2023 Volume 11 Pages 106-120
2023 Volume 11 Pages 106-120
Helleborus is a small genus of the Ranunculaceae family and comprises about 19 species of herbaceous perennials. These perennial plants have a long flowering period and are mainly evergreen. Helleborus cultivars, including H. niger (commonly called Christmas rose), are a highlight in winter gardens and bloom from winter until early spring, at a time when few other flowers are in bloom. Taxonomy of the genus Helleborus was previously based only on morphological characteristics; however, molecular studies have been done in the past 20 years and further such research will provide comprehensive genetic information. This genus has a rich and diverse group of flower shapes. This review provides a general introduction to the genus Helleborus, focusing on the two different taxonomic methods: morphological and molecular. Several molecular tools used for phylogenetic studies are summarized and evaluated for their applicability in future studies of Helleborus taxonomy.
Helleborus is a small genus of the Ranunculaceae family and comprises about 19 species of herbaceous perennials. In scientific classification of the Ranunculaceae, Helleborus belongs to the subfamily Ranunculoideae [1]. Helleborus is divided into two subgenera, Helleborus and Helleborastrum, and each of these is divided into three sections. According to morphological characteristics, Helleborus was previously divided into caulescent species and acaulescent species [2]. Caulescent species belong to the subgenus Helleborus and acaulescent species belong to Helleborastrum. Although the taxonomy of the genus Helleborus was formerly based only on morphological characteristics, now cytological and molecular analyses are available for providing additional information and greater accuracy in determining phylogenetic relationships.
1.2 DistributionThe majority of Helleborus species are naturally widely distributed over different parts of Europe, with only one species (H. thibetanus) native to western China [3]. The great majority are found in the mountainous regions of Southern and Central Europe. They are mostly native to open oak woodlands and grassy meadows and rocky slopes.
In the wild distribution of the genus Helleborus, different species generally gather in different regions, but several species grow side by side. For instance, H. foetidus is widespread in Western Europe; H. argutifolius is endemic to the islands of Corsica and Sardinia; H. viridis shows scattered distribution in Britain, France, and Spain; H. atrorubens, H. multifidus, H. torquatus, and H. hercegovinus show scattered distribution in Croatia, Bosnia and Herzegovina, and Montenegro; and H. orientalis is found around the Mediterranean and Black Seas. Several species, e.g. H. dumetorum, H. multifidus, H. odorus, and H. viridis, have overlapping habitats in the northwestern Balkans. Figure 1 shows the distribution maps of 18 Helleborus species in Europe, with reference data from Nonokuchi [4].
Interspecific hybrids appear in some regions, such as natural hybrids between H. odorus and H. atrorubens [5, 6]. The northwestern Balkans is an interesting region for Helleborus studies because many natural hybrids have been confirmed and the variation within the genus appears to be extremely high [7, 8, 9]. Collecting data about morphological traits and available genetic resources of wild Helleborus will be useful for breeding and marketing.
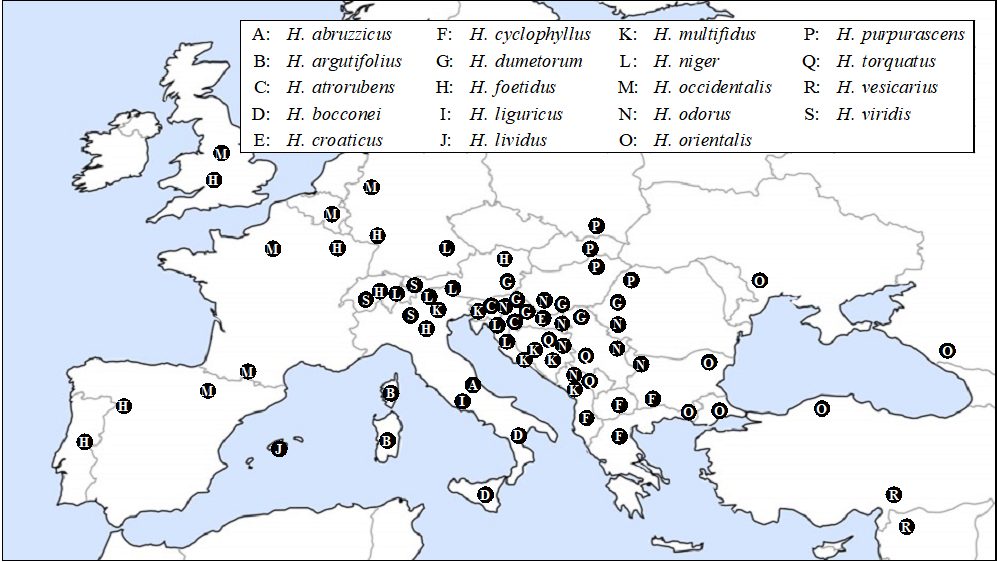
Figure 1: Distribution map of Helleborus species in Europe
Ddrawn using data in [4].
