2025 Volume 13 Issue 2 Pages 1-19
2025 Volume 13 Issue 2 Pages 1-19
The intricate dance between plants and their surroundings is governed by a complex set of adaptive behaviors, many of which are orchestrated at the epigenetic level. These epigenetic processes, including DNA methylation, modifications to histones, and the action of small RNAs, are crucial in adjusting how plants express their genes when faced with environmental stresses, both living (biotic) and non-living (abiotic). These changes not only help plants manage current adversities but also allow them to retain a memory of past challenges, potentially offering better protection against similar threats in the future. The advent of advanced epigenomic technologies and the introduction of CRISPR tools for epigenetic editing have greatly enhanced our grasp of the epigenetic mechanisms that underpin plant responses to stress. This review explores the intricate world of epigenetic regulation in plants, especially how it influences their ability to withstand stress, spotlighting significant discoveries and considering the role of epigenetic inheritance across generations in plant adaptation and evolution. Moreover, it discusses how integrating epigenomic information with other types of omics data can reveal detailed regulatory networks. Looking ahead, the review considers the hurdles and opportunities in applying our epigenetic knowledge towards improving crops, with a special focus on the promise of epigenetic engineering in boosting plant defense mechanisms against environmental challenges. This could play a pivotal role in promoting sustainable farming and ecosystem management. In sum, this paper emphasizes the vital importance of epigenetics in plant science, offering exciting prospects for enhancing agricultural methods and deepening our understanding of ecological interactions.
The ability of plants to flourish across a wide array of environments, often under severe conditions, showcases their extraordinary adaptability [1, 2, 3]. Yet, the growing threats posed by climate change, including rising temperatures, shifting rainfall patterns and new pathogens, present plants with challenges like never before [4, 5, 6, 7, 8, 9]. These environmental stressors not only jeopardize plant survival but also threaten global food security, considering our dependence on key agricultural species. Abiotic stresses such as drought, salinity and extreme temperatures directly affect plant physiological functions, reducing their growth and yield [10, 11]. Biotic stresses, including attacks by pathogens and herbivores, force plants into a relentless fight against damage and disease, further depleting their resources and diminishing crop yields [12, 13].
In this context, epigenetics has become a vital field for understanding how plants navigate and counter these stresses. Epigenetics refers to the study of inheritable changes in gene function that occur without alterations to the DNA sequence [14]. These changes, resulting from modifications to DNA and histone proteins, can affect chromatin structure and gene expression in ways that are both dynamic and reversible [15, 16]. Key epigenetic mechanisms include DNA methylation, histone modification and the activity of small RNA molecules [17, 18, 19], allowing plants to adjust their gene expression in response to environmental signals, thus enabling a more versatile and resilient stress response [20, 21, 22].
Exploring plant epigenetic responses to stress is not just a theoretical pursuit but is increasingly seen as critical for agricultural innovation [23]. By gaining insights into how epigenetic mechanisms drive stress responses and adaptation, we can identify new methods for enhancing crop resilience [24, 25]. This knowledge is crucial for breeding programs and biotechnological approaches aimed at creating stress-tolerant plant varieties, essential for ensuring agricultural productivity amid climatic fluctuations [23, 26].
Moreover, understanding the epigenetic landscape of plant stress responses sheds light on the possibility of transgenerational stress memory. This could allow plants to ‘prime’ their progeny for stronger stress responses based on the experiences of their parents, offering profound possibilities for plant breeding and crop enhancement strategies [23, 26, 27, 28]. This approach could lead to the development of crops that are not only resilient to current environmental pressures but also adaptable to future challenges.
The investigation into plant epigenetic adaptations to stress marks a new horizon in plant biology and agricultural research, opening up exciting prospects for boosting crop resilience. This is vital for safeguarding food security in the face of an increasingly unpredictable climate. This review aims to unravel the mechanisms behind plant epigenetic adaptations to stress, focusing on DNA methylation, histone modifications and small RNAs, and to explore how these adaptations can inform crop improvement and promote sustainable agriculture.
