2019 年 8 巻 p. 1-14
2019 年 8 巻 p. 1-14
Dried persimmon (Diospyros kaki Thunb.) is one of the most popular fruit products in East Asia, but its properties and consumer acceptance strongly depend on the drying process and storage conditions that are used. The quality of dried persimmon depends on its physical (color, texture) and chemical (tannin levels, sugar content, the presence of white powder on the surface) properties, which are drastically affected by the decrease in moisture content and composition changes that occur during the drying process and storage. Therefore, in the present report, the changes in the physico-chemical properties of dried persimmon that occur during the drying process and its quality degradation during storage are reviewed for providing fundamental information on the production of high-quality dried persimmon and storage management.
Persimmon (Diospyros kaki Thunb.) originated in China, where it has been cultivated for centuries, and it has since spread to Korea and Japan as well as the Mediterranean coasts of France, Italy, and Algeria. Persimmon has been a popular fruit in China, Korea, and Japan for the last century because of its unique flavor and taste (Celik and Ercisli, 2007; Luo et al., 2005; Sugiura and Taira, 2008; Suntudprom, 2014), with these countries producing 3.93 million tons, 0.35 million tons, and 0.23 million tons, respectively, in 2016 (FAO, 2016).
Persimmon cultivars can be classified into non-astringent and astringent types based on the presence of soluble tannins (catechin, catechin-3-gallate, gallocatechin, and gallocatahin-3-gallate) in the mature stage (Novillo et al., 2016; Vidrih et al., 1994; Yonemori and Matsushima, 1987). Non-astringent persimmon cultivars lose their astringency during maturation, and they can be eaten crisp like apples, whereas astringent cultivars contain approximately 2% soluble tannins at harvest time, and do not normally lose their astringency until they are fully soft and ripe (Akyildiz et al., 2004; Kumar et al., 2018). Astringent persimmons have a limited consumption, with some consumers being reluctant to buy the fresh fruit due to the rough “sandpapery” or dry sensation they create in the mouth when the flesh is consumed. Therefore, an improvement of the features of these persimmons through technological processes is required (Besada et al., 2014).
Astringency can be removed from astringent persimmon fruit in a number of ways, such as treating with ethylene, treating with ethanol vapor, placing in a sealed chamber containing carbon dioxide gas for several days, soaking in limewater for 2–3 days, immersing in warm water, freezing, and altering the drying process (Hu et al., 2010; Taira and Ono, 1997). Among these methods, the most interesting industrial application for astringency removal is the drying technique because drying is not only a traditional method for food preservation, but it is also a way by which the value of persimmon fruit can be improved, and it is a good option for consumers who live in areas where persimmon is not produced.
Dried persimmon is a special product of astringent persimmon fruit and contains higher concentrations of bioactive components due to the decrease in water content that occurs during the drying process. The main components of Japanese raw and dried persimmon per 100 g (USDA, 2016) are shown in Table 1. Fresh persimmon fruit are generally a good source of ascorbic acid, vitamin A, fiber, and minerals, particularly potassium, and it has been suggested that their consumption is good for human health, with these fruit being widely consumed in China to combat health issues such as coughs, hypertension, and dyspnea (Butt et al., 2015; Lia et al., 2013). The drying process increases the carbohydrate, dietary fiber, and nutrient and phytochemical contents of persimmon, while causing a loss of vitamins (Table 1), all of which contribute to the palatability, taste, color, nutritive, and consumption attributes of the dried product (Arnal and del Río, 2004; Celik and Ercisli, 2007). In addition, the drying process causes softening of the fruit (Kim et al., 2014), as well as moisture evaporation, sugar crystal formation on the surface, and gelled pectin formation, the level of each of which varies between different treatments and affects the quality of the final product (Nicoleti et al., 2007; Sugiura and Taira, 2008; Yamada, et al., 2009).
