2020 Volume 8 Pages 354-366
2020 Volume 8 Pages 354-366
Cyanide is a toxic substance that can be lethal to humans and is present in nature in several superior plants, called cyanogenic plants, with the capacity to generate significant amounts of cyanide (CN) from the cyanogenic glycosides (GCs) present in a natural state. Among the most important GCs are linamarin, lotraustralin, dhurrin and amygdalin. Cassava, sorghum, almonds, apricots, peaches, apples, cherries, alfalfa, bamboo, among others, are examples of these plants. The potential to generate CN varies with each plant. This paper reviews the literature related to the amounts of cyanide produced by these plants, their effects on humans, as well as their toxicological implications.
The term cyanide (CN) is used to describe compounds that contain the -C≡N group and technically, CN is defined as a triple bond molecule with a negative charge, consisting of a carbon atom in the oxidation state +2 and a nitrogen atom in the oxidation state -3 (Kuyucak and Akcil, 2013), it is a compound that existed on earth before the beginning of life and it was the basis for the formation of amino acids, which are the precursors in the evolution of life forms on earth (Logsdon et al., 2001). The CN exists omnipresent in nature, is released into the atmosphere by the burning of biomass and fuel, use of pesticides, insecticides, fertilizers, cosmetics and natural biogenic processes of superior plants, bacteria, algae and fungi (Logsdon et al., 2001; WHO, 2004a; Kuyucak and Akcil, 2013). However, the human body has the natural ability to detoxify small amounts of cyanide (Zottola, 2009).
CN exists naturally as cyanogenic glycosides (CGs) in more than 2000 plant species and is used as a self-defense mechanism against its predators (Møller, 2010; Bradbury et al., 2011, Chaouali et al., 2013; Kassim et al., 2014). CGs are secondary metabolites stored in cellular vacuoles of plant tissues and are composed of an aglycone type α-hydroxynitrile and a sugar like D-glucose (Vetter, 2000; Barceloux, 2009; Abraham et al., 2016). When the tissues are broken by mechanical action (crushing, chewing), the CGs make contact with endogenous lyase enzymes such as β-glucosidases and α-hydroxynitrile that result in the production of HCN (Bolarinwa et al., 2016; Bhalla et al., 2017); The HCN released by these cyanogenic plants protects them from attack, microbial, fungal, insects and herbivorous animals (INTA, 2011b; Kassim et al., 2014).
Among the varieties of plants with significant capacities to generate CN are cassava, sorghum, sweet potato, cabbage, linseed, millet, bamboo, alfalfa, beans, nuts, peanuts, cashews and seeds and berries of some fruits such as cherry, peach, apricot and apple (Logsdon et al., 2001; Møller, 2010; WHO, 2004a; Bradbury et al., 2011). Vegetables can vary widely in their concentration of cyanogenic glycosides depending on their age, genetic and environmental factors, location, season, and soil types (WHO, 2004a), as well as water stress, which limits the growth of the plant but increases its toxic power due to the higher concentration of cyanogenic glycosides (INTA, 2011b). In vegetables, the term "total cyanide" (total CN) is used to describe cyanide content as a sum of cyanogenic glycosides, cyanohydrins and free CN (Abraham et al., 2016); Free CN, includes cyanide ion (CN-) and hydrogen cyanide (HCN) (Aranguri-LLerena and Reyes-Lázaro, 2019).
The release of HCN during the hydrolysis of cyanogenic substances is highly toxic for all aerobic organisms, including humans, since HCN reacts with the iron ions of hemoglobin forming cyanohemoglobin, making it impossible to transport oxygen to the blood (FAO, 2007); similarly it can form complexes with the trivalent iron of the cytochrome C-oxidase and with some enzymes that contain copper ions, blocking the absorption of oxygen in mitochondrial respiration, causing respiratory difficulties and toxic effects, even death (Jaszczak et al., 2017¸ Latif and Müller, 2015). In a complementary way, Abraham et al. (2016) refer that when cellular respiration at the mitochondrial level stops, an increase in anaerobic metabolism occurs, with excess lactic acid formation and metabolic acidosis; finally, cell death occurs due to energy deprivation due to the lack of synthesis of adenosine triphosphate (ATP).
For these reasons, the objective of this paper was to organize the information that exists related to the main species of plants that generate CN, as well as to review the investigations that have been carried out in relation to their effects on human beings.
Cassava is an important food source for humans and particularly important in the daily diet of tropical regions. Its world production in 2017 reached 278 million tons, with Nigeria being the largest producer with 57 million tons, Thailand the main exporter with 8.6 million tons and China the largest importer (FAO, 2017). However, in Nigeria near large-scale cassava processing zones, HCN has been detected at levels of 20–46 mg/m3 in the air; and may have a slight effect on humans, since according to IPCS (2004), concentrations in the atmosphere of 120-150 mg/m3 of HCN can cause death later 0.5 to 1 h of exposure.
