2023 Volume 11 Pages 259-270
2023 Volume 11 Pages 259-270
Sorghum [Sorghum bicolor (L.) Moench] synthesize cyanogenic glycoside known as dhurrin. Fresh vegetative sorghum will rapidly liberate hydrogen cyanide from dhurrin upon disruption of cells in which they are stored in the plant tissue. Dhurrin production has been reported in Sudan grass (Sorghum sudanense), Johnsongrass (Sorghum halepense (L.) Pers) and Columbus grass (Sorghum almum). It is synthesized from amino acid tyrosine by the sequential action of two cytochrome P450 enzymes (CYP79A1 and CYP71E1). Dhurrin is believed to play a role in defense against pathogens, insect pests, herbivores and in regulation of metabolic processes. The metabolic processes highlighted in this review are those associated with plant growth and development and regulation of germination. It appears that dhurrin production in sorghum could be developmentally and environmentally regulated and controlled at the transcriptional level. This review focuses on dhurrin synthesis pathway, roles in sorghum, the main signaling molecule and research gaps.
Cyanogenic glycosides are phytotoxins synthesized as secondary metabolites in plants and consist of α-hydroxynitrile aglycones and a sugar moiety [1]. The toxin is naturally occurring in some plant species and is widely distributed in the plant kingdom, being present in more than 2,500 species [2]. They are derived from aliphatic protein amino acids which include L-valine, L-isoleucine, or aromatic amino acids L-phenyl alanine and L-tyrosine or from aromatic non-protein amino acids (2-cyclopentenyl)-glycine as precursors [3]. Most reports associate the toxin synthesis with the plant’s protective defense mechanism against predators. However, the trigger for synthesis and the role of the metabolite in plants under abiotic stress remains vaguely understood [4, 5, 6]. The metabolite is known to produce an undesirable taste which deters some predators and also releases highly potent hydrogen cyanide following cell disruption during foraging by animals [7]. Cyanogenic glycosides produced by cultivated plants include: linamarin in cassava (Manihot esculenta) and in red clover (Trifolium repens), dhurrin in Sorghum bicolor, amygdalin in rosaceous plants and lotaustralin in birdsfoot trefoil (Lotus corniculatus) [1] (Figure 1).
Sorghum is becoming a significant crop in most parts of the world due to its resilience to increasing soil moisture deficit occasioned by climate change. It is a suitable livestock feed that can be fed directly as green chop and/or grazed as forage. However, high levels of dhurinn can be fatal to livestock and this limits its use among livestock farmers. This review discusses the proposed roles of dhurinn in sorghum and possible triggers with the aim of shedding light on how it can be managed to make sorghum safe for livestock.

Figure 1: Chemical structures of cyanogenic glycosides produced by cultivated plants
Cultivation of forage sorghum (Sorghum bicolor (L.) Moench) is widely practiced over other forage crops due to its adaptability to wide variations in soils and climatic conditions. Sorghum also has desirable attributes which include fast growth, high biomass and drought tolerance [8]. Despite these favourable qualities, the use of sorghum as forage is limited by high levels of dhurrin in its tissues which poses a health threat to ruminants. Evidence of dhurrin in sorghum is widely reported [4, 8, 9, 10, 11, 12]. Besides Sorghum bicolor (L.) Moench, dhurrin is also found in Sudan grass (Sorghum bicolor cv. Sudanese), Johnson grass (Sorghum halepense (L.) Pers) and Columbus grass (Sorghum x almum Parodi). The concentration of the cyanogenic glycoside above a threshold of 200 ppm (equivalent to 20 mg%) on a fresh weight basis can kill a ruminant [13]. Dhurrin content varies among sorghum genotypes and even within genotypes, there is variation across crop developmental stages.