In the genus Helleborus, petals have developed into nectaries and sepals look like petals. The flowers of the genus characteristically have an outer whorl of five sepals and an inner one of up to 32 nectaries. Therefore, the ornamental structure in Helleborus is phytomorphologically sepals, and these persist on the plant for well over a month allowing the blossoms to last a long time [10, 11, 12]. The sepals are also responsible for the bulk of photosynthesis and provide assimilates for the developing fruit [13]. Flowers of the genus Helleborus are hermaphroditic (having functional male and female sexual organs); they are self-compatible but self-fertilization is likely naturally rare due to their protogynous habit [14]. Helleborus species appear to be predominantly entomophylous and cross-pollinating in nature [15, 16].
1.4 Economic valueHelleborus can be garden or pot plants, and some species are also grown as cut flowers (e.g. H. niger and H. ×hybridus) [17, 18]. They are some of the most popular flowers at Christmas in Europe and are easy to cultivate in the garden. In the Netherlands, about 500,000 pot plants and 1.5 million cut flowers of Helleborus were sold during 2007–2009 [19]. In 2014, the Royal FloraHolland auction sold Helleborus plants worth 12 million euros, placing them in eighth place [20]. More than 1,000 novel Helleborus hybrids and cultivars have been produced by interspecific hybridization. The production of hybrids among Helleborus species is mostly related to improving flower color and size. Compared with traditional mainstream ornamental flowers, Helleborus have particularly interesting flowers, and the genus is becoming ever more important commercially.
The economic value of the genus Helleborus is not only in ornamental plants, but also for therapeutic benefits. Many studies have shown that natural Helleborus extracts are a potential valuable source for medical uses. For instance, H. viridis extracts exhibit high biological activity with lasting antalgic, myorelaxant, and blood vessel regulating actions [21]; extracts of H. niger have immunostimulatory properties and are used in anticancer therapy [22]; and H. bocconei contains active molecules such as bufadienolide heterosides (hellebrin) with activity against bacteria responsible for respiratory infections [23].
Research on the taxonomy of the genus Helleborus has a long history. Within the genus, various infrageneric groupings have been proposed based on different criteria. For the purposes of horticulture, early classification of the genus Helleborus was according to the Caulescentes and Acaules [3]. The species H. argutifolius, H. foetidus, H. lividus, and H. vesicarius are classified into the Caulescentes. The Caulescentes usually grow in the mild winter climate of the Mediterranean–Atlantic range, and the Acaules are distributed in the Mediterranean Sea to the south temperate zone. Although the caulescent and acaulescent criterion is still being debated, it is an important distinction for horticultural purposes.
Mathew [3] divided the genus directly into six sections according to plant structure, pollen morphology, and seed characteristics: Syncarpus Spach, Griphopus Spach, Chenopus Schiffner, Helleborus, Helleborastrum Spach, and Dicarpon Ulbrich (Table 1).
