1. Introduction
Hypercholesterolemia is a condition in which cholesterol concentration in the blood exceeds the standard limit. This condition increases lipid accumulation within arteria vessel wall tissues, followed by inflammation, cell death, and fibrosis, leading to atherosclerosis. Atherosclerosis causes degenerative diseases such as stroke and cardiovascular disease. It is estimated that hypercholesterolemia increases the risk of cardiovascular disease by 50% for men aged 50 years and 30% for women aged 60 years [1]. Cardiovascular disease is the number one cause of death in the world. By 2013, as much as 31% of deaths worldwide, equal to 17.3 million, is caused by cardiovascular disease. The death toll is estimated to increase to 23.6 million by 2030 [2].
So far, treatment of hypercholesterolemia includes the utilization of statin as a 3-hydroxy-3-methyl-glutaryl-CoA (HMG-CoA) reductase inhibitor. Statins drugs have been shown to lower LDL concentration in plasma and reduce the risk of cardiovascular disease [3]. However, the effectiveness of statins as drugs for cardiovascular disease is only 35%. Approximately 65% of people treated with statins still develop the adverse effects of cardiovascular disease [4]. Therefore, statin usage should be accompanied by other therapeutic interventions [5].
Commonly, drug treatment for cholesterol includes: 1) HMG-CoA reductase inhibitors such as statins, 2) bile acid sequestrants such as resins, 3) lipoprotein lipase activators such as fibric acid derivatives, 4) lipolysis and triglyceride inhibitor such as nicotinic acid derivative, and 5) cholesterol absorption inhibitor such as ezetimibe. However, some adverse effects of a statin such as headache, bowel indigestion, vomiting, nausea, and hepatotoxicity [6]. According to Bellosta [7], other side effects of statin are myopathy and rhabdomyolysis. Myopathy is the increased creatine kinase in the blood plasma that results from muscle weakness and pain. Rhabdomyolysis is the death of muscle fiber and the release of its protein content into the bloodstream. These side effects can occur within 0.1–0.2% of patients treated with a statin under medication with other drugs.
Therefore, other treatments with low side effects should be overlooked as an alternative to prevent and cure hypercholesterolemia. Diet modification is one of the safest alternatives to maintain cholesterol levels in the body. This diet modification includes consuming hypocholesterolemic food, such as probiotic products. Many studies reported the promising effect of probiotic consumption on cholesterol status. This review aims to examine the mechanism of action of probiotics in preventing the risk of hypercholesterolemia and reducing LDL cholesterol levels in the human body.
2. Probiotics
Probiotics are beneficial live microorganisms because they provide health benefits to the host after being consumed in sufficient quantities, primarily by increasing the proliferation of native digestive microflora. These properties have been found in various microorganisms, especially lactic acid bacteria (LAB). LAB is a group of bacteria that produce lactic acid as the main metabolic end product during carbohydrate fermentation. LAB is grouped into several generas, including Lactobacillus, Enterococcus, Streptococcus, Lactococcus, Leuconostoc, and Pediococcus. Generally, probiotic bacteria are non-pathogenic bacteria that are usually found in the human gastrointestinal tract, and provide protection to the intestines from pathogenic bacteria.
Probiotics are generally used as food adjuvants, and several compounds resulting from the metabolism of probiotic bacteria provide health benefits, including:
-
(a) Prevention of diarrhea by providing protection against enteropathogenic bacterial attachment on intestinal epithelial cells, and regulating the intestinal microbial environment [8].
(b) Prevention of constipation by lowering the pH in the large intestine through formation of short-chain fatty acids such as butyric acid, propionic acid, and lactic acid. Low pH can increase peristalsis in the large intestine and decrease colonic transit time [9].
(c) Stimulation of immune system through 3 (three) mechanisms, firstly by direct contact with gastrointestinal epithelial cells (IEC), which secrete various cytokines such as Interleukin-6 (IL-6) and initiate communication with other cells—surrounding immune cells. Secondly is through specialized epithelial cells namely M cells (MC) which are present throughout the digestive tract. Macrophages (MQ) or dendritic cells (DC) are the first cells under M cells to directly come into contact with probiotics or their antigen fragments and produce cytokines. And the last mechanism is that DCs in the lamina propria of the GI tract may elongate their dendrites between the IECs and may be able to directly uptake and process probiotics in the lumen of the GI tract [10].