The synthesis of dhurrin is controlled by three genes, CYP79A1, CYP71E1 and UGT85B1 which encode enzymes mediating dhurrin production [14]. The enzymes involved are cytochrome P450 and glycosyltransferase. Researchs have demonstrated the existence of variation in dhurrin content among genotypes, crop developmental stages and climatic conditions [1, 12]. The variation in dhurrin content among sorghum genotypes could be associated with the differences in expression of CYP79A1, CYP71E1 and UGT85B1 genes in each genotype. With regard to growth developmental stages, dhurrin content usually progressively increases after crop emergence and attains maximum levels at the early reproductive phase, followed by a progressive decline. It is widely established that a high concentration of dhurrin is at the early vegetative growth stages [7, 8, 15, 16]. The higher levels in young plants have been linked to a ready supply of N [17], expression of responsible genes [18] and the expression of enzyme-encoding genes CYP79A1 and CYP71E1, which are the highest at early crop growth stages [12]. The early growth stage is associated with active growth with canopy development which normally triggers increased uptake of nutrients from the soil. The canopy demand for plant assimilates is usually at its highest when the canopy is fully attained and that is at or towards the transitional developmental stage from vegetative to the reproductive phase. Nitrogen accumulation in sorghum canopy, which is largely composed of leaves and leaf sheaths, increases progressively to maximum towards the end of vegetative or at the early reproductive phase, followed by a decline [19]. Since dhurrin is a storage form of N in plants [11, 12], the physiological state of sorghum at different developmental stages could explain the observed variation in its content.
Dhurrin was reported to be localized in cell vacuoles within the epidermal cell [20]. However, through Raman hyperspectral imaging, dhurrin has been found surrounding epidermal, cortical and vascular tissue [21]. The compound was seen to be localized within the cytoplasm, although it was not possible to associate it with particular cell organelles or vesicles [12]. Dhurrin can be hydrolyzed to produce hydrogen cyanide in the presence of β-glucosidase and α-hydroxynitrilase enzymes [22] which are localized in the chloroplast and cytosol of mesophyll cells. The spatial separation of dhurrin and the enzymes limits any possible reaction which only occurs when the leaves are injured. Foraging on sorghums by animals results in plant cell disruption which facilitates contact of dhurrin with the enzymes leading to the release of hydrogen cyanide (HCN) which is toxic to ruminants (Figure 2) [22]. The reaction mainly occurs in the rumen after ingestion of masticated sorghum and the generated HCN is rapidly absorbed into the bloodstream through the rumen walls. Hydrogen cyanide inactivates cellular respiration and the animal can die suddenly within 1–2 hours after ingestion of sorghum fodder with high amounts of dhurrin. Concentrations of about 200 ppm (on a wet basis) and above are lethal to animals [13], but even though lower dhurrin concentrations may not be harmful to the animals, it is not allowed to circulate in the system. The low levels of dhurrin in the bloodstream can rapidly be detoxified in the liver by the rhodanase enzyme which converts cyanide to thiocyanate (SCN). Rhodanase is a mitochondrial enzyme that also facilitates the transfer of sulfur atoms from a sulfane-sulfur donor to cyanide (Figure 2).
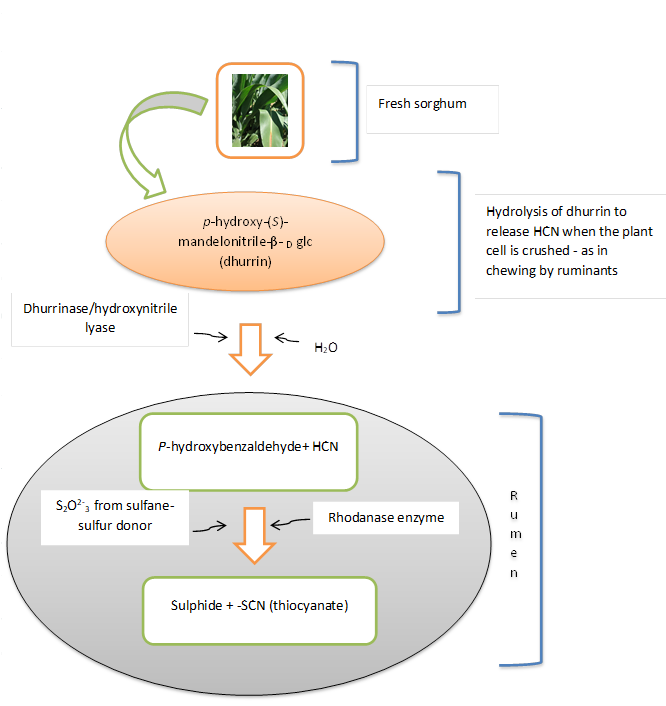
Figure 2: Hydrolysis of dhurrin to liberate HCN and dissociation of HCN in the rumen to release thiocyanate ion (Modified from Busk and Moller [12])
Dhurrin is synthesized from amino acid tyrosine by a sequential action of two cytochrome P450 enzymes: CYP79A1 and CYP71E1 [12, 23]. The biosynthesis follows a general pathway that involves the conversion of tyrosine to hydroxyphenyl-acetaldoxime via the two multifunctional cytochrome P450 enzymes (CYP79A1 and CYP71E1). CYP79A1 catalyzes the conversion of tyrosine to p-hydroxyphenyl acetaldoxime while CYP71E1 catalyzes the conversion of p-hydroxyphenylacetaldoxime to S-hydroxymandelonitril [23] as shown in Figure 3.