Following detailed study of morphological characteristics and growth forms, Werner and Ebel [24] proposed a hypothetical phylogenetic tree of the genus Helleborus; this was the first focus on the structure of hypsophylls (bracts or bracteoles) and divided Helleborus into two subgenera: Helleborus and Helleborastrum. The subgenus Helleborus, including sections Griphopus, Chenopus, and Helleborus, has undivided bracteole hypsophylls. Subgenus Helleborastrum includes sections Syncarpus, Dicarpon, and Helleborastrum, and has divided frondose hypsophylls. Subgenus Helleborastrum is paraphyletic to the subgenus Helleborus.
According to the structure of hypsophylls and considering morphological character progressions and factors relating to the environment and distribution, a hypothetical phylogenetic tree of the genus has been proposed [24]. The genus is divided into two grade groups: Caulescentes and Scapigeri. The Caulescentes grow in the mild winter climate range and the Scapigeri grow in sub-Mediterranean to south temperate deciduous forests.
| Section | Species | Types of leaves | Leaf shape | Follicle number | Remarks |
|---|---|---|---|---|---|
| Syncarpus Spach | H. vesicarius | both basal leaves and cauline leaves | leaves with 3–5 coarsely jagged leaflets | 3 | dying down in summer |
| Griphopus Spach | H. foetidus | sub-shrubby stems bearing cauline leaves | leaves with 7–10 broad leaflets | 3 | evergreen |
| Chenopus Schiffner | H. argutifolius, H. lividus | sub-shrubby stems bearing cauline leaves | leaves with 3 broad leaflets | 3–6 | evergreen |
| Helleborus | H. niger | basal leaves only | pedate with 7–9 leaflets | 5–8 | leaves overwintering |
| Helleborastrum Spach | H. atrorubens, H. cyclophyllus, H. dumetorum, H. multifidus, H. odorus, H. orientalis, H. purpurascens, H. torquatus, H. viridis | basal leaves only | pedate with 7–many leaflets | 4–7 | leaves winter-deciduous or overwintering |
| Dicarpon Ulbrich | H. thibetanus | basal leaves only | pedate with 7–10 leaflets | 2 | leaves winter-deciduous |
Created using data in [3]
Traditionally, leaf shape, flower color, plant height, and the overall bearing of the plant are some of the characteristics used to designate different species. Based on published books on Helleborus, brief descriptions of 17 species are shown in Table 2 [3, 25, 26] and leaf morphology of 15 major species is shown in Figure 2 [26, 27].
Detailed descriptions of morphological characteristics and geographical separation of the subspecies of three species (H. niger, H. orientalis, and H. multifidus) and H. torquatus are given below. These four species contain subspecies and show distinct regional differences that are frequently confounded among subspecies.
Helleborus niger: H. niger is a very variable species, both in the wild and cultivated stock. It has two currently recognized subspecies: subsp. niger Linn and subsp. macranthus (Freyn) Schiffner. The leaves of subsp. niger are very obviously pedate and comprise seven to nine broadly wedge-shaped leaflets with coarsely toothed margins. Flowers are up to 8 cm in diameter. Subsp. niger is very widespread and grows in an area stretching across the northern side of the Alps. Subsp. macranthus is similar to subsp. niger but distinguished by leaf segments; their leaf segments are broadly lanceolate and slightly serrated, with flowers 8–11 cm in diameter. They are distributed only in northern Italy, Slovenia, and Croatia.
Helleborus orientalis: H. orientalis is the most colorful and floriferous species and divided into three subspecies: subsp. orientalis, subsp. abchasicus (A. Braun & Sauer) B. Mathew, and subsp. guttatus (A. Braun & Sauer) B. Mathew. The three subspecies show many clear differences in flower color and native range. Subsp. orientalis usually has white flowers with some green or cream color, and the nectaries are usually green. Subsp. abchasicus has pink- to plum-colored flowers with tinted green interiors, and the nectaries are also green or plum to dark purple. Flowers of subsp. guttatus are similar to those of subsp. orientalis, except that they are spotted to varying degrees with red or purple, and usually three sepals have more spots than the other two. The native range of subsp. orientalis is northwestern Turkey, southern Ukraine, and in the Caucasus. Subsp. abchasicus has a limited range in Georgia and perhaps Romania. Subsp. guttatus has a very limited range in Ukraine.