| Nutrient | Unit | Raw persimmon (per 100 g) | Dried persimmon (per 100 g) |
| Water | g | 80.32 | 23.01 |
| Carbohydrates | g | 18.59 | 73.43 |
| Energy | kcal | 70.00 | 274.00 |
| Total lipid (fat) | g | 0.19 | 0.59 |
| Protein | g | 0.58 | 1.38 |
| Fiber, total dietary | g | 3.60 | 14.50 |
| Potassium, K | mg | 161.00 | 802.00 |
| Phosphorus, P | mg | 17.00 | 81.00 |
| Iron, Fe | mg | 0.15 | 0.74 |
| Sodium, Na | mg | 1.00 | 2.00 |
| Magnesium, Mg | mg | 9.00 | 31.00 |
| Calcium, Ca | mg | 8.00 | 25.00 |
| Zinc, Zn | mg | 0.11 | 0.42 |
| Vitamin A, RAE | ug | 2167.00 | 38.00 |
| Vitamin A, IU | IU | 1627.00 | 767.00 |
| Vitamin C (total ascorbic acid) |
mg | 7.50 | -- |
| Vitamin B1 | mg | 0.03 | -- |
| Vitamin B2 | mg | 0.02 | 0.03 |
| Vitamin B3 | mg | 0.10 | 0.18 |
| Vitamin B6 | mg | 0.10 | -- |
RAE: retinol activity equivalents; IU: international units; --: not detected
The effects of the drying process on the quality of dried persimmon in terms of properties such as the viscoelasticity, sugar content, color, white powder formation on the surface, astringency, membrane formation on the surface, and presence of bioactive compounds have previously been described (Hayashi, 1989a; Ishii and Yamanishi, 1982; Karakasova et al., 2013; Manabe et al., 1978; Nicoleti et al., 2005; Park et al, 2006; Tulek and Demiray, 2014), knowledge of which is also important for understanding the storage behaviors. However, while it is known that the physical, biochemical, microbiological and physiological reactions contribute to the deterioration of dried persimmon and are largely dependent on the storage conditions, few studies to date have considered the storage condition management of dried persimmon and quality changes during this period. Therefore, in the present review, we focus on the chemical changes that occur in persimmon fruit during the drying process (with a particular emphasis on sugars, pectin, and tannin) and their effects on the various physical properties (texture, color) of dried persimmon and also summarize quality deterioration during the storage period to assist in the development of methods that improve and maintain the quality of the dried product.
Drying extends the shelf life of persimmon, allowing consumers to benefit from this fruit throughout the year. The traditional and most common method that is used to produce dried persimmon in Japan and China is sun drying in the open air (Sugiura and Taira, 2008), which brings nutritional and health benefits. However, technological advances have allowed this drying method to be improved, with hot-air cabinet drying, solar drying, oven and vacuum oven drying, microwave drying, and freeze-drying having been tested in recent years (Karakasova et al., 2013; Kursun and Karaca, 2018). Each of these drying methods has a different effect on the physico-chemical properties of the final product, as do parameters such as the persimmon cultivar, pre-treatment, drying temperature, air flow rate, humidity around the fruit, and drying period (Ayuko et al., 2017; Cho et al., 2017; Demiray and Tulek, 2017; Karakasova et al., 2013; Yamada et al., 2009). An overview of studies on the effect of drying process on the physico-chemical properties of dried persimmon was shown in Table 2. While the exact procedure that is used to produce dried persimmon in Asia varies among production areas (Ayuko et al., 2017; Kawaguchi et al., 2012; Sugiura and Taira, 2008; Tulek and Demiray, 2014), the general procedure is relatively consistent (see Fig. 1). Here, a traditional procedure for producing dried persimmon (Diospyros kaki) in Japan (Dojo-hachiya kaki as an example) was shown in Fig. 2
| Samples | Drying method | Sugar | Tannin | Pectin | Color | References |
