Fig. 4B); colonies on PDA white (oac909) to pale cream-white (oac815 to 816), thin to thick, dense, downy to cottony with abundant aerial hyphae (Fig. 4C1). Aerial hyphae producing hyphal strands on MA and PDA. Hyphal strands on PDA producing fan-shaped, synnema-like structures bearing numerous arthroconidia in the middle to basal regions (Fig. 4C1). Growth (five replicates of three strains) after 28 d in the dark was 16-26 mm (radius) on MA, 11-15 mm on MYG, and 18-22 mm on PDA.

Micromorphology: Arthroconidia (3.0-)4.7-8.2(-11.8) × (1.9-)2.4-3.1(-3.9) µm, average 5.8-7.1 × 2.5-2.9 µm, Q = (1.15-)1.64-3.23(-4.97), Qm = 2.08-2.68 on MA; (2.9-)4.4-8.0(-11.1) × (2.1-)2.4-3.0(-3.5) µm, average 5.8-6.7 × 2.5-2.8 µm, Q = (1.10-)1.60-3.04(-4.39), Qm = 2.24-2.38 on PDA (90 conidia from three strains on each medium) cylindrical to kernel-shaped, smooth, hyaline, thin- to thick-walled (up to 0.7 µm), dikaryotic, secession mode schizolytic, sometimes rhexolytic (Fig. 4D-J; Supplementary Table S3). Conidiogenous hyphae intricately entwined (Fig. 4F, G). Arthroconidia frequent on MA, absent on MYG, abundant on PDA. Chlamydospores variable in shape and size depending on media; on MA (10.7-)12.1-23.5(-28.0) × (5.6-)6.5-10.6(-12.9) µm (15 chlamydospores from 3 strains), globose to clavate or subclavate, sometimes ellipsoid to peanut-shaped, smooth, sometimes covered with crystals, thin- to thick-walled (up to 1.5 µm), produced in the mid to distal portions of hyphae, hyaline (Fig. 4K); on MYG (8.7-)9.8-14.6(-18.4) × (7.2-)7.5-10.8(-13.0) µm (15 chlamydospores from three strains), mostly globose to subglobose, sometimes clavate or subclavate, rarely chaining two cells, smooth, sometimes covered with crystals, thin- to thick-walled (up to 1.5 µm), produced in the mid to distal regions of hyphae, hyaline. Chlamydospores frequent on MA, more frequent on MYG, absent on PDA. Hyphae 1.5-5 µm in diam., similar among media, cylindrical, smooth, thin-walled, hyaline, clampless. Cystidia absent.
Cultures examined: TUFC 101584, isolated from basidioma tissue of TUMH 63383; TUFC 101840, isolated from basidiospores of TUMH 63956; TUFC 101893 (ex-holotype), isolated from basidioma tissue of TUMH 64253.
3.2.2. Gerhardtia foliicolaMacromorphology: After 28 d in the dark, colonies on MA white (oac909) to pale pinkish-white (oac816), very loose, woolly, aerial hyphae abundant, hyphal strands loose; colonies on MYG pale grayish-white (oac808-809), dense, pellicular, without aerial hyphae or hyphal strands; colonies on PDA pale pinkish-white (oac816), dense, cottony, aerial hyphae present, hyphal strands few or absent. Growth (five replicates of two strains) after 28 d in the dark was 26-28 mm (radius) on MA, 19-20 mm on MYG, and 18-19 mm on PDA.