Figure 3: The biosynthetic pathway for the cyanogenic glycoside dhurrin in sorghum: Adapted from Busk and Moller [12]
The biosynthesis of dhurrin begins with the conversion of tyrosine to (Z)-p-hydroxyphenylacetaldoxime by the substrate-specific CYP79A1 enzyme [12, 24]. The second step involves the subsequent conversion of (Z)-p-hydroxyphenylacetaldoxime to (S)-p-hydroxymandelonitril by CYP71E1, an enzyme with low substrate specificity. The final step is the conversion of (S)-p-hydroxylmandelonitrile to dhurrin by a soluble UDP-Glc p-hydroxy-mandelonitrile glycosyltransferaseT85B1 (Figure 3). These reactions take place in the cytosolic surface of the endoplasmic reticulum, followed by the transfer of dhurrin to the vacuole of the epidermal cells where it is stored. Most chemical defense metabolites in plants are toxic to the plant and this could be the reason why dhurrin is transported from the cytoplasm to the vacuole where the pH is low to favour the reduction in self-toxicity [25]. Tissue disruption in sorghum facilitates contact of vacuolar dhurrin with glucosidase from the chloroplast of mesophyll cells and hydroxyl nitrile lyase from the cytosol of mesophyll cells where dhurrin is broken down to hydrogen cyanide and p-hydroxybenzaldehyde [26] as elaborated above.
As discussed in the previous sections of this review, recent studies on cyanogenic glycoside metabolism indicate that these compounds are products of normal plant metabolism. They are derived from amino acids and are used as defense against pathogens, insect pests and herbivores, and as a regulation of metabolic processes. Among the metabolic processes highlighted by various authors include those associated with response to environmental stress, storage and transportation of nitrogen, and regulation of germination.
4.1 Dhurrin as a defense mechanism against phytopathogens and herbivoresDhurrin is hydrolyzed through cleavage of β-glucosidase to provide the corresponding hydroxynitrile, which spontaneously dissociates into sugar, p-hydroxybenzaldehyde and a toxic hydrogen cyanide gas (HCN) (Figure 2) at pH values above 6 [27]. The toxic HCN produced during hydrolysis of dhurrin acts as phytoanticipin in plants which is believed to provide immediate defense against pathogens or insects attack [4]. For instance, induced cyanogenesis in barley leaf by single-cell expression of dhurrinase-2 from sorghum, led to a significant reduction of powdery mildew colonization which suggests that hydrogen cyanide in sorghum could hinder fungal diseases in plants [27]. Cyanogenic glucosides have also been shown to reduce herbivore damage on crops [28]. Despite the evidence supporting the deterrent effect of cyanogenic glycosides, a number of studies have shown little or no effect of the compound on herbivores. For instance, the selection of chanana shrub (Turnera ulmifolia) as a source of food and as an oviposition site by the American butterfly (Euptoieta hegesia), was found to be aided by some concentration of cyanogenic glycosides [29]. Gleadow and Woodrow [30] reported a significant inverse correlation between cyanogenic glycoside content in the young leaves of Eucalyptus cladocalyx and the amount of damage by herbivores. Dhurrin is also reported to be an oviposition activator to insect pests in certain instances. In a study to evaluate 14 sorghum varieties for susceptibility to Atherigona socata and Chillo partellus, it was observed that susceptible cultivars; CSH-1, Swarna and IS 10795 had more dhurrin which suggests dhurrin as an oviposition activator for the pests [31].
These contentious findings point to some underlying role of dhurrin in sorghum, other than defense against insect and pathogen attacks, and that their synthesis could be triggered by some factor(s). It is not wholly convincing that sorghum produces the compound for defense against insect and pathogen attacks. The herbivores or sucking insects still find the plants palatable. The herbivores only succumb to the toxic chemical effect after ingesting and any other animal or insect will still forage on the target, oblivious of the dangers. The above observations could be incidentals and the reason(s) for the production of dhurrin in sorghum is much more than is currently claimed.