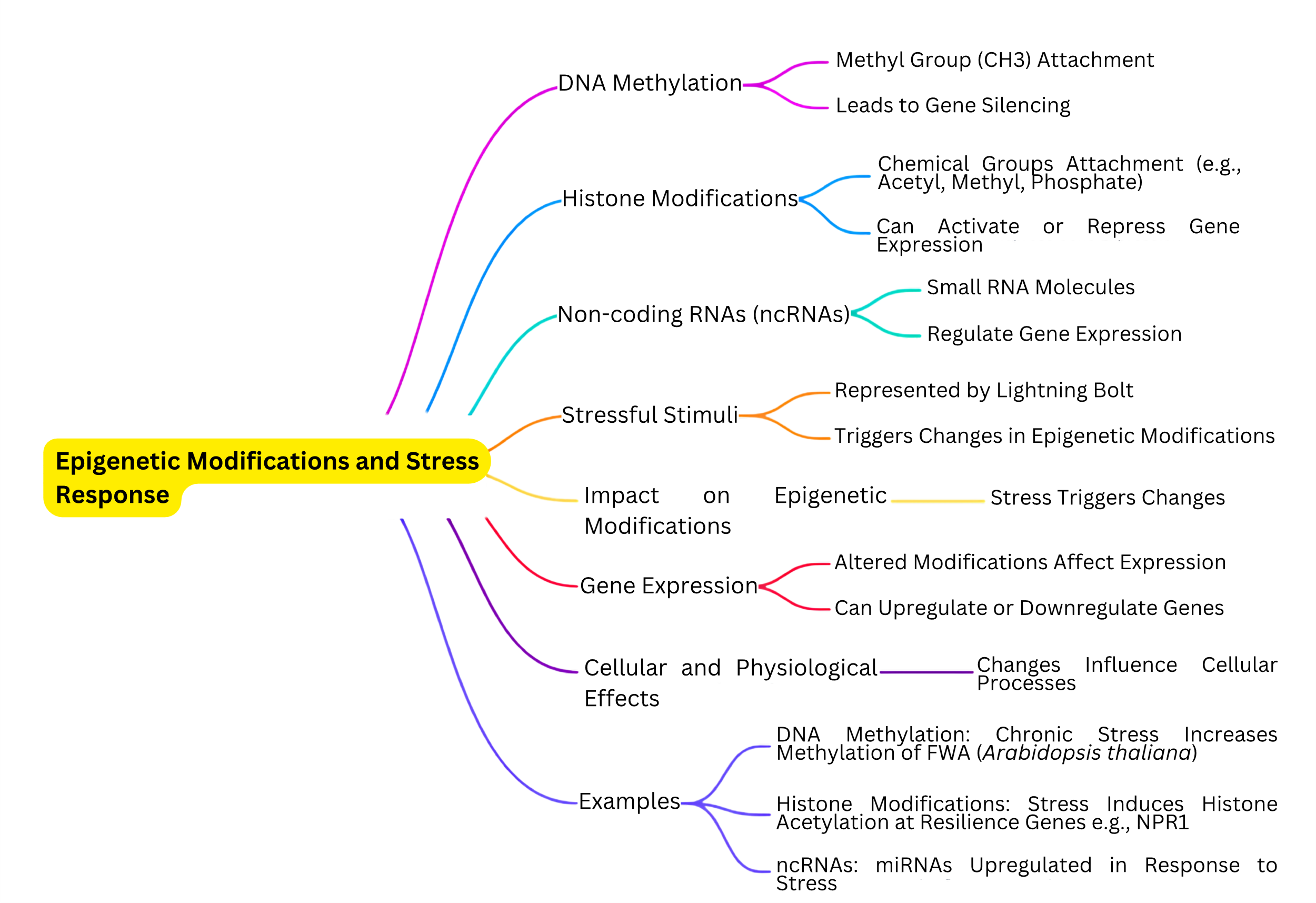
2. Epigenetic modifications and their role in stress responseThe intricate web of epigenetic mechanisms plays a fundamental role in how plants adapt and withstand environmental stressors. Key players in this process include DNA methylation, histone modifications and the influence of small RNAs, which together fine-tune gene expression in reaction to external challenges [16, 19, 29]. These epigenetic adjustments do not change the DNA sequence but have a profound effect on gene activity (Fig. 1). This allows plants to quickly and flexibly respond to changes in their environment, a capability that is essential for stress tolerance, growth and the initiation of defense strategies against diseases and pests [1].
The impact of epigenetic modifications goes beyond just dealing with immediate threats. They enable plants to ‘remember’ past stress encounters. This memory, carried through epigenetic marks, primes plants for a more robust response to similar stresses in the future, playing a critical role in their adaptation and survival [30, 31]. The ability of these epigenetic changes to be passed down through generations highlights their evolutionary significance, offering a pathway for selecting and breeding crop varieties that are better equipped to handle stress [32, 33].