Micromorphology: Arthroconidia on MA (3.4-)4.7-7.2(-8.8) × (2.0-)2.4-3.2(-3.9) µm, Q = (1.1-)1.6-2.8(-3.7) (60 conidia from two strains); on PDA (4.0-)5.2-8.6(-10.5) × (2.3-)2.4-3.5(-4.3) µm, Q = (1.2-)1.7-3.1(-4.1) (30 conidia from one strain), cylindrical to kernel-shaped, smooth, hyaline, thin- to thick-walled (up to 1 µm), dikaryotic, chain straight or curved, secession mode schizolytic or rhexolytic (Supplementary Table S3). Conidiogenous hyphae not intricately entwined. Chlamydospores on MA 14-23 × 6.5-12.5 µm, clavate to subclavate, smooth or covered with granules or crystals, thin- to thick-walled (up to 1.5 µm), hyaline; on MYG 5.5-20.5 × 4.5-11 µm, clavate to subclavate, sometimes globose to subglobose, smooth or covered with granules or crystals, thin- to thick-walled (up to 1.5 µm), produced in the mid to distal portions of hyphae, hyaline. Hyphae 1.5-5 µm in diam., similar among media, cylindrical, smooth, thin-walled, hyaline, clampless. Cystidia absent.
Cultures examined: TUFC 100078, isolated from basidioma tissue of TUMH 60927; TUFC 101392, isolated from basidioma tissue of TUMH 101392.
3.3. Molecular phylogenetic analysesWe obtained seven sequences from each ITS and LSU region. The LSU dataset consisted of 24 sequences of 659 gap-included characters, 51 of which were parsimony-informative sites. The ML and MP analyses generated identical topologies (Fig. 5). The log likelihood in the ML analysis was -1,454.64. In the MP analysis, a total of eight equally parsimonious trees were recovered. The tree length, consistency index (CI), retention index (RI), and rescaled consistency index (RCI) were 0.845361, 0.930233, and 0.786382, respectively. Japanese G. venosolamellata, North American G. highlandensis, and Chinese G. sinensis comprised a monophyletic clade with a strong support (MLBS/MPBS = 99/100). Within this large clade, seven specimens of G. venosolamellata formed a single clade with moderate support (94/75). Gerhardtia highlandensis was positioned between G. venosolamellata and G. sinensis. Gerhardtia borealis, G. citrinolobata, G. foliicola, G. pseudosaponacea, and G. yunnanensis each formed independent clades.

The ITS dataset consisted of 25 sequences of 635 gap-included characters, 146 of which were parsimony-informative sites. The ML and MP analyses generated identical topologies (Fig. 6). The log likelihood in the ML analysis was -2,238.79. A total of eight equally parsimonious trees were recovered in the MP analysis. The tree length, CI, RI, and RCI were 291, 0.852234, 0.910603, and 0.776046, respectively. Seven G. venosolamellata specimens formed a single clade with strong support (95/99). Three North American G. highlandensis specimens, including the isotype, formed a single clade with strong support (91/96) and a sister clade with the Japanese G. venosolamellata clade, with moderate support (72/-). Four Chinese G. sinensis specimens, including the holotype, comprised a single clade with moderate support (78/99), which was sister to the Japanese G. venosolamellata-North American G. highlandensis sister clade, with strong support (99/100). Gerhardtia borealis, G. cibaria, G. citrinolobata, G. foliicola, G. pseudosaponacea, and G. yunnanensis each formed independent clades.