| Whole persimmon fruits | Sun drying | N/A | Decreased markedly during 7 to 9 day’s drying | New membrane formed | N/A | Manabe et al., 1978 |
| Whole persimmon fruits | Temperature: 0℃, 5℃, 10℃, 30℃, 40 ℃, 50 ℃. | N/A | Astringency removal was about 20 days at 5°C and 10°C; 10 days at 30°C; and 3 days at 40°C and 50°C. | Hydrochloric acid soluble pectin decreased and water-soluble pectin increased at 5°C to 40 °C section. | N/A | Manabe et al, 1980 |
| Whole persimmon fruits | Sun drying | Sucrose disappeared after about 10 days. Sugar was about 60% in dry matter during drying. | Soluble tannin and astringency disappeared in 7 days. | N/A | N/A | Ishii and Yamanishi, 1982 |
| Whole persimmon fruits | Sun drying | Sugars were fructose, glucose and sucrose in persimmon. Ratios of fructose to glucose in different cultivates were different. | N/A | N/A | N/A | Hirai and Yamazaki 1984 |
| Whole persimmon fruits | Constant temperature and humidity machine | N/A | N/A | A secondary surface formed due to the existing of pectin | Water content of the secondary skin influence the yellowing | Hayashi, 1989 |
| Sliced persimmon fruits | Cabinet drier (Temperature: 60℃, 75℃, and 90±2℃) |
N/A | N/A | N/A | Color values of samples were measured but the visual variations between them was not quite clear. | Akyildiz et al., 2004 |
| Whole persimmon fruits | Sun drying | N/A | Soluble tannin content decreased faster with hand-softened treatment. | N/A | Appearance of hand softened fruit was darker than the controlled. | Kawaguchi et al, 2012 |
| Whole persimmon fruits | Sun drying | The application of persimmon peel extracts increased the glucose content significantly. | N/A | N/A | Sprayed with 10% persimmon peel extract for 2 min at 20±1°C could exhibit higher acceptance. | Kim et al., 2014 |
| Sliced persimmon fruits | Solar drier | The content of glucose and fructose increased in samples pre-treated with K2S2O5. | Loss of astringency taste due the process of freezing | N/A | Sulphiting treatment prevented color deterioration during dehydration. | Karakasova et al., 2013 |
| Whole persimmon fruits | Cabinet drier (Temperature: 55℃, 65℃, and 75℃) | N/A | N/A | N/A | Color changes occurred in accordance with the first-order kinetic model. | Tulek and Demiray, 2014 |
| Sliced persimmon fruits | Tray drier (Temperature: 50°C for 6 h, 65°C for 4 h, and 80°C for 3 h using 20 cycles/min) |
N/A | N/A | N/A | Samples treated with sodium metabisulfite and dried at 65°C had the highest L* value. | Bolek and Obuz, 2014 |
| Whole persimmon fruits | Sun drying | N/A | Tannin content was significantly higher in bigger fruits than smaller ones. | N/A | Color value was affected by the fruit size. | Cho et al., 2017 |
| Whole persimmon fruits | Air-dried | Extracts from plant-derived compounds increased the sugar concentration of samples. | N/A | N/A | Color change revealed a decreasing tendency in the value of ΔE. | Kim et al., 2018 |
| Sliced persimmon fruits | Convective drier (Temperature: 45, 50, 55, 60 and 65°C ) |
N/A | N/A | N/A | Drying at 65°C improved the colour retention of samples. | Senadeera et al., 2020 |
N/A: Not mentioned in the reference

Fig. 1 General procedure for producing dried persimmon (Diospyros kaki) in Asia

Fig. 2 A traditional procedure for producing dried persimmon (Diospyros kaki) in Japan (Dojo-hachiya kaki as an example)
The drying process leads to changes in the moisture content of the fruit and consequently affects its macroscopic characteristics. The evaporation of moisture and increase in sugar concentration that occur during drying result in white powder forming on the surface of dried persimmon and can also damage and disrupt the cell walls and even collapse the cellular tissue, all of which affect the quality of the final product.