2.1 DNA methylationDNA methylation stands as a cornerstone epigenetic mechanism, significantly influencing plant life by regulating gene expression, which in turn affects growth, development and environmental stress responses [18, 34]. This process entails adding methyl groups to cytosine bases in DNA, altering chromatin structure in ways that methylation hinders gene expression while demethylation could enhance transcription (Fig. 2). The ability to modify DNA methylation dynamically is key to adaptation of plants, enabling them to turn stress response genes on or off as needed [35, 36, 37, 38, 39].

The orchestration of DNA methylation is carried out by enzymes known as DNA methyltransferases [40], which are responsible for attaching methyl groups to DNA. In plants, methylation can occur in three specific contexts: CG, CHG and CHH (H represents A, T, or C), with each context being targeted by different enzymes [18, 29]. This intricate system allows for sophisticated control over gene expression, preparing plants to both confront and memorize stress events. Notably, the methylation landscape within a genome of plant is fluid, evolving in response to stresses like drought, high salinity and pathogen invasion, highlighting the adaptive value of methylation [41, 42].
The role of DNA methylation in managing gene activity and stress adaptation is complex. Typically, higher methylation levels mean gene silencing, whereas demethylation triggers gene activation [43]. This regulatory mechanism enables plants to fine-tune their stress responses. For instance, under drought conditions, genes that bolster drought resistance might be activated through demethylation, aiding the survival strategy of plant [44, 45]. On the other hand, genes that could hinder stress management might be silenced through methylation to save energy and resources [46, 47].
Research into DNA methylation patterns under various stress conditions has revealed that stress like high salinity can cause the hypermethylation of genes critical for ion balance and osmotic control, aiding in salt stress adaptation [42, 48]. Temperature fluctuations also prompt significant methylation changes, impacting genes linked to temperature resilience [49, 50]. These insights highlight pivotal role of DNA methylation in adjusting plant physiological and molecular responses to stress.
2.2 Histone modificationsHistone modifications add another crucial dimension to the epigenetic regulation landscape, significantly influencing how plants react to environmental stresses. Histones are the proteins around which DNA is coiled, creating structures known as nucleosomes. The modifications made to histone tails after they are produced—such as acetylation, methylation, phosphorylation and ubiquitination—play a key role in altering chromatin structure and, consequently, gene expression [51, 52, 53]. These changes do not modify the DNA sequence itself but can drastically affect how genes are turned on or off, allowing plants to adjust their growth, development, and responses to stress in flexible and reversible ways [16, 54].
The intricate patterns of histone modifications, along with their combined effects, create a complex “histone code” that dictates gene activity [55]. For example, adding acetyl groups to histone tails typically leads to an open chromatin configuration and active gene expression by reducing the positive charge of histone, which decreases their binding strength to the negatively charged DNA [56]. Methylation effects vary; they can either encourage or inhibit gene expression depending on which lysine residue is methylated and the degree of methylation (mono-, di-, or tri-methylation) [57]. These processes are controlled by various enzymes, including histone acetyltransferases (HATs), deacetylases (HDACs), methyltransferases (HMTs) and demethylases (HDMs), each essential for finely tuning gene expression in response to environmental pressures [16, 58].
Studies have underscored the significance of histone modifications in how plants deal with stress. For instance, increased histone acetylation in response to drought stress activates genes that help the plant manage water scarcity [59, 60]. Changes in histone methylation have been linked to cold stress responses, with certain modifications regulating cold-responsive genes and aiding in the development of cold tolerance [61, 62]. These instances highlight the pivotal role of histone modifications in plant adaptation to challenging environments, pointing to their potential as targets for improving crop resilience.
2.3 Small RNAsSmall RNAs (sRNAs) are crucial for the epigenetic regulation of gene expression, playing a key role in how plants respond to environmental stresses [63]. These tiny, non-coding RNAs, which are usually 20–24 nucleotides long, include microRNAs (miRNAs) and small interfering RNAs (siRNAs) [64]. Each type has a unique role in defending the plant against both abiotic (non-living) and biotic (living) stress factors [65, 66]. Fig. 3 depicts the process through which small RNAs operate involves the conversion of double-stranded RNA precursors into mature sRNAs. These sRNAs then guide the RNA-induced silencing complex (RISC) to target specific mRNA molecules, leading to their degradation or the inhibition of their translation. This mechanism allows plants to quickly adapt their gene expression in light of changing environmental conditions, enhancing their ability to tolerate stress.