| Species | Flower color | Flower diameter (cm) | Plant height (cm) | Flowering period |
|---|---|---|---|---|
| H. argutifolius | Apple-green | 2.5–5 | 75–120 | January–May in the wild,January–March in cultivation |
| H. atrorubens | Purple-green | 4–5 | 30–45 | March–April in the wild, January–February in cultivation |
| H. croaticus | Purple, red or green | 4–5 | 10–20 | February–March |
| H. cyclophyllus | Green | 5–6 | 40–55 | March–May in the wild, January–March in cultivation |
| H. dumetorum | Green | 2.5–4 | 20–30 | February–March |
| H. foetidus | Apple-green | 2 | 75–120 | January–June |
| H. lividus | Apple green inside and pink or purple outside | 2.5–4 | 40–60 | December–March |
| H. multifidus | ||||
| H. multifidus subsp. multifidus Visiani | Green | 1–2 | 20–30 | April–May in the wild, January–April in cultivation |
| H. multifidus subsp. bocconei (Tenore) B. Mathew | Green | 4.5–6 | 20–30 | March–April in the wild, January–March in cultivation |
| H. multifidus subsp. hercegovinus (Martinis) B. Mathew | Yellow-green | 4–5 | 45–70 | March–April in the wild, February–March in cultivation |
| H. multifidus subsp. istriacus (Schiffner) Merxmuller & Podlech | Yellow, green and showy | 4–5.5 | 20–30 | February–April |
| H. niger | ||||
| H. niger subsp. niger Linn | White, or fading to pink | 4–8 | 15–20 | March–June in the wild, December–March in cultivation |
| H. niger subsp. macranthus (Freyn) Schiffner | White, or fading to pink | 8–11 | 15–20 | March–June in the wild, December–March in cultivation |
| H. occidentalis | Green | 3–5 | 10–20 | February–April |
| H. odorus | Green | 4–6.5 | 50 | February–April in the wild, January–March in cultivation |
| H. orientalis | ||||
| H. orientalis subsp. orientalis | White with some green | 6–7 | 45 | February–May in the wild, January–April in cultivation |
| H. orientalis subsp. abchasicus | Pink to plum | 6–8 | 45 | April in the wild, January–April in cultivation |
| H. orientalis subsp. guttatus | Red or purple | 6–9 | 45 | March–April |
| H. purpurascens | Plum-purple to sea-green | 5–7 | 10–20 | February–May in the wild, January–April in cultivation |
| H. thibetanus | Pink with dark veins | 5–6.5 | 30–50 | March–May in the wild |
| H. torquatus | Dark purple on the outside, inside the same color or green | 3–6 | 20–40 | March–May in the wild, January–March in cultivation |
| H. versicarius | Green with banded in brick redat | 7.5 | 45 | February–April |
| H. viridis | ||||
| H. viridis subsp. viridis | Deep apple green | 4–5 | 30 | March–April in the wild, February–March in cultivation |
| H. viridis subsp. occidentalis (Reut.) Schiffner | Deep apple green | 3–4 | 30 | March–April in the wild, February–March in cultivation |
Helleborus multifidus: H. multifidus is a variable and beautiful species. There are four subspecies: subsp. multifidus Visiani, subsp. bocconei (Tenore) B. Mathew, subsp. hercegovinus (Martinis) B. Mathew, and subsp. istriacus (Schiffner) Merxmuller & Podlech. In adult plants of all subspecies, leaves have many lobes, the overall shape of a leaf is pedate, and segments are lanceolate and leathery. For subsp. multifidus, basal leaves are glabrous to sparsely pubescent, pedate with all segments divided into multiple additional segments, for a total of 20–45, each 12 cm long and about 1.5 cm wide. Conical and cup-shaped flowers are usually 2–4 cm in diameter. This subspecies is distributed in the central region of the Adriatic coastal mountains. Subsp. bocconei is similar to subsp. Multifidus, but basal leaves are nearly glabrous, and pedate with segments divided for only half of their length, and each segment is less than 6 mm long. The few large drooping flowers are similar to those of H. odorus. Flowers are usually little more than 4 cm across in the wild. This subspecies grows from Sicily up into central Italy. Subsp. hercegovinus is a pretty plant, with leaflets divided into extremely narrow lobes, pedate with segments similar to subsp. multifidus, but the number of segments is as much as 45–100, and the thin segments are less than about 6 mm wide. Basal leaves are pubescent and flowers are usually 3–5 cm in diameter. It has a distinct range in Herzegovina and Montenegro. Subsp. istriacus can be easily distinguished from other subspecies, as the leaves are the least divided of all, with typically 10–14 divisions. Leaves are pedate with segments occasionally completely undivided, or divided for only half their length, and finely to coarsely serrated. Basal leaves are pubescent. The flowers are relatively large (4–5.5 cm), and may be purple tinted on the outside. This subspecies grows in northwestern Croatia, Slovenia, and northeastern Italy.