2.1 Free sugar content of dried persimmonSugar is an important component of persimmon fruit, with the ratio of total sugars determining the sensorial properties of the product (Karakasova et al., 2013) – indeed, in ancient times, dried persimmon was used not only as a confectionery item but also as a substitute for sugar. During the drying process, sugar crystals form over the surface of the fruit, creating an appealing product. A number of studies have demonstrated that the free sugars in persimmon fruit include fructose, glucose, and sucrose, as well as galactose and arabinose as minor components (Testoni, 2002). The sucrose content in the flesh of persimmon fruit is influenced by the cultivar, harvesting time (usually disappearing at full maturity), and method of astringency removal that is used – it is particularly noteworthy that mature, over-mature, and dried persimmon have a negligible sucrose content (Hirai and Yamazaki, 1984). In some cultivars, mannitol has also been detected during the drying process (Ishii and Yamanishi, 1982). Only fructose and glucose were detected in “Hachiya” cultivars during sun drying process (Jia et al., 2018).
Studies have shown that the sucrose content of persimmon fruit disappears approximately 10 days after the beginning of sun drying, whereas the fructose and glucose contents initially decrease and then increase during the drying process due to the decomposition of sucrose (Yamada et al., 2009). Total sugars make up approximately 60% of the dry matter throughout the drying process (Ishii and Yamanishi, 1982), while the ratios of glucose to fructose are generally 1.3–1.4 in the raw flesh, 1.2 in over-mature flesh, and 1.0–1.42 in dried flesh (Hirai and Yamazaki, 1984). The evaporation of moisture from the surface of persimmon fruit causes the sugars to crystalize and form a characteristic white powder (Ishii and Yamanishi, 1982), which consists of glucose and fructose in a ratio of 1.68–6.55 depending on the cultivar, product, and drying method that is used (Table 3).
Mass transfer is an important process during fruit and vegetable drying (Helel and Boukadida, 2008). Water moves from the tissues toward the surrounding air as a result of a number of mechanisms, such as capillary flow, the diffusion of water due to concentration differences, surface diffusion, vapor diffusion in the pores due to the presence of a pressure gradient, and water vaporization–condensation, some of which may interact with each other, making drying a very complex process (Upadhyaya et al., 2012). Water transfer and evaporation cause changes in the sugar concentration and the crystallization of sugars on the surface of the fruit, both of which are important to the sensory quality of persimmon because sweetness is a key factor contributing to its processing quality (Chen et al., 2016). However, it is not currently clear if some sugar diffusion or transfer in the flesh also occurs during the drying process.
| Cultivar | Dried persimmon | White powder on the surface | Reference |
| Glucose:fructose | Glucose:fructose | ||
| Hyakume | 1.00 | 4.30 | Ishii and Yamanishi, 1982 |
| Hachiya | 1.10 | 3.50 | |
| Ichida A | 1.30 | 3.90 | Hirai and Yamazaki, 1984 |
| Ichida B | 1.24 | 3.90 | |
| Kayabayashi | 1.36 | 3.78 | |
| Super-hiratane | 1.32 | 3.69 | |
| Koeda | 1.07 | 4.24 | |
| Gesso | 1.30 | 3.50 | |
| Saijo | 1.20 | 4.05 | |
| Hachiya | 1.42 | 5.06 | |
| Yamato-Hyakume | -- | 3.94 | Sugiura and Taira, 2008 |
| Hiratanenashi | -- | 6.16 | |
| Yotsumizo | -- | 6.47 | |
| Ichida | -- | 5.11 | |
| Gionbo | -- | 6.55 | |
| Saijo | -- | 5.95 | |
| Dojo-Hachiya | 1.15 | 1.68 | Jia et al., 2018 |
| Sangju Doongsi | 1.06 | -- | Kim et al., 2014 |
--: Not mentioned in the reference
Soluble tannins make up 0%–4% of the fresh weight of persimmon fruit depending on the cultivar and degree of ripening (Akyildiz et al., 2004; Karakasova et al., 2013) and it is their presence that causes the astringent sensation (Ahn et al., 2002). It has also been shown that the tannin content is significantly higher in larger fruit than in smaller fruit (Cho et al., 2017). Soluble tannins coagulate and become insoluble during the ripening process (softening) or astringency removal treatment so that they are no longer detectable by consumers (Taira and Ono, 1997). Therefore, tannin removal is an important consequence of the drying process that improves the quality of the final product.