4.2 Role of hydrogen cyanide in nitrate assimilationThe hydrogen cyanide produced when dhurrin is hydrolyzed plays a key role in primary plant metabolism. A recycling pathway for the products of cyanogenic pathway has been proposed following research evidence [32]. The synthesized cyanogenic glycosides avoid the release of HCN following cyanogenesis. Instead, the reduced nitrogen and carbon are recovered from the nitrile group of ammonia and carbondioxide and are utilized in primary plant metabolism.
Busk and Møller [12] also demonstrated a link between dhurrin increase in sorghum seedlings and an increase in nitrate supply. The increase in dhurrin following nitrate supply suggests dhurrin is a nitrate sink in sorghum. Dhurrin is usually high during seedling growth stage where it constitutes 30% of dry mass of the shoot and decreases as the plant matures. It is possible that sorghum is utilizing stored nitrogen in the form of dhurrin during its growth and developmental stages [33]. In an experiment with a cyanogenic sorghum line, Jørgensen et al. [34] observed a significant reduction in plant metabolism when cyanogenic glycoside was removed at a certain crop growth stage. A study conducted on the growth and nitrogen partitioning of two sorghum mutants with varying cyanide content seems to confirm the observation. Sorghum cyanide deficient (tcd1) mutant which does not synthesis dhurrin and another sorghum mutant (acdc1) with cyanide but decreasing as the plant ages, were grown at different concentrations of nitrogen. Higher growth of acdc1 and slower growth in tcd1 plants was observed during early developmental stages, which suggests an involvement of dhurrin in growth and development of sorghum [35, 36]. The involvement of dhurrin in growth of sorghum is clear and can be attributed to the proposed recycling pathway in which nitrogen and carbon are released to support metabolism.
In a related study, results by Jørgensen et al. [34] show retarded growth, slender stems, long internodes, small leaves and poor root development in cassava when cyanide content in the plant was reduced to <25%. The growth of cassava was partly restored when nitrogen was added to the growth medium, signifying the influence of cyanide in the internal nitrogen supply.
4.3 Role of hydrogen cyanide in plant response to stressThe physiological release of ethylene in plants is associated with biotic stress as in pathogenic attack and abiotic stress as observed in mechanical injury, flooding and/or drought [37]. Siegen and Bogatek [26] argued that since hydrogen cyanide is a co-product of ethylene synthesis, it could be playing a significant role in responding to stress stimuli alongside ethylene. Enhanced synthesis of cyanogenic glycosides as observed in plants under stress conditions could suggest a strategy by the plant to use it as a source of nitrogen for metabolism when photosynthesis is low as occasioned by soil water deficit [38].
Ethylene is an important plant hormone that is involved in root growth, stem elongation, senescence, fruit ripening and response to biotic stress stimuli, including pathogen attacks [39]. Similarly, Bjarnholt et al., [38] reported hydrogen cyanide as playing a regulatory (perhaps signaling) function in many physiological processes: seed germination, nitrate assimilation, or plant responses to some environmental stimuli. The role of cyanogenic glycoside in plant stress regulation is not fully explored even though evidence exists that supports their dual toxic and signaling role. Cyanide that is produced during ethylene synthesis is normally detoxified in the plant tissues [26] hence its toxic role during this process is nullified. In other studies, cyanide production in stressed plants has been shown to be stimulated by 1-aminocyclopropane-1-carboxylic acid synthase (ACS) activity in ethylene biosynthesis and was reported in soybean (Glycine max) seedlings exposed to 2,4-dichloro phenoxyacetic acid (herbicide) and barnyard grass (Echinochloa crus-galli), exposed to 3,7-dichloro-8-quinolinecarboxylic acid [40, 41]. The concentration of endogenous cyanide in these plants was elevated up to 30-50 mM due to herbicide-stimulated ACS activity during ethylene biosynthesis. Thus, a temporary increase in HCN concentration in a small region of plant tissue may take place and in this case, cyanide may act as a signaling cellular molecule, which triggers the events consequently leading to the acquisition of stress resistance [32]. In tobacco leaves, it has been demonstrated that a non-lethal concentration of cyanide enhances the resistance to tobacco mosaic virus (TMV) [42]. Similar results were also reported in experiments with Arabidopsis thaliana which showed that cyanide can induce resistance to turnip vein-clearing virus (TVCV) [43]. Nahrstedt and Rockenbach [44] performed a study in Olinia species and results indicated that cyanogenic glycosides may be involved in modulating oxidative stress by scavenging reactive oxygen species such as hydrogen peroxide (H2O2) in chlorotic leaves. The role of cyanide in stress tolerance could be by providing a ready source of N to mitigate oxidative stress [27, 32, 45]. The signaling role of cyanogenic glycosides in ameliorating abiotic stress is not fully understood.