Helleborus torquatus: H. torquatus is not divided into subspecies, but is one of the most variable species in the genus. This species is distributed in two distinct and geographically separated areas: northwestern Bosnia and Hercegovina and Croatia, and the other in southeastern Montenegro. The former population tends to have almost linear leaflets, whereas the latter tends to have broader leaflets.
In addition to these, H. atrorubens is considered merely a subspecies of H. dumetorum [5, 28].
2.3 Summary of morphological taxonomy within the genus HelleborusSpecies identification of the genus Helleborus according to morphological and anatomical characteristics is still difficult because of the presence of intraspecific morphological variation forms. The main morphological characteristics generally considered for the identification of the various taxa are blade division, serration and pubescence of the leaves, flower diameter, width of the tepals, and the follicle ratio; however, these characteristics are highly variable, even within the same population [6, 8]. For instance, H. viridis is a variable species distributed in western and southern Europe, with outstanding gorgeous forms. Servettaz [8] found differences in the shapes of basal leaves from a single specimen in Lombardy (region in northern Italy), indicating that this characteristic is useless for determining H. viridis taxonomy, and safely concluded that a species present in Lombardy, H. odorus subsp. laxus, could also be identified as H. viridis.
Identifying the species based on morphological characteristics is difficult, especially because of problems associated with variable species and wild growing conditions, which easily cause confusion. Research on the classification and phylogeny of Helleborus has previously been mostly restricted to morphological characteristics. Although classification of some Helleborus species based on morphologic characteristics remains difficult, it is undeniable that morphologic characteristics also play an important role.
Morphological characterization and investigation of the phylogenetic relationships among Helleborus species are important for conservation of plant genetic resources and could be improved. Some morphological traits of the species are valuable for horticultural improvement of Helleborus, and could be introduced by interspecific hybridization. As a result, advanced studies are required using more robust characteristics than morphology.
Herbarium specimens have played a key role in morphological taxonomy, but it is noteworthy that leaf sequence changes during plant development and leaves exhibit their definitive characteristics at the time of maximum extension. Usually, flowers and leaves are collected simultaneously, but the leaves during the flowering phase are too small (incomplete information) to exhibit their definitive characteristics. For example, confusion of H. viridis with Lombardy and H. odorus subsp. laxus from the Eastern Alps is common because of consideration only for fundamental characteristics in herbarium specimens [8]. Advanced taxonomic studies of the genus Helleborus need to be combined with molecular analysis in the future.
There are limitations to taxonomical study of the genus Helleborus by relying solely on traditional morphological characteristics because of the variability of morphological characteristics and to transitional forms. To clarify Helleborus genetic relationships, there have been phylogenetic studies of some representatives in the past two decades using different molecular markers.
3.1 Analysis of phylogenetic relationships by molecular techniqueAnalysis of the phylogenetic relationships within the genus Helleborus was first carried out by Sun et al. [2], using plastid DNA (trnL-F and matK) and nuclear ribosomal ITS DNA sequences. The combined data of trnL-F, matK, and ITS provide strong support for the monophyly of Helleborus. All six sections (Dicarpon, Chenopus, Griphopus, Helleborus, Helleborastrum, and Syncarpus) are monophyletic; however, the high-level bootstrap support for H. thibetanus (section Dicarpon) would allow it to be included in section Helleborastrum.