The removal of astringency from persimmon fruit is achieved through the condensation or polymerization of soluble tannins into insoluble, non-astringent forms by acetaldehyde, which is produced in the flesh during treatment (Pesis et al., 1988). Sun drying is one method of astringency removal, with studies showing that soluble tannins and astringency disappear in approximately 7–9 days with this treatment (Ishii and Yamanishi, 1982; Manabe et al., 1978). The application of a hand softening treatment during the drying process also assists with the removal of astringency (Kawaguchi et al., 2012). Sun drying is effective in removing persimmon astringency due to the formation of a membrane on the fruit surface and the degradation of fruit tissues, which cause incomplete respiration to occur and the production of acetaldehyde (Manabe et al., 1978). However, Taira and Ono (1997) also found that soluble tannins adhere to the cell wall fragments, causing them to become insoluble and potentially also contributing to the astringency reduction in persimmon fruit. The speed of removal of astringency during the drying process is also related to temperature, increasing at higher temperatures (Manabe et al., 1980).
2.3 Pectin changes during the drying processFruit softening, which characterizes ripening, occurs during the drying process due to structural and compositional changes to the cell wall carbohydrates, mainly as a result of the action of cell wall-degrading enzymes (Ali et al., 1998). Among these cell wall-degrading enzymes, pectin-degrading enzymes have received the most attention as potential causal agents of the softening of ripening fruit (Cutillas-Iturralde et al., 1993). Asgar et al. (2003) revealed that the sun-drying process causes softening-associated changes due to solubilization of the high molecular weight pectin polymer into cold-water-soluble pectic polysaccharide 3 (CWP3), which is separated from CWP by one fraction, and the subsequent depolymerization of CWP3. Furthermore, it has been shown that the molecular size distribution of CWP3 is affected by the degradation of arabinogalactan and arabinan side-chains (Asgar et al., 2004).
Pectin is a complex mixture of polysaccharides but mainly consists of d-galacturonic acid molecules with glycosidic linkages (Vincke et al., 2003). This polymer can form gels under different conditions – high-methoxy (HM) pectins form gels in the presence of high concentrations of soluble solids and low pH levels (pH 2.5–3.4), while low-methoxy (LM) pectins form gels in the presence of Ca2+ ions, which act as a bridge between pairs of carboxyl groups in the pectin molecules and help to form intermolecular ionic junction zones (Lofgren et al., 2002). Heating and the evaporation of moisture from the surface of the fruit cause the flesh of the surface layer to become harder than the inner part due to the gelatinization of the pectin it contains (Hayashi, 1989b), which ranges from 0.7% to 1% of the fresh weight (Karakasova et al., 2013). This gelatinized layer, which is known as a secondary surface, can then be easily separated from the inner part by extending the drying process, affecting the quality of the product, particularly the texture of the flesh.
The effects of the interaction between soluble pectin and soluble tannins on the astringency of persimmon have been reported by many authors. The finding that the total pectin content decreases with no associated increase in water-soluble pectin during softening of the flesh of intact fruit suggests the formation of pectin–tannin complexes (Taira et al., 1997). Furthermore, Chen et al. (2016) found that pectin can enhance the viscosity of persimmon pulp, and Mamet et al. (2017) reported that persimmon tannins enhance the gel properties of HM and LM pectin. However, the mechanisms underlying this strong enhancement ability remain unclear.