(d) Reduce lactose intolerance by production of galactosidase enzymes which hydrolize lactose [11].
(e) Prevention of the risk of colon cancer by preventing changes in the intestinal microflora, inactivating carcinogenic compounds, competing with pathogenic bacteria, increasing the host immune response, providing anti-proliferative effects through regulation of apoptosis and cell differentiation, fermenting undigested food, inhibiting tyrosine kinase signaling pathways [12].
(f) Reduce allergy symptoms, by acting as potent activators for the innate immune system [13].
(g) Facilitates mineral absorption by increasing mineral solubility due to increased production of antibacterial compounds in short-chain fatty acids [14].
(h) Lowering cholesterol levels [15].
LAB that have potential as probiotic agents are Lactobacillus bulgaricus, Lactobacillus casei, Lactobacillus plantarum, Lactobacillus acidophilus, and Streptococcus thermophilus [16, 17, 18]. Some of the requirements needed to make a strain of LAB as a probiotic agent are that the strain is a native microorganism in the human digestive tract, has resistance to gastric acid, some antibiotics and lysozyme, remains alive after passes through the digestive tract, can grow and attach to the epithelial cells of the human intestine, has a beneficial effect on the intestine, produces acid in large and fast quantities, can create antimicrobial components in the form of bacteriocin, diacetyl, and reuterin which are effective in inhibiting other unwanted bacteria, especially pathogenic bacteria [19, 20]. According to FAO, standard probiotic products have 106–107 cfu/g active and live probiotic microorganisms [21]. Therefore, the critical factors for the effective use of probiotic properties are to maintain the viability and activity of probiotic bacteria during the storage stage and to survive the conditions of gastric acid, enzymes, and bile salts in the small intestine [22].
3. Cholesterol synthesis and regulation
Cholesterol is a major essential component for membrane cells in the human body. It is also used for synthesis of bile acids, vitamin D, and sex hormones such as testosterone, progesterone, androsterone, and estradiol. It is also essential to maintain nerve cells [4, 6]. The cholesterol concentration should be kept in the body to avoid over-accumulation. The excess cholesterol level in the blood plasma contributes to adverse health effects. The concentration of normal cholesterol within blood plasma is below 200 mg/dL. It becomes borderline within 200 to 239 mg/dL and high within 240 mg/dL or above [6]. The metabolism of cholesterol is one of the most important biochemical reactions in the body to maintain the homeostasis balance of cholesterol. The metabolism of cholesterol can be divided into cholesterol intake and cholesterol excretion. The source of cholesterol intake comes from de novo cholesterol biosynthesis in the various tissues and cholesterol absorbed by the intestine from the dietary and biliary sources. The excess cholesterol in the human body is then disposed through cholesterol excretion in unaltered form through the digestive tract and skin, and in altered form to other products (bile acids and steroid hormones) [4].
De novo synthesis of cholesterol is a complex and complicated biochemical process. It is composed of 30 cellular reactions involving more than 15 different enzymes through human cellular tissues [23, 24]. It consists of five significant steps: 1) the conversion of acetyl-CoA into 3-hydroxy-3-methyl-glutaryl-CoA (HMG-CoA), 2) the conversion of HMG-CoA into mevalonate, 3) the conversion of mevalonate into isopentenyl pyrophosphate (IPP), 4) the formation of squalene from the IPP precursor, and finally, 5) the conversion of squalene into cholesterol.
Our diet contributes to the absorption of 300–500 mg of cholesterol daily. Epithelium of the intestinal mucosal provides around 300 mg/day of cholesterol, and another 800–1200 mg/day of cholesterol comes from bile. The absorption occurs mainly on the duodenum and proximal jejunum [25]. The difference of 800 mg/day in dietary cholesterol gives only a 6% poof variation in total plasma cholesterol [26]. The total amount of cholesterol absorption in the intestine varies greatly depending on the absorption efficiency and the amount consumed daily. It is estimated that cholesterol from bile reabsorption in the intestine accounts for two-thirds of cholesterol intake originating from the intestine [27].