In consideration of sequence, divergence in section Helleborastrum was extremely low. Sun et al. [2] indicated that more sensitive markers such as AFLP (amplified fragment length polymorphisms) were feasible and an appropriate technique in the next step for dividing section Helleborastrum. The combined data showed a remarkably low level of sequence divergence in section Helleborastrum, leading to the relationships among the poorly differentiated species in the section remaining unclear. Section Helleborastrum has been considered to show high intraspecific morphological variability. For instance, H. multifidus taxon shows extremely polymorphic morphological characteristics.
Meiners et al. [29] used AFLP markers to analyze the taxonomic subdivision using 40 genotypes from 20 Helleborus species. A dendrogram showed two major clades with minor clusters representing the six sections, consistent with previous studies [24]. In this dendrogram, sections Helleborastrum, Dicarpon, and Syncarpus composed the first clade, and the second clade comprised sections Chenopus, Helleborus, and Griphopus; H. foetidus (section Griphopus) alone formed an independent clade. Based on the AFLP analysis, section Dicarpon was placed next to section Helleborastrum. This result is contrary to Sun et al. [2] who suggested that Dicarpon be assigned to Helleborastrum, but consistent with Mathew [3]. The taxa H. abruzzicus and H. liguricus were described as new species by 2008, which was the first time that these species were included in taxonomic analysis of the genus Helleborus; they were grouped into section Helleborastrum and placed next to H. multifidus subsp. Multifidus and H. multifidus subsp. Hercegovinus in the dendrogram, respectively [29]. The result was a very similar classification to that of Hang [2], but with higher bootstrap support than in the previous study.
Meiners et al. [30] also used AFLP markers to analyze the taxonomic subdivision of 24 genotypes from 19 Helleborus species, with similar genetic relationships to a previous report [29]. These results showed that the AFLP technique was a good choice for species discrimination and evaluation of genetic relationships within the genus Helleborus.
The taxonomic resolutions of some species are still debated among taxonomists, including many uncertainties for H. multifidus. The traditional morphological characteristic classification recognizes four subspecies within the complex of H. multifidus: subsp. Multifidus, subsp. Bocconei, subsp. Hercegovinus, and subsp. Istriacus. However, these subsequently became controversial. Subsp. Hercegovinus is considered as a separate species (H. hercegovinus) based on the amount of nuclear DNA and multi-locus AFLP data [29]. The H. multifidus taxon shows extreme polymorphy of morphological characteristics, possibly because of nucleotide variation as a result of geographic distance and spatial isolation; H. multifidus is widely distributed in Italy, Slovenia, Croatia, Bosnia and Herzegovina, Montenegro, and Albania.
Lasić et al. [31] used molecular genetic markers (trnL region and matK of chloroplast DNA and nuclear ITS1 and ITS2 region) to genetically characterize H. multifidus in three localities in Bosnia and Herzegovina. The results showed differences between the populations from Herzegovina and Bosnia, and indicated that the two populations did not belong to the same taxon. The taxonomy of H. multifidus in Bosnia and Herzegovina and adjacent regions remains a genuine issue, and further molecular genetic analyses and expanded sampling locations are necessary to resolve their genetic relationships.
3.2 Application of molecular markers to taxonomic identificationThe main morphological characteristics are generally considered for taxonomic identification; however, some species are highly variable even within the same population. Within the genus Helleborus, variability of the diacritic characteristics and the presence of transitional forms in some species have made species classification based only on morphological characteristics difficult. For example, H. odorus is a wide-ranging species found in the region from Slovenia to Hungary, Romania, and Bosnia; and H. viridis is native to Spain, France, Switzerland, and Italy in open woods. It should be noted that, although the distinction of H. odorus from H. viridis is obvious, there is some confusion in the wild populations in north Italy because of morphological variation. The population commonly named H. viridis in Lombardy had morphological characteristics different from both H. viridis subsp. Viridis and H. viridis subsp. Occidentalis, and that fell within the variation range of H. odorus subsp. Laxus [32].