The properties of pectin gels are influenced not only by pectin and tannins but also by other coexisting components of the food matrixes, such as proteins, polyphenols, and polysaccharides. However, pectin and tannin complexes in the fruit have only been investigated in vitro to date, with no analysis of the properties of the pectin–tannin complexes or the gel properties (their interaction with each other) in dried persimmon fruit, or of how the gel properties influence the formation of white powder, all of which would be interesting research areas in the future.
2.4 Color changes during the drying processColor is one of the main quality attributes of dried food products and is known to play an important role in consumer acceptability. The color of a product may change during the drying process, storage, and distribution due to a number of chemical and biochemical reactions, such as the type and amount of carotenoid change, the Maillard reaction, and ascorbic acid browning (Chen et al., 2016; Cho et al., 2017; Kim et al., 2014; Perera, 2005), and the retention of total color can be used as a quality indicator to evaluate the extent of deterioration due to thermal processes (Avila and Silva, 1999).
During the production of dried persimmon, sulfur dioxide is applied after peeling to inhibit microbiological spoilage and oxidation, but this fumigation also causes the surface color of the dried persimmon to become a light brown or amber color (Rosello et al., 1993; Sugiura and Taira, 2008). A number of studies (shown in Table 4) have also demonstrated that pre-treatment with various solutions affects the color of the product – for example, pre-treatment with potassium metabisulfite produces significantly brighter dried samples than no treatment or pre-treatment with citric acid and vitamin C (Carcel et al., 2010; Karakasova et al., 2013); pre-treatment with sulfite solution prevents color deterioration during dehydration (Akyildiz et al., 2004); and pre-treatment with sodium metabisulfite increases the appearance score (Bolek and Obuz, 2014). Furthermore, while immersion of the fruit in a 20% sucrose solution decreases the drying time, it also reduces the color quality compared with fruit that are dipped in water (Tulek and Demiray, 2014).
| Pre-treatments | Samples | Descriptions | References |
| Dipping in 8% sodium metabisulphite solution and water ( as control) for 5 min. | Sliced persimmon fruits | Non-sulphited samples dehydrated at 60°C received a highest score of color. | Akyildiz et al., 2004 |
| Dipping in 1% sodium sulfite (30 min); 1% critic acid (20 min). | Whole persimmon fruits | Oxidative and non-oxidative degradation of ascorbic acid contributed to the browning of samples. | Yamada et al., 2009 |
| Dipping in 3% potassium metabisulphite solution (15min); 3% critic acid (15 and 35 min respectively). | Sliced persimmon fruits | Potassium metabisulphite solution generated significantly brighter dried samples; critic acid was useful to limit the color changes during storage period. | Carcel, et al., 2010 |
| Dipping in 5% critic acid (5 min); 3% potassium metabisulphite (5 min); 2% ascorbic acid (5 min). | Sliced persimmon fruits | Pre-treated by potassium metabisulphite produced dried products with the best sensorial properties. | Karakasova et al., 2013 |
| Dipping in 3% ascorbic acid (5 s); 3% sodium metabisulphite (5s). | Sliced persimmon fruits | Treated with sodium metabisulphite had the highest L* value; but given the lowest sensory scores. | Bolek and Obuz, 2014 |
| Soaked in 70% prethanol A solution; soaked in Medilox (60 ppm Hypochlorous acid); sprayed with 10% persimmon-peel extract; burned with sulfur powder; and so on. | Whole persimmon fruits | Treated with 10% persimmon-peel extract exhibited the highest overall acceptance value. | Kim et al., 2014 |
| Peeling and dipping in 20% sucrose solution (15min); water at 80 °C (15min). | Whole persimmon fruits | Dipping in water at 80 °C got better color characteristics. | Tulek and Demiray, 2014 |
| Dipping in 20% sucrose solution (15min); water at 70 °C (15min). | Whole persimmon fruits | Dipping in 20% sucrose solution resulted in increased drying rate. | Demiray and Tulek, 2017 |
| Dipping in different extracts from plant‐derived compounds (Glycyrrhiza uralensis, Cinnamomum cassia, Syzygium aromaticum, and Cnidium officinale) at different ratios. | Whole persimmon fruits | With the best combination ratios, the evaluation of color change revealed a decreasing tendency in the value of △E during the drying period. | Kim et al., 2018 |
In an investigation of the mechanism that causes browning to occur during the processing of semi-dried persimmon, Yamada et al. (2009) observed changes in several components that are related to both enzymatic and non-enzymatic browning and found that browning develops when the water content of the fruit decreases to 50% or less. It has also been shown that “hand massage” treatment may promote the development of browning (Kawaguchi et al., 2012; Yamada et al., 2009).