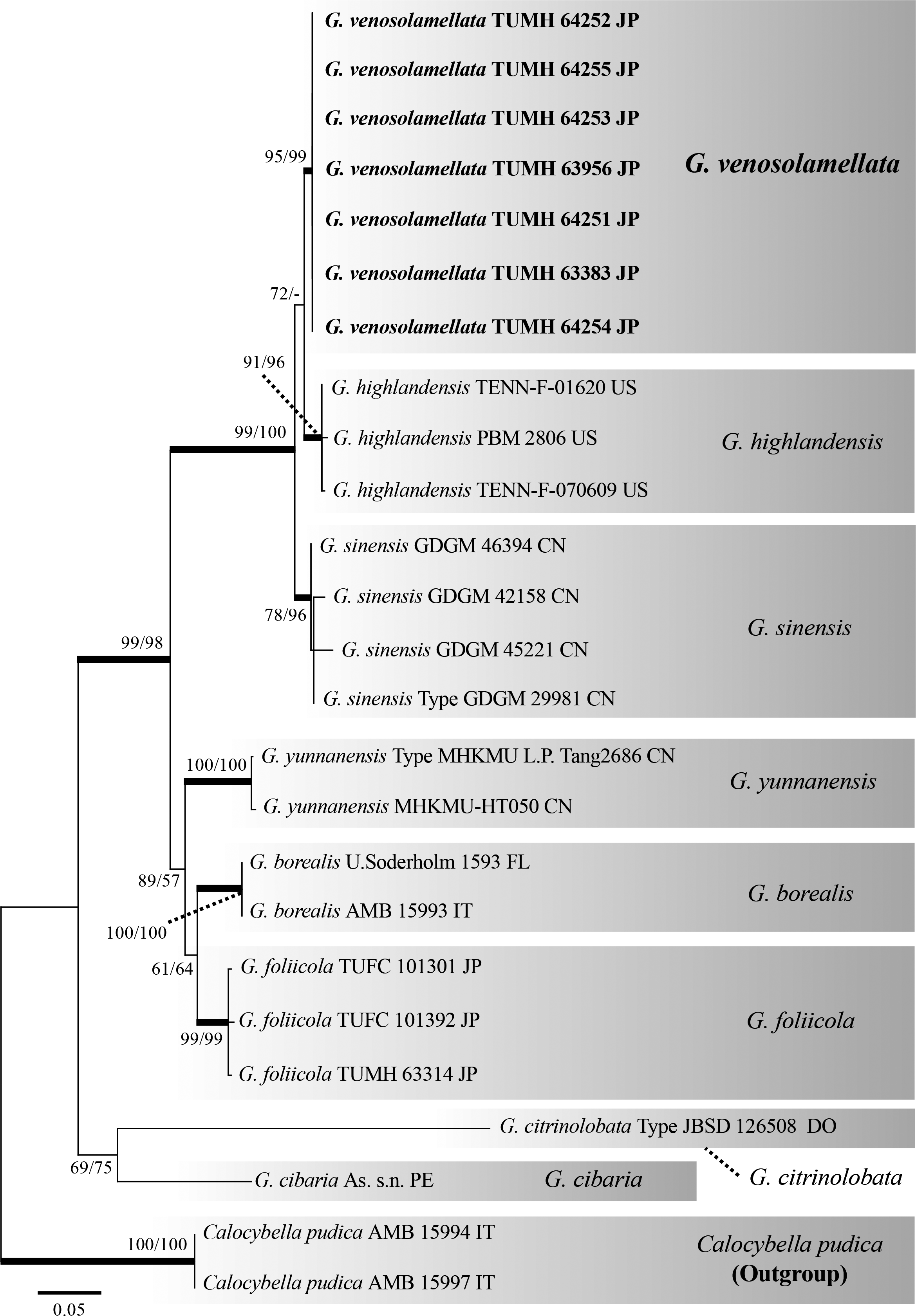
Basidiomata morphology and molecular data indicate that G. venosolamellata is a new species. Morphological differences between G. venosolamellata and other Gerhardtia species are shown in Table 3.