The creation of miRNAs starts with their genes being transcribed into primary miRNAs (pri-miRNAs), which are then processed into precursor miRNAs (pre-miRNAs) and eventually mature miRNAs [67]. These mature miRNAs, once incorporated into the RISC, can target mRNAs to either cut them or block their translation, affecting gene expression post-transcriptionally [68]. miRNAs are vital for plant development and the response to stress, with certain miRNAs being specifically regulated by stress conditions to target genes that are crucial for stress resistance and signaling pathways [19, 69].
The siRNAs, meanwhile, derive from longer double-stranded RNAs and are processed into 21–24 nucleotide long siRNAs. These molecules are involved in silencing transposable elements, defending against viruses and regulating genes [70]. In stress response contexts, siRNAs help silence genes or genomic regions that may be harmful under certain conditions, aiding the plant in adjusting its physiological and developmental strategies to better manage environmental stresses [71, 72, 73]. Notably, siRNAs are also involved in directing DNA methylation and histone modifications at specific genomic locations, thereby connecting RNA silencing with other forms of epigenetic regulation [29].

The role of small RNAs in stress response is both dynamic and complex. For example, certain miRNAs have been found to regulate drought-responsive genes by targeting transcription factors and other regulators involved in water stress tolerance [74, 75]. Plants also adjust the expression of specific miRNAs in response to salt and temperature stresses, targeting genes that control ion balance, osmotic stability and cold stress responses [24]. This underscores the essential role of sRNAs in plant adaptation to abiotic stresses.
Moreover, small RNAs play a part in the epigenetic memory of stress, allowing plants to “remember” past stress exposures and respond more effectively to similar future challenges [76, 77]. This memory mechanism, partly mediated by sRNAs, might involve stable changes in sRNA expression patterns across generations, offering a way for stress adaptation traits to be inherited [31, 50]. Such insights point to the potential of small RNAs as targets for breeding programs aimed at creating crops with enhanced stress tolerance, highlighting the need for further exploration into sRNA-mediated regulatory networks.
The concept of epigenetic memory, where plants retain a “memory” of past environmental stresses that shapes their future reactions to similar challenges, is a captivating aspect of plant adaptation [78, 79]. This memory enables plants to respond more quickly and effectively to repeated stressors, improving their chances of survival and overall fitness. Epigenetic memory is facilitated by stable changes in epigenetic markers such as DNA methylation, histone modifications and specific small RNAs [30, 31]. These changes can persist through cell divisions and, in some instances, be passed down through generations. The capacity of plants to remember and adapt to past environmental conditions holds profound implications for their adaptation, evolution, and the development of stress-tolerant crops.
Transgenerational epigenetic inheritance refers to the transmission of epigenetic information, not encoded in the DNA sequence itself, from one generation to the next, potentially affecting the phenotype of offspring [32, 80]. This process suggests that the environmental experiences of parent plants, particularly stress exposures, can influence the stress responses and adaptability of their offspring. This form of inheritance challenges traditional notions of heredity and adaptation by showing that acquired characteristics, through epigenetic modifications, can be passed down and impact the fitness of future generations.
The transgenerational inheritance of epigenetic marks can occur via meiotic and mitotic cell divisions, ensuring the preservation of DNA methylation patterns, histone modifications and small RNA levels across generations [25, 81]. This preservation allows the epigenetic state related to specific stress responses to be maintained in the germ line and reactivated in offspring when faced with environmental triggers [32, 82]. However, the stability of these epigenetic marks across generations can vary, depending on factors such as the developmental stage of plant, the type of stress encountered and the epigenetic mechanisms involved [83].
Growing evidence supports the existence of transgenerational epigenetic inheritance, with research showing that plants subjected to abiotic stresses like extreme temperatures, salinity and drought can produce offspring with modified stress responses. These alterations are often linked to changes in DNA methylation, histone modifications, and the expression of small RNAs in the progeny [44, 47]. For instance, offspring of Arabidopsis plants exposed to cold stress have shown enhanced freezing tolerance, a trait linked to inherited changes in histone methylation patterns [84]. Similarly, rice plants exposed to drought stress have offspring with modified DNA methylation patterns that provide increased drought resilience [44].
Drought stress poses a significant threat to plant survival and agricultural productivity, impacting vast areas of crops globally [85]. The capacity of plants to adapt to water scarcity is essential for sustaining agricultural output and ecosystem health. Epigenetic mechanisms are key to enabling plants to navigate drought conditions by modulating gene expression without changing the DNA sequence itself.