Today’s consumers demand high-quality products with an attractive appearance, flavor, taste, texture, and nutritional value. Consequently, optimizing preservation technologies for perishable foods to maintain their initial high quality is one of the main goals of the food industry. Although the drying process extends the shelf life of dried persimmon, quality deterioration of dried persimmon also occurs during storage and transport (Fig. 3). Therefore, it is important to maintain the quality of the product until all of the dried products have been sold. An overview of studies related with the storage of dried persimmon was shown in Table 5.
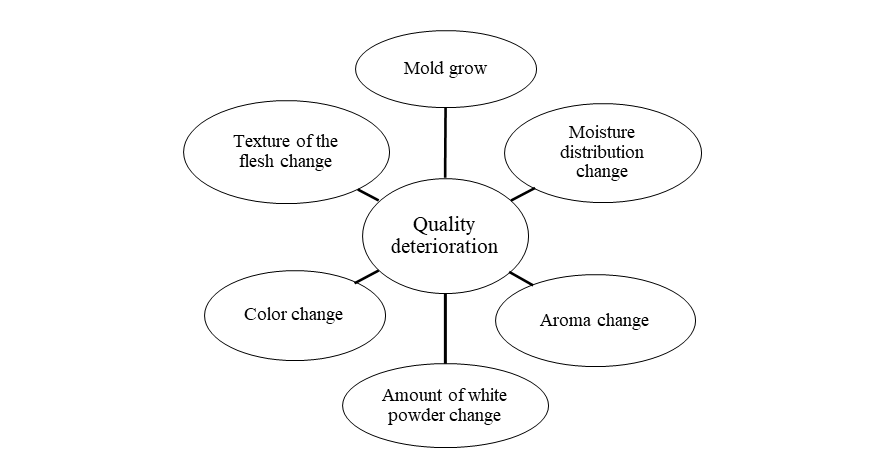
Fig. 3 Quality deterioration of dried persimmon (Diospyros kaki) during storage and transportation
| Treatments | Storage conditions | Descriptions | References |
| Various packing methods and materials (Polyethylene, Nylon, PA/PE package, PET/Al./CPP) | Polyethylene + room temperature | 1 month | Park et al., 1989 |
| Nylon + room temperature | 1.5-2.5 month | ||
| PA/PE package + 5 ℃ | 5 months | ||
| PET/Al./CPP+5 ℃+Carbon dioxide or Nitrogen | 8 months | ||
| Oxygen scavenger (used or not), Package material (KON/PE; Cellophane), Moisture contents (60%, 47%, 35% d.b.) | Temperature: 12 ℃, 16 ℃ and 20 ℃ | Using oxygen scavenger and 12-16℃ were proper condition for storage. | Hayashi, 1990 |
| Pre-treated by 3% potassium metabisulphite solution (15min); 3% critic acid (15 and 35 min); packed with glass containers for storage | Stored at 2, 10, 18 and 28 ℃ | 3% critic acid (15 min) and 18 ℃ were an option to maintain the color characteristic. | Carcel, et al., 2010 |
| Nylon film package | Stored at -20, -10, 0 and 10 ℃ | Self-life of semidried persimmons stored at -20 ℃ was 203 days. | Choi et al., 2017 |
| Packed with Carbon dioxide, Nitrogen, Argon gases | Stored at -10 and 10 ℃ | Nitrogen and Argon modified packaging (-10 ℃) was the most desirable. | Choi et al., 2018 |
| Polyethylene film and plastic box (polyethylene terephthalate) | Stored at -20, 5, 12 and 25°C | Visual appearance and color index can be used for determining the shelf-life of dried persimmons. | Hyun et al., 2019 |
The most important factor in food storage management is temperature, with lower temperatures generally extending the storage life by minimizing nutrient losses and property changes (Perera, 2005). During an investigation of the microbiological, physico-chemical, and visual quality properties of dried persimmons of the cultivar “Cheongdo-Bansi” during storage at various temperatures, Hyun et al. (2019) found that the visual appearance and color index were useful indicators for determining the shelf life of the product. The two main methods that are available for preserving the quality of dried persimmon fruit during storage are frozen storage and storage under low temperature conditions in the presence of an oxygen scavenger, which is generally used in dried persimmon preservation, because the low temperatures prevent microorganisms from growing and slow down chemical activity, delaying degradation of the fruit.