| Species name | Pileus color | Lamellae | Stipe base | Basidiospores | Pileipellis | Type locality | References |
| Gerhardtia borealis | Reddish brown | Crowded | 6-8.5 × 4-5 µm | Cutis/Trichodermium | Germany | Bon (1994) | |
| G. cibaria | Fuscous | Very crowded or rarely close | Glabrous | 4.8-6.4 × 3.2-4.8 µm | Cutis | Peru | Matheny et al. (2017) |
| G. citrinolobata | Lemon yellow | Close | 4.1-5.0 × 2.9-3.4 µm | Ixocutis | Dominican Republic | Vizzini et al. (2017) | |
| G. venosolamellata | Off white to pale salmon pink | Subdistant, intervenose | Tomentose to Strigose | 4.5-6 × 2.5-3 µm | Cutis | Japan | This study |
| G. foliicola | Reddish brown | Very crowded | Glabrous | 3.5-5.5 × 2-3 µm | Ixocutis | Japan | Endo et al. (2019) |
| G. highlandensis | White to pale pinkish cinnamon | Close to subdistant, not intervenose | Tomentose to Strigose | 4.5-6.5 × 2.5-3 µm | Cutis | US | This study; Bigelow and Smith (1969) |
| G. leucopaxilloides | Verona brown to russet | Crowded to close | Glabrous | 4-6 × 2-3 µm | Cutis | USA | Bigelow and Smith (1969) |
| G. marasmioides | Ochraceous | Distant | 4.8-5.5 × 2.7 μm | Cutis | Argentina | Singer (1952) | |
| G. piperata | Cream to dirty yellow | Crowded | Glabrous, fibrillose to finely tomentose | 5.3-7.8 × 2-2.8 µm | Cutis | US | Mešić and Tkalčec (2009) |
| G. pseudosaponacea | Buff | Close | Glabrous | 4.9-6.3 × 2.6-3.2 μm | Cutis/Hymeniderm | New Zealand | Cooper (2014); Vizzini et al. (2015) |
| G. sinensis | White to pale yellow | Subdistant, Intervenose | Tomentose | 5.2-6.2 × 3.0-3.4 µm | Cutis | China | Li et al. (2017) |
| G. suburens | Grayish | Close | 5-6 × 2-2.5 µm | US | Clémençon (1968) | ||
| G. yunnanensis | Yellowish to reddish brown | Subdistant to crowded | Glabrous | 5.0-6.0 × 2.5-3.0 µm | Cutis | China | Mu et al. (2021) |
Gerhardtia highlandensis (= Clitocybe highlandensis), originally described from North Carolina, is closest to G. venosolamellata with respect to ITS phylogeny; we were able to examine the isotype specimen of this species (TENN-F-016020), which is stored at TENN. Because the original morphological description of G. highlandensis was insufficient for comparison with G. venosolamellata, we have also provided a detailed morphological description of the type specimen of G. highlandensis. We found that G. highlandensis has denser, more decurrent lamellae without lateral veins, and slightly larger basidiospores compared to G. venosolamellata (Table 3). In addition, according to Bigelow and Smith (1969) and a note attached to the isotype, G. highlandensis appears to prefer coniferous litter, whereas G. venosolamellata appears to prefer broad-leaf litter.
Gerhardtia sinensis, originally described from China, is morphologically closest to G. venosolamellata. We requested a loan of the holotype specimen of G. sinensis (GDGM 29981) from the herbarium at the Guangdong Institute of Microbiology, Guangdong Academy of Science (GDGM); however, our request was unsuccessful due to COVID-19-related closures (Ting Li, personal communication, Feb 2021). Thus, we compared G. venosolamellata and G. sinensis with the original description of the latter species (Li et al., 2017). Gerhardtia sinensis has whiter basidiomata and slightly broader basidiospores (5.2-6.2 × 3.0-3.4 µm) with Q values not exceeding 2.0, whereas the basidiospores of G. venosolamellata often have Q values exceeding 2.0 (Table 3; Li et al., 2017).
Gerhardtia marasmioides, which Co-David et al. (2009) recently transferred to Clitopilus without morphological or molecular phylogenetic analyses, was initially described from Argentina as Rhodocybe marasmioides Singer (Singer, & Digilio, 1951). Li et al. (2017) proposed that this species be treated as Gerhardtia because it has cyanophilic basidiospores and cyanophilic basidia with siderophilous granules (Baroni, 1981; Contu & Consiglio, 2004). Gerhardtia venosolamellata is similar to G. marasmioides. Although we requested the isotype specimen of G. marasmioides from MICH, we were unfortunately unable to obtain a loan because the specimen is too small for destructive sampling. However, according to the original description of G. marasmioides by Singer and Digilio (1951), this species has distinct ochraceous pileus and white distant lamellae (Table 3). Fresh material of this species should be sampled from the type locality for molecular phylogenetic analyses.