Fico [33] used the RAPD (randomly amplified polymorphic DNA) technique to investigate an initial taxonomic differentiation of Helleborus species growing in northern and central Italy, and revealed a clear discrimination between the four species (H. odorus, H. viridis, H. multifidus subsp. Bocconei, and H. niger) and eight populations, with all the species characterized by green flowers. Although samples from Lombardy (portion of northwestern Italy) were classed as H. odorus in a previous morphological analysis [8], these populations were considered to belong to H. viridis using the RAPD analysis. The RAPD results also obtained more piece of critical information about the taxonomic position of H. odorus and H. viridis. A previous hypothesis, that populations from the Maritime Alps belong to H. multifidus subsp. Bocconei, was also disproved and differs from the results by RAPD analysis [33]. Thus, RAPD analysis is a potentially useful tool for Helleborus species discrimination and morphological variation definition.
Difficulties in species recognition caused by highly variable morphological characteristics can be tackled using molecular markers. It is of interest to explore the potential of some molecular markers as a diagnostic feature of these taxa.
3.3 Summary of molecular taxonomy within the genus HelleborusSummarizing the taxonomy within the genus Helleborus using molecular tools, the classification into six sections recognized by Mathew [3] is supported by most researchers based on both morphological characteristics and molecular analysis. However, taxa of section Helleborastrum remain less clear. Section Helleborastrum has been considered a group of closely related taxa. Hybridization (highly heterozygous) among species easily occurs in this section. The degree of variability shown by individual taxa is another problem for this section, e.g. H. torquatus in northwest Bosnia and southeastern Montenegro, and different geographical regions of H. odorus show several geographical separations [2]. To clarify the ambiguity in section Helleborastrum, further studies require increased numbers of individuals per species and collecting samples from different origins. The taxonomic resolution of many Helleborus species is still debated among taxonomists.
Molecular techniques have been used for the genus Helleborus in the past 20 years, including DNA barcoding, RAPD, and AFLP. These techniques differ in their resolving power in detecting genetic differences, the type of data they generate, and their applicability to taxonomic levels.
DNA barcoding is an effective and reliable tool for identification and taxon classification of species by sequencing a very short standardized and universal DNA sequence in a well-defined gene [34, 35, 36]. The common molecular data used in plant systematics come from two sources: chloroplast DNA (e.g. rbcL, trnL-F, matK, psbA, trnH, psbK, and ndhF) [37, 38, 39, 40, 41, 42] and nuclear ribosomal DNA (e.g. ITS) [43, 44].
Previous studies using DNA barcoding discriminated many plant species. Suitable DNA sequences vary among genus and purpose, and thus selecting a gene of sufficient length and substitution rate is an important step [45]. For example, the matK gene was used for Rheum spp.; however, for Dendrobium spp. or Galphimia spp., it should be combined with the rbcL gene [37, 46, 47]. The psbA–trnH and rbcL intergenic region is a suitable DNA marker for species identification in medicinal pteridophytes [39]; and the ndhF gene provided more phylogenetic information than rbcL in major clades of the Asteraceae family [43].
Chloroplast DNA (cpDNA) has been extensively used as a source of data in plant phylogenetic analysis and species differentiation; however, the low evolutionary rate of these sequences limits the power of cpDNA for the assignment at some genus or species levels [48, 49]. According to the literature, chloroplast genome sequences are very well-suited for differentiation of old varieties, but less so for modern cultivars because they are often too closely related [41, 50].
In the past two decades, the nuclear ribosomal internal transcribed spacer region has revolutionized species-level plant phylogenetics. Nuclear ribosomal DNA has largely been presumed to undergo concerted evolution, and direct sequencing of nuclear ribosomal DNA is possible using most systems [51, 52]. The nuclear genome typically evolves faster than cpDNA and provides greater variation at lower taxonomic levels [53, 54]. Therefore, the positive attributes of using ITS sequence data include rapid rate of evolution and availability of universal primers for amplification. However, owing to the biparental inheritance characteristic, nuclear DNA is not reliable for reconstructing hybrid speciation [55].