Changes in DNA methylation are among the primary epigenetic responses to drought, with studies indicating both increases and decreases in methylation across different plant species [41, 44]. These changes affect genes involved in water transport, osmotic regulation and stress signaling, thereby allowing plants to modify their physiological strategies to conserve water and improve drought resilience [35, 86, 87]. For example, in maize, drought conditions have been shown to cause hypermethylation of genes associated with water stress response, suppressing these genes in favor of activating alternative pathways that bolster drought tolerance [45, 46, 86].
Histone modifications also significantly influence plant responses to drought by altering chromatin structure to either facilitate or restrict gene transcription [88, 89]. Research in species like Arabidopsis and rice has pinpointed specific histone modifications linked to the activation or suppression of drought-responsive genes [59, 60]. An increase in histone acetylation at the promoters of these genes, for instance, has been observed, promoting their expression and aiding the adaptation of plants to drought [89, 90].
Small RNAs, including microRNAs (miRNAs) and small interfering RNAs (siRNAs), are vital in regulating gene expression under drought stress [71, 91]. By targeting mRNA for degradation or translation inhibition, these sRNAs adjust the expression of stress response genes [75, 92]. The miRNAs in particular, are crucial for drought tolerance, targeting transcription factors and signaling pathways that influence water use efficiency, root development and stomatal behavior. The specific miRNAs that change in expression due to drought highlight their potential as indicators of drought tolerance and as genetic engineering targets to improve crop resistance to water stress [75, 92].
4.2 Temperature stress (heat and cold)Temperature stress, which includes both heat and cold extremes, significantly affects plant growth, development and survival [35, 93]. With the changing global climate, understanding the adaptation of plants to temperature variations through epigenetic mechanisms is crucial for agricultural resilience and food security.
4.2.1 Heat stressHeat stress triggers a variety of epigenetic changes that help plants adapt by altering gene expression [78, 94]. DNA methylation patterns shift in response to high temperatures, influencing genes related to heat shock response, cellular protection and homeostasis maintenance [95, 96, 97, 98]. For instance, Arabidopsis experiences dynamic DNA methylation changes near genes linked to temperature stress response under heat stress, aiding in their expression adjustment to improve heat tolerance [41, 54].
Histone modifications are also vital during heat stress. Modifications such as acetylation and methylation of histones can alter chromatin structure, making genes more accessible or repressed in response to heat [99, 100]. This enables the swift activation of heat shock proteins (HSPs) and other protective measures [101]. Increased histone acetylation, for example, has been associated with the activation of HSP genes, crucial for mitigating the adverse effects of elevated temperatures [49, 102].
4.2.2. Cold stressCold stress, on the other hand, leads to unique epigenetic changes. DNA methylation levels vary with low temperatures, resulting in the demethylation and increased transcriptional activity of cold-responsive genes [103]. This demethylation promotes the expression of genes essential for cold acclimation, such as those involved in membrane stabilization and cryoprotectant accumulation [46, 61].
Histone modifications during cold stress, like enhanced histone acetylation and specific methylation patterns, are linked to the activation of C-REPEAT BINDING FACTOR (CBF) genes and other cold-responsive genes [78, 104]. These modifications help open up the chromatin structure, facilitating the activation of cold tolerance pathways. Such epigenetic adjustments are crucial for acclimation of plants and survival in freezing temperatures [60, 62].
Small RNAs, including miRNAs and siRNAs, play a key role in regulating temperature stress responses by targeting mRNA for degradation or translational repression [105, 106]. They adjust the expression of temperature stress tolerance genes, including those involved in heat shock responses and cold acclimation [107, 108, 109, 110]. The specific miRNAs that change expression in response to temperature stresses highlight their role in fine-tuning plant responses to extreme temperatures and offer targets for developing temperature-resilient crops [75, 92].
4.3. Salinity stressSalinity stress significantly impacts plant growth, development and productivity, especially in arid and semi-arid regions [111, 112]. For agriculture and food security, the ability of plants to adapt and thrive under high salinity conditions is essential. Epigenetic mechanisms, such as DNA methylation, histone modifications and small RNA regulation, play critical roles in adjusting plant responses to salinity stress, helping them physiologically and biochemically cope with high salt levels [113, 114].
DNA methylation changes are widely observed in plants under salinity stress, influencing gene expression related to ion balance, osmotic regulation and stress signaling [35, 113]. These epigenetic adjustments can silence genes that are harmful under salt stress or activate genes crucial for salt tolerance [35]. For example, in rice, salinity stress leads to the differential methylation of genes involved in salt tolerance, including those encoding ion transporters [42, 48]. These changes are vital for maintaining ion equilibrium and preventing the harmful buildup of sodium ions [115].