Wang et al. [4] suggest three significant pathways of cholesterol excretion. Reverse cholesterol transport from plasma, reverse cholesterol transport via lymphatic vessels, and transintestinal cholesterol excretion. The first two pathways are also called the biliary pathways since the cholesterol is altered to bile form, excreted to the intestine, and later as fecal matter as the end product. These two pathways use HDL as the main cholesterol scavenger. HDL can absorb the lipid from extrahepatic tissues, including the arterial wall, and transport it back to the liver for bile excretion. The HDL can move freely in the human body through the blood vessels in plasma and lymphatic vessels. Meanwhile, transintestinal cholesterol excretion is also called the non-biliary pathway since it can excrete the cholesterol directly via enterocyte onto the intestine and disposed of as fecal matter. It is estimated that under basal conditions, 35% of fecal-neutral sterol originated from the TICE pathway in non-biliary form, and 65% originated in biliary secretion [28].
As cholesterol is a hydrophobic molecule and insoluble in the water, it needs a carrier to be transported into various human body tissues. Therefore, cholesterol is packaged as water-soluble lipoproteins in the bloodstream and lymphatic vessels. The lipoprotein structure consists of the core and the shell. The center is filled with hydrophobic lipids such as cholesterol esters. This core is surrounded by a body of polar lipids (the phospholipids). The surface of the lipoprotein also contains a wide variety of apoproteins that increase molecular stability and enhance solubility. Furthermore, apoprotein also has other functions, such as activating specific enzymes for metabolism and acting as ligands that target specific receptors for certain functions.
Based on the density, the lipoproteins can be classified into four major classes, which are: 1) chylomicrons, 2) very low-density lipoprotein (VLDL), 3) low-density lipoprotein LDL), and 4) high-density lipoprotein (HDL). The first three classes play a role in lipid delivery from the liver into the tissues. Precisely, LDL delivers cholesterol to cells. Meanwhile, HDL reverses cholesterol transport, collecting excess cholesterol from various tissues into the liver.
4. Mechanism of probiotic in lowering cholesterol
Hypercholesterolemia is a condition in which serum level concentration is elevated significantly. This hypercholesterolemia is characterized by high plasma levels of LDL and low plasma concentration of HDL [4]. The total cholesterol level is considered high if it exceeds 200 mg/dL. This increase in serum is one of the predictors of dyslipidemia and increases the risk of coronary heart disease. Previous research has shown that every 1% decrease in total cholesterol can reduce the risk of cardiovascular disease by 2% [29]. Consequently, monitoring and reducing total cholesterol levels is essential to do. Dietary regulation and diet modification are one way to help suppress the increase in blood lipid levels. The recommended dietary arrangement is to limit the consumption of foods containing cholesterol and saturated fat. In addition, eating foods that have benefits for lowering cholesterol levels is also necessary.
Several theories of how probiotics remove host cholesterol have been put forward. They are mainly: 1) the ability of probiotics to convert cholesterol into coprostanol, 2) the deconjugation of cholesterol by probiotics enzyme, which leads to cholesterol precipitation and assimilation, 3) the probiotics regulation of protein expression related to cholesterol metabolism, and 4) the production of SCFA by probiotics that can increase apolipoprotein expression responsible for reverse cholesterol transport [30, 31, 32, 33].
4.1 Conversion of cholesterol to coprostanol
Three pathways are proposed regarding the conversion of cholesterol to coprostanol by probiotics [33]. These three pathways are shown in Figure 1. The first is a direct pathway (Figure 1 a), which reduces the 5.6 double bonds of cholesterol into a single bond, resulting in coprostanol synthesis. The second pathway is the isomerization of cholesterol to allocholesterol, which is further reduced by species of probiotics into coprostanol (Figure 1b). However, only a few pieces of evidence support the second pathway. The third one is an indirect pathway via the production of intermediate products, including cholestenone and coprostanone (Figure 1 c). According to Juste and Gerard [31], the third pathway is the most substantial with ample evidence. It is reported that the identification of neutral fecal sterols has found the presence of coprostanol, cholestone and coprostanone in human and animal faeces. All three metabolites are absent in germ-free animals.