Frozen storage and distribution also cause ice crystals to form and grow throughout the flesh of the fruit, however, some of which may combine with the cell walls. Ice re-crystallization and re-sublimation during the storage period cause changes in the microstructure and properties of the tissue (Adapa et al., 2000), with the former rupturing the cell structure and subsequently degrading the quality of the food (Ullah et al., 2014) and the latter causing a reduction in weight, shrinkage of the tissue structure, and freeze burn (Reid and Perez Albela, 2006). In addition, temperature fluctuations during the storage period enhance the rate of ice crystal growth and resizing, which are the main causes of frozen food quality degradation during storage (Ablett et al., 2002). The effects of temperature fluctuations on the quality of foods have been described for bread, meat, potatoes, and green vegetables (Giannakourou and Taoukis, 2003; Javid et al., 2014; Phimolsiripol et al., 2011). However, few studies have reported the effects of frozen storage and temperature fluctuations on quality changes in dried persimmon, with Sobral et al. (2001) presenting the glass transitions and a phase diagram of freeze-dried persimmon but not relating these to changes in quality.
The presence of an oxygen scavenger, moisture content of the dried persimmon, and packaging material that is used have also been shown to affect quality changes during the storage period (Hayashi, 1990). For instance, Choi et al. (2017) mentioned that semi-dried persimmons that are stored under freezing conditions may expire within 6 months when packaged in normal paper boxes but the shelf life increases to 1 year when stored in modified atmosphere packaging. However, few studies have investigated the quality changes that occur in dried persimmon during storage and transportation, highlighting the need for further research in this field.
The drying process and storage conditions have a large impact on the quality of dried persimmon. The drying of persimmon fruit causes a change in color and an increased amount of white powder on the surface, both of which affect consumer acceptance and commercial value, while quality deterioration during storage affects the sensory properties of the product. While a large numbers of studies have investigated composition changes in dried persimmon during the drying process, few have considered the association between composition changes and quality properties and none have investigated the mechanism of quality deterioration during storage. Since storage condition management, particularly temperature management, is important for reducing the deterioration kinetics and maintaining the high quality of dried persimmon, this is a potential area of interest for current and future research. Furthermore, an increased understanding of the impact of physico-chemical changes on the quality of dried persimmon could have industrial applications, allowing uniform dried persimmon products to be produced and alleviating the quality degradation of dried persimmon throughout the storage period.