Histone modifications also play a significant role in gene expression regulation in response to salinity stress [114, 116, 117, 118]. Alterations in histone acetylation and methylation can either facilitate transcription by opening up the chromatin or suppress it by compacting the chromatin [119, 120]. This allows for the swift activation or repression of specific gene sets under salinity stress 121. In Arabidopsis, for instance, increased histone acetylation at stress-responsive gene promoters under salt stress promotes their expression, aiding the plant’s adaptation to challenging conditions [58, 59].
Small RNAs, including miRNAs and siRNAs, are crucial in regulating gene expression under salinity stress, targeting mRNAs of proteins involved in salt stress responses for degradation or translational repression [71, 122, 123]. Certain miRNAs have been identified that regulate transcription factors and signaling molecules within salt tolerance pathways [66, 75]. This regulatory layer through small RNAs enhances the ability of plant to finely tune its response to salinity, offering potential targets for genetic engineering aimed at improving salt tolerance in crops.
Plants are constantly under threat from a wide array of pathogens, including viruses, bacteria, fungi and nematodes, which can significantly impact plant health and agricultural yield. The capacity of plants to defend against or tolerate these pathogen attacks is critical for their survival. Epigenetic mechanisms are central to modulating plant defense responses, enabling the activation or repression of specific genes crucial for combating pathogen challenges through the dynamic regulation of DNA methylation, histone modifications and small RNA pathways.
DNA methylation changes play a pivotal role in plant responses to pathogen invasion. Alterations in methylation patterns upon pathogen detection can lead to the downregulation of genes that make plants susceptible or the upregulation of defense-related genes, thus preparing the plant to launch an effective defense [29, 41]. For example, the hypermethylation of promoter regions of certain genes can silence them, preventing pathogens from hijacking the plant’s cellular machinery. On the flip side, the demethylation of promoters of defense genes can enhance their expression, strengthening the plant’s defense arsenal against pathogens.
Histone modifications, such as acetylation and methylation, are also crucial in regulating gene expression in the face of pathogen attacks. These modifications can alter chromatin structure, thereby making genes more transcriptionally active or repressed [16, 54]. Increased histone acetylation at defense gene loci, for instance, can boost the transcription of genes that produce antimicrobial compounds or signaling molecules essential for defense activation. Likewise, specific histone methylation marks have been linked to the priming of plant immune responses, enabling plants to react more quickly and effectively to pathogen invasions [27, 124].
Small RNAs, including microRNAs (miRNAs) and small interfering RNAs (siRNAs), are key players in the regulation of gene expression during plant-pathogen interactions. These small RNAs can silence both pathogen-derived genes and plant genes that contribute to susceptibility, as well as regulate defense signaling genes [125, 126]. This regulation allows for a nuanced defence response, optimizing the ability of plant to fend off pathogens while minimizing self-damage. The identification of pathogen-responsive miRNAs and siRNAs has paved the way for deeper insights into plant immunity and the development of innovative disease resistance strategies.
5.2. HerbivoryHerbivory, where animals feed on plants, poses a significant biotic stress that can drastically affect plant growth, survival and reproduction. In response, plants have developed various defense strategies, many of which are epigenetically regulated. Through changes in DNA methylation, histone modifications and small RNA pathways, plants can modify gene expression to activate defenses that make them less palatable or deter further herbivore attacks.
DNA methylation changes in response to herbivory can influence the expression of genes related to both direct and indirect defense mechanisms. Direct defenses include the production of toxins, thorns and trichomes, while indirect defenses may involve releasing volatile organic compounds to attract herbivore predators. For instance, herbivory has been shown to cause hypermethylation at gene loci linked to the biosynthesis of defensive secondary metabolites in some plants, leading to increased expression of these genes and enhanced resistance [29, 41].
Histone modifications are also key in orchestrating plant responses to herbivory. Changes in histone acetylation and methylation can quickly turn defense-related genes on or off, allowing plants to rapidly respond to herbivore presence. Increased histone acetylation at defense gene promoters, for example, has been observed to trigger the transcription of genes that produce compounds deterring herbivores [16, 54]. Similarly, certain histone methylation patterns are associated with the activation of genes responsible for producing volatile signals that attract herbivore predators [27, 124].