The indirect pathway of cholesterol to coprostanone consists of three steps (Figure 1c). The first step is the oxidation of cholesterol to cholestenone. This oxidation is catalysed by the cholesterol oxidase enzyme and steroid dehydrogenase. The enzymes first oxidise the cholesterol and then isomerise the double bond in the steroid ring of the cholesterol. The second step is the alteration of cholestenone into coprostanone. This process requires steroid reductase enzyme and Nicotinamide adenine dinucleotide hydrogen (NADH) as cofactors. Finally, coprostanone is converted into coprostanol. It is suggested that this last step requires a 3-beta-hydroxysteroid dehydrogenase enzyme [31].
Although the multi-omics study shows that there are frequent and abundant microbiome resources in human biota that can produce 3-beta-hydroxysteroid dehydrogenase enzyme, only a few active strains exhibit the ability to convert cholesterol into coprostanone has been isolated. It indicates that the conversion process is carried out by several microbiomes that require the challenging task of isolation [31]. Lye et al. [32] have isolated four probioticstrains from the human gastrointestinal tract, such as L. acidophilus ATCC 314, L. acidophilus FTCC 0291, L. acidophilus FTCC 0411, L. bulgaricus FTDC 1311, and L. casei ATCC 393. The four probiotic bacterial strain isolates can lower cholesterol because they can produce 3-beta-hydroxysteroid dehydrogenase enzyme to convert cholesterol into coprostanone and coprostanol [32].

Sekimoto et al. [30] are the first to report an inverse correlation between cholesterol level in the serum and coprostanol content within faeces. This finding suggests that converting cholesterol into coprostanol can reduce the total cholesterol level within blood plasma. The conversion of cholesterol into coprostanol is catalysed by cholesterol reductase [31]. Lactobacillus strain can produce cholesterol reductase inside and outside of the cell membrane. Therefore, the alteration of cholesterol into coprostanol can occur inside and outside the cell can be intracellularly and extracellularly. Coprostanol is less absorbed than cholesterol in the human intestine. It causes an increase in fecal excretion and cholesterol reduction within blood plasma [32].
4.2 Cholesterol deconjugation by Bile Salt Hydrolase (BSH) enzyme and cholesterol assimilation
Bile salt hydrolase (BSH) is an enzyme expressed mainly through intestinal bacteria living in the bile environment, such as Bifidobacterium spp., L. acidophilus, L. plantarum, and so on. However, it is not found in strains that live in non-bile environments, such as S. thermophilus and L. delbruecki [34]. This enzyme is produced outside the cell membrane, can tolerate oxygen, and has the highest activity in an acidic environment, which is 5–6. The action of BSH is also inducible by the density of bile acid as a substrate. A higher concentration of bile acid can increase the activity of BSH [35]. This enzyme can deconjugate the bile salts, thus negating the toxicity effect of bile salt.
The coprecipitation of deconjugated bile salt occurs at low pH. Initially, the pH of the intestine is neutral to slightly alkaline, which is unsuitable for coprecipitation reaction. However, dietary fibre fermentation by intestinal microbe produces short-chain fatty acids. Thus, the pH becomes acidic and suitable for coprecipitation reaction [36]. After coprecipitation, the deconjugated bile salt is removed from enterohepatic circulation and excreted in the faeces. This, in turn, will lower the emulsification and absorption of cholesterol intake. The new bile salts are formed by the body using cholesterol storage. Specifically, the increased BSH activity reduces the farnesoid X receptor (FXR) activity that promotes the downregulation of small heterodimer partner (SHP). This downregulation of SHP activates the liver X receptor (LXR), promoting reserved cholesterol transport into bile. Then, bile salts are synthesised, and the cholesterol concentration of the body is reduced [37]. The mechanism process of cholesterol deconjugation is shown in Figure 2.
Furthermore, bile salts are needed as the prerequisite for assimilation reaction, mainly through the modification of membrane lipid composition [35,38]. Murga et al. [39] showed that cultured bacteria grown under a bile salt environment contained extraordinary fatty acids such as C 18:0, 10 OH, and C 18:0, 10-oxo on the plasma membrane. The changes in plasma lipid act as a homeostasis mechanism to preserve flexibility and rigidity of the membrane cell, thus increasing the cells' survivability [32].
This assimilation reaction is not pH-dependent and generally occurs at a higher reaction rate in the growth phase of cells. Cells within the resting phase show no significant cholesterol assimilation activity. Moreover, Jarocki et al. [40] found that the release of deconjugated bile acids by BSH led to increased hydrogelation of certain bile salts. This phenomenon may significantly impact many factors, such as biofilm formation, which support the bacterial colonization of the human digestion tract. It also has been shown that BSH-positive Bifidobacterium is more tolerant to antibiotic treatment. Therefore, it is suggested that BSH also increases the survivability of the strains through the formation of a natural hydrogel shield.