Although successfully used in many phylogenetic studies, there is one limitation in using chloroplast markers and ITS sequences: for most species, chloroplast genes are maternally inherited, and ITS sequences represent only one or two loci in the genome. Phylogenetic hypotheses inferred from a single gene should be viewed with caution, as gene and organismal phylogenies may not overlap [54, 56]. Generally, use of two or more candidate barcodes has been advocated as best for species taxonomic identification and promising interpretations [41, 57].
When both cpDNA and ITS sequencing fail to resolve phylogenies, the molecular marker approach has the potential to solve such difficulties, particularly among closely related species, or at the intra-specific level [58]. Molecular markers are grouped by their different abilities of showing homozygosity (dominant marker) or heterozygosity (co-dominant marker). The most common dominant DNA markers in plants are RAPD markers [47], inter-simple sequence repeat (ISSR) [59], and AFLP [60], whereas the most commonly used co-dominant markers are restriction fragment length polymorphism (RFLP) [61], microsatellite (SSR) [62], sequence characterized amplified region (SCAR) [63], and single nucleotide polymorphism (SNP) [64].
An ideal marker does not exist for use in all studies. Each technique has both advantages and limitations, and will be suited to a range of investigations [65]. For instance, the AFLP technique analyzes a large number of polymorphisms compared with other markers, and has been successfully used to evaluate genetic relationships or diversity in plants including Brassicaceae [66], Prunus [67], Betula [68], Rosa [69], and Ranunculaceae [70]. Within the genus Helleborus, AFLP markers have been used for analysis of taxonomic subdivision, and high bootstrap values were obtained in dendrograms [29]. However, AFLP markers did not show strong differentiation when used in evaluating H. niger genetic diversity within naturally occurring Slovenian populations [9].
The genus Helleborus in the wild is probably highly heterozygous, and dominant markers have the capacity for analyzing homozygosity but are limited for heterozygosity [71]. These factors may explain why AFLP markers could not explain a high proportion of variation among H. niger individuals in Slovenia [9]. For effective evaluation of genetic diversity of wild Helleborus, it would be helpful to combine with other co-dominant markers in future study. Co-dominant markers can distinguish between homozygous and heterozygous plants. Use of AFLP has an advantage in efficiency of simultaneous analysis of a large number of bands, but a recent study showed that the polymorphic information content for AFLP is inferior to that for SSRs [72].
The genus Helleborus is becoming a very economically important ornamental plant. The classification of Helleborus has progressed from traditional morphological classification to using molecular markers. The parameters for morphological data are limited for some taxonomic classification, and it is difficult if relying solely on morphological characteristics. Therefore, molecular markers have become favored and are the most suitable means for taxonomic analysis of Helleborus.
Molecular genetics has made great progress over recent years and provides a range of techniques useful for plant conservation. This also provides support for using molecular technology in studying the taxonomy of the genus Helleborus, and provides a range of new techniques for easy and reliable identification of species in the conservation of plant genetic resources.
The genus Helleborus has received relatively little attention in genetic studies, knowledge of genetic relationships is limited, and natural variation of the genus has not yet been fully exploited. Various molecular markers are well established among many plants and their advantages and limitations are apparent, but their application to the genus Helleborus requires future study to improve genetic information. With the combination of molecular marker technology and traditional morphological approaches, such taxonomic study will improve.
As phenotypic diversity among wild Helleborus populations is relatively high, germplasm collection is useful for breeding purposes and eventual marketing [6]. Therefore, assessing genetic diversity and genetic structure from different regions in some species is increasingly vital for the genus Helleborus, as shown by previous study of genetic diversity of H. niger in natural local populations located in different geographical regions [9]. Collecting more information about existing variation and establishing genetic resources for the genus Helleborus will help horticultural breeding programs.
We express our great appreciation to Dr. Weijun Wu and Mr. Junlin Huang for help in preparing this manuscript.