Small RNAs, such as miRNAs and siRNAs, play a crucial role in regulating gene expression during plant-herbivore interactions. These small RNAs can downregulate genes that hinder plant defense or enhance the expression of genes important for defense activation. By influencing the expression of transcription factors, signaling molecules and enzymes involved in defense compound biosynthesis, small RNAs help fine-tune plant defensive responses to herbivory [125, 126]. The discovery of small RNAs responsive to herbivory opens up new avenues for understanding plant defense mechanisms and identifying potential targets for improving crop resistance to herbivore pests.
The concept of transgenerational inheritance of epigenetic adaptations highlights how epigenetic changes, triggered by environmental stimuli, can be passed down from one generation to the next. This inheritance does not alter the DNA sequence but can significantly influence the phenotype of the offspring. This mechanism extends the scope of evolutionary adaptation, allowing offspring to benefit from the environmental experiences of their ancestors and providing a swift means of adjusting to changing environments.
This form of inheritance involves the transmission of epigenetic marks—such as DNA methylation, histone modifications and small RNA regulation—through both meiotic and mitotic cell divisions. These epigenetic modifications are preserved during gamete formation and passed on to the next generation, ensuring that offspring inherit a “memory” of their parents’ environmental conditions, which may offer adaptive advantages under similar circumstances [32, 80]. The processes that maintain and inherit these epigenetic marks are intricate, relying on a complex network of enzymes and regulatory mechanisms to preserve epigenetic information across generations.
Key mechanisms underpinning transgenerational epigenetic inheritance include the replication of DNA methylation patterns during DNA replication, the inheritance of histone modifications (though the mechanisms here are less clear due to histone replacement during DNA replication and gametogenesis) and the role of small RNAs in establishing and maintaining epigenetic marks across generations [25, 81]. These elements collectively influence gene expression patterns in the progeny.
The ecological and evolutionary implications of transgenerational epigenetic inheritance are profound. It offers a rapid adaptation mechanism to environmental changes, potentially improving survival and reproductive success under stress. This inheritance mode also contributes to phenotypic variation, serving as a substrate for natural selection. Furthermore, it can impact the dynamics of various ecological interactions, such as plant-pathogen relationships, predator-prey dynamics and competition, thereby affecting community structure and ecosystem functionality.
The stability of epigenetic marks across generations varies, influenced by the nature of the epigenetic modification, environmental conditions and species-specific regulatory mechanisms. While some epigenetic marks are stably inherited across multiple generations, others may be reset during gametogenesis or early embryonic development, limiting their long-term impact [127, 128]. Understanding the factors that determine the stability and reversibility of epigenetic modifications is key to unraveling the significance of epigenetic inheritance in adaptation and evolution.
The realm of epigenetics has seen significant technological progress in recent years, transforming our comprehension of how epigenetic mechanisms affect gene expression, development and adaptation to environmental changes. These advancements have not only deepened our ability to explore and define the epigenome but also paved the way for innovative methods to modify epigenetic marks, impacting fields ranging from agriculture and medicine to ecology.
The advent of high-throughput sequencing technologies has vastly expanded our capability to analyze epigenetic modifications across genomes. Key techniques such as bisulfite sequencing (BS-seq) for DNA methylation mapping, Chromatin Immunoprecipitation sequencing (ChIP-seq) for histone modifications, and small RNA sequencing for small RNA profiles have become foundational in epigenetics research. These methods enable detailed examination of epigenetic landscapes, helping to uncover epigenetic markers linked to gene regulation, stress responses, and developmental processes across diverse plant species.
The adaptation of CRISPR/Cas systems for epigenetic editing marks a major innovation, offering the ability to precisely modify epigenetic states without changing the DNA sequence itself [129, 130]. CRISPR-based technologies allow for the targeted recruitment of epigenetic modifiers to specific genomic regions, facilitating accurate editing of DNA methylation and histone modifications. This approach provides a potent means to epigenetically manipulate plant genomes, enhancing our capacity to investigate gene functions, control gene expression, and improve crop characteristics related to stress resilience and productivity (Fig. 4).

Combining epigenomic data with other omics layers, such as transcriptomics, proteomics, and metabolomics, is essential for decoding the intricate regulatory networks that govern plant responses to environmental stresses. This multi-omics strategy enables the association of epigenetic changes with alterations in mRNA, protein, and metabolite levels, offering a comprehensive view of the mechanisms driving plant adaptation [102, 131]. Such integrated approaches are poised to enrich our understanding of the interactions among the genome, epigenome, and environmental factors, identifying crucial regulatory elements for crop enhancement.