4.3 Probiotics alter protein expression related to cholesterol biosynthesis
Probiotics have several pathways of protein expression regulation related to cholesterol metabolism as seen in Figure 3. Chen et al. [41] showed that live LAB could reduce the expression of HMGCR via the influence on the nuclear factor-κB (NF-κB) family in human liver HepG2 cells. The reduced HMGCR expression can lower the biosynthesis of cholesterol. HMGCR is involved in converting HMG-CoA into mevalonic acid, the initial stage of cholesterol synthesis [41]. NF-κB is a transcription factor that regulates the broad spectra of genes involved in the immune system, inflammation process, cell survival, cell proliferation, and cell migration [42, 43]. NF-κB is composed of five family structures: p50, p52, p65, RelB, and c-Rel [44]. They bind to specified DNA elements and mediate target gene transcription. This NF-κB can be stimulated via different stimuli, including cytokines, DNA damage, and viral and microbial metabolites. According to Kim et al. [45], gene expressions can be stimulated by some lab components, such as polysaccharides on the cell wall, lipopolysaccharide, and proteins.
Chen et al. [41] also show that two main structures of NF-κB that play a role in the downregulation of HMGCR expression are p50:p65 and p50:p50. The downregulation happens within human liver HepG2 cells through the NF-κB binding site at nt-265 bp. Lew et al. [46] found that Lactobacillus plantarum DR7 can regulate the expression of HMGCR via the adenosine monophosphate-activated protein kinase (AMPK) activation pathway. AMPK regulates the metabolism of energy in the body. It is a crucial regulator for mitochondrial biogenesis, protein synthesis, glucose, and lipid metabolism [23, 47]. This upstream kinase is activated through phosphorylation, especially the phosphorylation of threonine-172 (Thr172) in the alpha subunit activation loop. The activated AMPK will increase the production of ATP and decrease its utilization [48, 49]. Furthermore, the activation of AMPK has been shown to inhibit the activity of HMGCR via phosphorylating serine-871 (Ser871) both in vitro and in a cell line [50].
Lactobacillus strains have been shown to regulate the mRNA expression levels of liver enzymes related to cholesterol metabolism, including the down-regulation of acyl-CoA: cholesterol acyltransferase (ACAT) and the upregulation of cholesterol 7α-hydroxylase (also known as cytochrome P450 7A1 or CYP7A1) [51]. These cholesterol-lowering probiotics possess a therapeutic effect on non-alcoholic fatty liver disease (NAFLD) in rats by up-regulating Cholesterol 7 alpha-hydroxylase (CYP7A1), LDL-R, FXR mRNA, and Peroxisome proliferator-activated receptor alpha (PPAR-α) protein produced in the process of fat metabolism while down-regulating the expression of HMGCR, Peroxisome proliferator-activated receptor gamma (PPAR-γ) and sterol regulatory element-binding protein 1c (SREBP-1c), and through normalizing the intestinal dysbiosis and improving the intestinal mucosal barrier function [52]. CYP7A1 is expressed in the endoplasmic reticulum of hepatocytes and is regulated by the bile acids in terms of cholesterol homeostasis [53]. LDL-r is a receptor that picks up the LDLs circulating in the bloodstream. Therefore, it lowers LDL in blood plasma. FXR is a nuclear hormone receptor that when activated, can lower blood cholesterol via increasing the macrophage reverse cholesterol transport (RCT) [54]. The Sterol Regulatory Element Binding Proteins (SREBPs), which regulate the transcription of HMG CoA reductase, also hold the transcription of genes encoding many other enzymes in the cholesterol biosynthetic pathway including HMG CoA synthase farnesyl diphosphate synthase, and squalene synthase [55].