Other Gerhardtia species are morphologically distinct from G. venosolamellata (Table 3). Gerhardtia borealis, G. cibaria, G. citrinolobata, G. foliicola, G. leucopaxilloides, G. suburens, and G. yunnanensis have denser lamellae (Clémençon, 1968; Bigelow & Smith, 1969; Matheny et al., 2017; Endo et al., 2019; Mu et al., 2021). Gerhardtia foliicola, G. leucopaxilloides, and G. yunnanensis have distinct brownish pilei. Gerhardtia citrinolobata has distinct lemon-yellow pilei (Vizzini et al., 2017). Gerhardtia pseudosaponacea has smooth basidiospores and a hymeniderm pileipellis (Cooper, 2014; Vizzini et al., 2015). Gerhardtia piperata has larger basidiomata and narrower basidiospores (Mešić & Tkalčec, 2009).
We found that the basidiomata of G. venosolamellata produce asexual spores (arthroconidia) on hyphal strands at the stipe base, and sexual spores (basidiospores) on the hymenophore. Arthroconidia production was also confirmed in G. highlandensis based on the isotype specimen. Species in the genus Asterophora (Lyophyllaceae) produce chlamydospores on their basidiomata for asexual reproduction (Thompson, 1936; Singer, 1986). For example, A. lycoperdoides (Bull.) Ditmar produces numerous chlamydospores on the pileus, whereas A. parasitica (Bull.) Singer produces chlamydospores on the hymenophore (Singer, 1986; Bessette, Bessette, & Fischer, 1997). Recently, Baroni et al. (2007) reported two new genera, Arthromyces and Blastosporella, which produce conidia on the basidiomata. Although not a member of the Lyophyllaceae, it is also well-established that Dendrocollybia racemosa (Pers.) R.H. Petersen & Redhead [Tricholomataceae, order Agaricales (Hofstetter et al., 2014)] produces conidia on the tip of the racemose branch on the stipe (Lennox, 1979). Our results suggest that additional species that produce conidia on their basidiomata may be identified via investigation of other Gerhardtia species, as well as other taxa in the Lyophyllaceae and Agaricales. We observed no clear morphological differences between G. venosolamellata and G. highlandensis with respect to the arthroconidia on hyphal strands at the stipe base.
Among the Lyophyllaceae, the genera Asterophora, Calocybe, Fibulochlamys, Hypsizygus, Ossicaulis, Sagaranella, Sphagnurus, and Termitomyces have anamorphic life cycles at the vegetative mycelium stage (Brunner & Miller, 1988; Nagasawa & Arita, 1988; Walther et al., 2005; Madrid, Cano, Stchigel, Gené, & Guarro, 2010). In Gerhardtia, the microscopic characteristics of cultured mycelia have been documented for three species (G. suburens, G. leucopaxilloides, and G. foliicola), all of which produce numerous arthroconidia exhibiting both schizolytic and rhexolytic secession (Clémençon, 1968; Endo et al., 2019). In this study, live cultures of G. venosolamellata were also observed to produce numerous arthroconidia with schizolytic and rhexolytic secession. As mentioned in Endo et al. (2019), Nagasawa and Arita (1988) reported that the arthroconidia of Hypsizygus and Ossicaulis exhibited only schizolytic secession. Further investigations, including of the cultured mycelia of other Lyophyllaceae taxa, are required to confirm whether the culture characteristics of Gerhardtia are unique, stable traits characteristic of the genus.