These technological advances in epigenetics research are revolutionizing our understanding of plant biology, providing new tools and methodologies for dissecting the complex interplay between genetic and epigenetic factors in shaping plant phenotypes and their responses to the environment.
Despite swift advancements, challenges in epigenetics research persist, including the demand for more affordable and higher-resolution epigenomic mapping techniques, the creation of effective epigenetic editing tools for a broader array of plant species, and a more profound grasp of epigenetic memory and inheritance mechanisms. Future studies must also explore the functional relevance of epigenetic variations, their generational stability, and their effects on plant phenotypes under evolving environmental conditions.
The ongoing evolution of epigenetic research promises exciting prospects for agriculture, offering new avenues for developing crops with improved stress tolerance, higher yields, and enhanced nutritional value. Moving forward, interdisciplinary efforts that merge epigenetics with other scientific fields will be vital for leveraging epigenetic insights for sustainable crop development and ecosystem management.
The exploration of epigenetic mechanisms in plant adaptation to environmental stresses has unveiled new dimensions in our understanding of plant biology and the complex interactions between plants and their environments. Through the dynamic regulation of gene expression via DNA methylation, histone modifications, and small RNAs, plants demonstrate remarkable adaptability and resilience against both abiotic and biotic stresses. This epigenetic flexibility not only equips plants to tackle immediate challenges but also enables them to “remember” past stresses, potentially conveying these adaptive traits to future generations through transgenerational epigenetic inheritance.
The advent of high-throughput epigenomic techniques and CRISPR-based epigenetic editing tools has transformed our capacity to investigate and manipulate epigenetic mechanisms, providing deep insights into the epigenetic underpinnings of stress tolerance. The integration of epigenomic data with other omics analyses allows researchers to dissect the intricate regulatory networks governing plant stress responses, setting the stage for the creation of crops that are more resilient to environmental pressures.
Despite these technological strides, challenges persist. The processes by which epigenetic marks are established, maintained, and inherited in response to environmental stresses remain to be fully elucidated. Additionally, the functional relevance of numerous epigenetic modifications and their generational stability warrants further exploration to fully leverage their potential for enhancing crop performance.
Looking ahead, the realm of plant epigenetics promises significant advancements for agriculture and ecosystem management. Developing crops with improved stress tolerance through epigenetic engineering could markedly bolster food security amidst climate change and growing global populations. Furthermore, insights into epigenetics in plant adaptation and evolution are invaluable for understanding ecosystem resilience and guiding conservation efforts.
In summary, the study of epigenetic adaptations to environmental stresses is a dynamic and rapidly progressing field that merges fundamental biology with practical applications. As technological innovations continue to enrich our epigenetic research toolkit, the prospects for devising novel solutions to agricultural and ecological challenges are increasingly promising. Embracing the complexities of epigenetic regulation and its implications for plant biology opens the door to a future where epigenetic manipulation plays a pivotal role in sustainable agriculture and biodiversity conservation.
・Epigenomic profiling techniques, like bisulfite sequencing and ChIP-seq, have enabled unprecedented insights into the epigenetic landscapes that shape plant stress responses, uncovering regulatory mechanisms involving DNA methylation, histone modifications, and small RNAs.
・Innovative CRISPR-based tools are revolutionizing epigenetic editing, allowing precise manipulation of DNA methylation and histone marks, thus opening new avenues for enhancing crop resilience through epigenetic engineering.
・Multi-omics approaches that integrate epigenomic data with transcriptomic, proteomic, and metabolomic information are decoding the intricate regulatory networks governing plant adaptation to environmental stresses.
・Mounting evidence highlights the capacity of plants to pass on epigenetic stress memories across generations, potentially priming offspring for improved stress tolerance through transgenerational epigenetic inheritance.
・How stable are stress-induced epigenetic modifications across multiple generations, and what factors govern their reversibility or preservation in the long term?
・To what extent do epigenetic variations contribute to phenotypic plasticity and adaptation in natural plant populations, and how do these mechanisms influence evolutionary trajectories?
・Can we develop robust epigenetic biomarkers or signatures that reliably predict stress tolerance and adaptive potential in crop plants?
・How can we optimize epigenetic editing tools and strategies to engineer resilient crop varieties that can withstand combined or novel environmental stresses?
・What are the ecological implications of transgenerational epigenetic inheritance, and how might it influence community dynamics, ecosystem functioning, and species interactions under changing climatic conditions?
・Can we leverage plant epigenetic mechanisms to design innovative approaches for sustainable agriculture, ecosystem restoration, and biodiversity conservation?