SCFAs are fatty acid hydrocarbon groups comprising C1 until C6 hydrocarbon bones. The members of this group are formic acid (C1), acetic acid (C2), propionic acid (C3), butyric acid (C4), valeric acid (C5), and hexanoic acid (C6) [62]. In the human digestion tract, the majority (95%) of SCFAs found are acetic acid, propionic acid, and butyric acid. Formic acid, valeric acid, and hexanoic acid comprise the rest (5%) of SCFAs [56]. SCFAs are produced in the colon and small intestines. However, only a tiny amount of SCFAs are found in small intestines since they are mainly made in the caecum and proximal colon [57]. The concentration of SCFAs in the intestinal lumen is between 2–30 mM. This concentration mainly consisted of acetate (10–20 mM) with only a tiny fraction of butyrate and propionate (1-5mM). SCFAs are efficiently absorbed into the colon and transported into the liver. It is estimated that only 5–10% of initial SCFAs are secreted into the feces [58].
The absorbed SCFAs are then metabolized in three sites: colonocytes, hepatocytes, and muscle cells. Butyrate is utilized as the primary energy source of colonocytes. It is estimated that 60–70% of colonocyte energy comes from butyrate metabolism. Butyrate, along with propionate, is also metabolized by hepatocytes. Hepatocytes utilize them for the gluconeogenesis process [59]. Eventually, muscle cells metabolize acetate as a residual compound for their additional energy sources [60]. The metabolism of SCFA is correlated with the concentration of Apolipoprotein AI (ApoA-I) produced in the body. ApoA-I is a lipoprotein that composes 70% of HDL particles [61]. ApoA-I is mainly produced in the liver (70%). The rest of ApoA-I is made in the intestine (30%). On the HDL particle, ApoA-I plays an essential role in RCT (reverse cholesterol transport) process as the acceptor of the cholesterol. It is due to its function as a ligand for the ATP-binding cassete transporter A1 (ABCA1) and ATP-binding cassette sub-family G member 1 (ABCG1) [62].
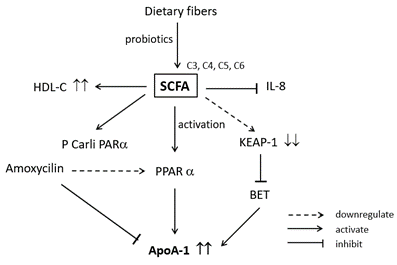
Zhao et al. [63] showed that SCFAs administration increases the HDL-C concentration in the serum of the experimental mouse. However, serum HDL-C concentration alone is not strongly correlated with increased Reverse Cholesterol Transport (RCT) activity and decreased cardiovascular diseases (CVD) risk. Other factors should be considered, such as the size of HDL and its functionality. Therefore, many researchers focused their studies on the mechanism of SCFAs to increase the serum HDL-C concentration. Kaptein et al. [64] and Malle et al. [65] found that the addition of butyrate enhanced the secretion of ApoA-I protein. Kaptain et al. [64] showed that adding 2 mM butyrate in human hepatoma cells (HepG2) increased the production of ApoA-I by 2.4 folds. This secretion happened after 48 hours of administration, and it was found that the secretion of ApoA-I mRNA also increased by 2.3 folds.
Therefore, they hypothesized that the mechanism of ApoA-I secretion happened via transcriptional regulation. In agreement with this result, Malle et al. [65] also found that the addition of butyrate increased the production of ApoA-I protein by threefold and the secretion of ApoA-I protein by 2.5-fold in HUH-7 hepatoma cell line compared to the control. Moreover, Tayyeb et al. [66] concluded that the enhanced ApoA-I transcription in HepG2 cells was not only by butyrate but also by other SCFAs. However, the increase of ApoA-I secretion in the presence of SCFAs is not found in the human colon adenocarcinoma (Caco-2) cells [62].
As for the mechanism, the whole process is shown in Figure 4. Based on Figure 4, it is suggested that SCFAs downregulate the Kelch-like ECH-associated protein 1 (KEAP1) and activate peroxisome proliferator-activated receptor alpha (PCarliPARα). KEAP1 downregulation inhibits bromodomain and extra terminal (BET), increasing ApoA-I expression. Meanwhile, the activation of PPARα also increases the expression of ApoA-I. It has been found that the secretion of interleukin 8 (IL-8), a cytokine that activates neutrophils on inflammation reaction, is significantly inhibited by the presence of SCFAs such as propionate, butyrate, and valerate. As for the effect of the antibiotics, amoxicillin has been found to suppress the expression of ApoA-I by lowering PPARα activation on HepG2 cells [62].