We compared the shape, size, color, and wall thickness of arthroconidia among different species (G. foliicola and G. venosolamellata) and media (MA and PDA), but observed no morphological differences in either comparison. Clémençon (1968) described the arthroconidia (“chlamydospores”) of G. leucopaxilloides on PDA as “4-6 µm long with 1-2 larger inclusions”, whereas those of G. suburens were described as “(4-)6-12 µm long with 3-7 smaller inclusions”. We observed no distinct morphological differences between G. venosolamellata and the aforementioned two species with respect to the arthroconidia produced by mycelia cultured on PDA. However, the conidiogenous hyphae of G. venosolamellata tend to entwine intricately, and it was difficult to observe the chaining of conidia on either MA or PDA. This characteristic has not been reported or observed in G. foliicola, G. leucopaxilloides, or G. suburens. We will continue to investigate whether the conidiogenous mode exhibited by G. venosolamellata is a species-specific characteristic.
We observed the traits of cultured G. venosolamellata using three different media, MA, MYG, and PDA, and found that the species exhibits a preference for PDA. Interestingly, the mycelia only produced fan-shaped, synnema-like hyphal strands on PDA. While the distal portions of these structures are mostly sterile, the basal to middle portions are covered with numerous arthroconidia. Clémençon (1968) reported the production of similar structures in G. suburens and G. leucopaxilloides mycelia cultured on PDA. However, G. foliicola did not produce such structures in our study, even when using PDA. The macromorphological characteristics of culture strains may be taxonomically relevant, but further investigations using various media are needed.
Clavate, granule-covered cells, which are rarely seen on the cultured mycelia of G. foliicola, were identified as cystidia in our previous study (Endo et al., 2019). This is because the granules that cover the cells were thought to be waste products or metabolites of the hyphae. In this study, we observed these cystidia in more detail. More thorough observation revealed that cells of G. venosolamellata and G. foliicola covered with granules or crystals exhibited substantial variation (e.g., globose, subglobose, ellipsoid, subclavate, or clavate) depending on the culture media (e.g., cells of both species were typically larger on MA). Furthermore, two such cells were often chained together, and were thick-walled (up to 1.5 µm) and produced in the middle of the hyphae. Owing to these characteristics, we now consider these cells chlamydospores rather than true cystidia. Nagasawa and Arita (1988) reported that the cultured mycelia of Hypsizygus marmoreus (Peck) H.E. Bigelow produced smooth chlamydospores. We also confirmed the same character in two TUFC strains of H. marmoreus stored and opened at FMRC (data not shown). Because there is little information on chlamydospore formation on cultured mycelia in the Lyophyllaceae, we will continue to investigate chlamydospores to clarify whether the abovementioned characteristics are among the taxonomic traits of the family.
Gerhardtia venosolamellata is a new species in the family Lyophyllaceae. This family contains numerous taxa whose cultured mycelia produce conidia and chlamydospores. When conducting taxonomic studies on species in this family, it is important to characterize the cultured mycelia, in addition to conducting molecular phylogenetic analyses and precise morphological observations of the basidiomata.
The authors declare no conflicts of interest. All experiments undertaken in this study complied with the current laws of the country in which they were performed. All of the authors contributed to the preparation and revision of the manuscript.
We great thank Dr. Patrick B. Matheny and Dr. Oliver Margaret at TENN for loaning the Gerhardtia highlandensis isotype specimen, Dr. Timothy Y. James in MICH for providing valuable information and advice to make manuscript, Dr. Ting Li, in GDGM for providing information about loaning specimen of Gerhardtia sinensis stored at GDGM. We also great thank Mr. Naoto Kinoshita, a caretaker at Tottori Deainomori, for facilitating our basidiomata sampling, Ms. Sachiko Ueta, Ms. Ayako Eriguchi, Ms. Mizuki Yokono, Ms. Masako Oka, and Ms. Kiko Hirata for the cryopreservation and maintenance of Gerhardtia strains. We also thank the staff of the Division of Instrumental Research, Research Center for Supports to Advanced Science, Shinshu University, and FASMAC Co., Ltd. (http://fasmac.co.jp) for technical support with DNA sequencing. We thank all the staff of Tottori University. This study was supported in part by a large research grant (Nitaro Maekawa) from the Institute for Fermentation, Osaka (IFO).