Environmental growing conditions are a significant abiotic contributor to dhurrin concentration in sorghum. Soil water deficit as one of the abiotic stresses has been shown to influence dhurrin content in sorghum. Drought stress heightens dhurrin content in sorghum [33, 46], but no suggestion as to whether it is involved in mitigating the stress. Water-stressed plant experience reduced growth and photosynthesis, the latter suffers severe reduction and could be halted if the stress prolongs [47]. Could the surge in dhurrin during water stress be explained on the basis of N assimilation? Nitrogen assimilation in plants requires a steady supply of oxoglutarate and glutamate [48], both of which are readily available under optimal photosynthesis. The reduction of photosynthesis under severe water deficit serves to reduce the carbon skeletons precursors, one of which is oxoglutarate, which is needed for N assimilation and hence reduced N demand in the process (Figure 4). This could partly explain the observed increase of dhurrin under drought stress. However, there is a need for confirmatory studies.
Besides soil moisture deficit, there are indications that growing temperature influence dhurrin levels in fodder sorghum (ongoing study), with high temperatures triggering an increase in dhurrin compared to low temperatures. A sorghum genotype grown in two locations of different temperature regimes will present different dhurrin levels and thus calls for a cautious recommendation of fodder sorghum on the basis of HCN-p content across locations with varying temperatures. Could the differences in dhurrin accumulation as influenced by temperature point to some response to environmental coping mechanism? The role played by cyanogenic glycoside in nitrogen metabolism and the influence of abiotic factors need more investigation, especially for the agronomic benefit of sorghum as fodder.

Figure 4: Possible dhurrin depletion process through the nitrogen assimilation pathway in plants. Accumulation of dhurrin under abiotic stress could be linked to the shortage of products of photosynthesis as a consequence of stress factors. GS, glutamine synthetase; GOGAT, glutamate synthase (Modified from Lu et al., [49])
Dhurrin production in sorghum is both developmentally and environmentally regulated but also varies between and within sorghum lines [17]. The enzymes that are involved in dhurrin synthesis pathway (Figure 2) are coded for by 3 structural genes in a gene cluster containing SbMATE2, which encode a transporter of multidrug and toxic compound extrusion (MATE), co-expressed with the biosynthetic genes [50]. Similar results by Nielsen et al. [11] indicates that the biosynthetic gene cluster encodes a MATE transporter (Sobic.001G012600) and a glutathione S transferase (GST) named as SbGST1 (Sobic.001G012500) of the plant specific phi sub family. These genes have been confirmed in a 9-day-old sorghum seedling shoots and found to be co-expressed with dhurrin biosynthetic genes with their expression being enhanced by abscisic acid and osmotic stress treatment [50]. The GSTs also function as a carrier protein for anthocyanins and other reactive molecules for vacuolar sequestration [51]. Such reactive molecules protect plants against various biotic and abiotic stresses [52] partially due to their powerful antioxidant properties. Does this suggest a protectant role of dhurrin against abiotic stress – since its production is triggered by factors similar to those that trigger the production of other plant antioxidants? More work is needed to unveil the involvement of dhurrin in abiotic stress such as moisture deficit and extreme temperatures during crop production and its implication for fodder sorghum.
Cyanogenic glycosides seem to play various roles in plants including acting as respiratory toxins, feeding deterrents, and mobile nitrogen storage compounds in the seeds of several plants. The surge in dhurrin production in sorghum during adverse environmental conditions, suggests that the compound could be playing a role in abiotic stress. Some work is needed to shed light on the possible role of dhurrin in plants under abiotic stress, factors that trigger increased production of dhurrin in sorghum, and their accompanying functions at different stages of plant development.
The authors have not declared any conflict of interest.
We are grateful to the Centre of Excellence in Sustainable Agriculture and Agribusiness Management (CESAAM) project, Egerton University for providing funds for this research. This work was part of the Ph.D Thesis of the first author.