In all its tissues, including its roots, the cassava plant contains CN in a variable quantity and in two forms of CGs, linamarin (bound or combined cyanide) of 85 to 90% and lotaustralin (free cyanide) of 10 to 15% (Morgan and Choct, 2016; Díaz-Sobac et al., 2019). The CGs can be hydrolyzed in an aqueous medium by the action of the linamarase enzyme of cassava, producing glucose and cyanohydrin. Cyanohydrin decomposes spontaneously at pH > 5, in acetone and HCN (Figure 1) (WHO, 2004a; Bradbury et al., 2011; Nambisan, 2011; Bradbury and Denton, 2014; Latif and Müller, 2015; Nzwalo and Cliff, 2011; Glanpracha and Annachhatre, 2016; Morgan and Choct, 2016; Pinto-Zevallos et al., 2016).
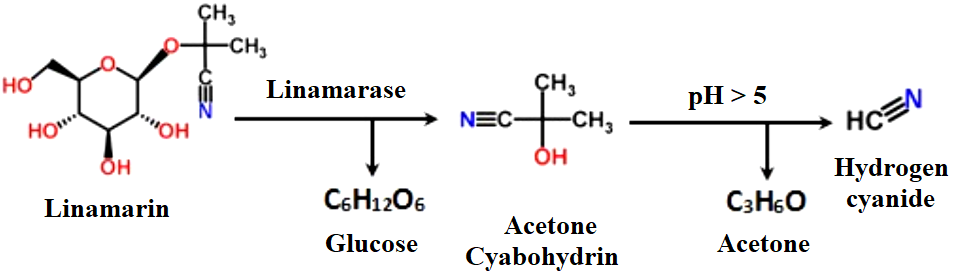
Figure 1: Reaction of cyanogenesis from linamarin contained in cassava (adapted from Nzwalo and Cliff, 2011)
Depending on the age of the plant, soil conditions, fertilizers and climate; the CN levels in cassava tubers can vary from 75 to 1000 mg/kg (Morgan and Choct, 2016; Imakumbili et al., 2019), it can even be between 1300 at 2000 mg/kg, and its leaves between 1000 and 2000 mg of GCs/kg of dry matter (WHO, 2004a). By spectrophotometry, Tivana et al. (2014) found that the concentration of HCN in the pulp and bark of cassava, averaged 200 mg/kg fresh weight.
According to FAO (2007), cassava varieties with an HCN content of less than 180 mg/kg on a dry basis are classified as sweet: between a range of 180 to 300 mg/kg as intermediate and those greater than 300 mg/kg as bitter and the highest proportion of HCN is found in the bark of the root and the husk; in the pulp its concentration decreases from its periphery to its central part, in its leaves the concentration of CN is greater in the fresh than in the adult.
The consumption of a high number of cyanogenic substances, contained in bitter cassava, can cause the Konzo, a neurological disease that causes an irreversible paralysis in the lower limbs and occurs mainly in children and women of childbearing age; in chronic cases the patient cannot walk, and speech is difficult (Cliff et al., 2011; Onojah and Odin, 2015; Jaszczak et al., 2017). This disease is associated with a monotonous diet of bitter cassava with insufficient supply of essential amino acids containing sulfur (S), an element used by the body to convert CN into thiocyanate (SCN) through the catalytic action of the enzyme rhodanase, to finally be excreted in urine (FAO, 2007; Cliff et al., 2011; Bradbury and Denton, 2014; Latif and Müller, 2015, Onojah and Odin, 2015). It is important to know that cassava has a low protein content, less than 2% (Nambisan, 2011; Morgan and Choct, 2016), but it is a high source of carbohydrates (Barceloux, 2009; Tivana et al., 2014).
Therefore, unprocessed cassava is cyanogenic and highly toxic (Burns et al., 2012; Tshala-Katumbay et al., 2016); its consumption without proper processing can cause a serious nutritional disease such as konzo or even death; according to Notimérica (2017), autopsies in Venezuela confirmed that the victims died of endogenous poisoning due to the inadequate consumption of bitter cassava. Food safety programs have been implemented in several parts of the world to prevent the Konzo, such as the distribution of less toxic varieties of cassava along with new processing methods, prolonged wetting, direct roasting, cassava scratching and the moistening of the flour (Cliff et al., 2011); an example of this is the so-called "gari", a product derived from cassava root; after grating it, dehydrate it, ferment it to finally toast the resulting mash in the form of grains (Barceloux, 2009).
By dipping cassava pieces in cold water before the cooking process, most of the free HCN can be removed after 4 to 5 hours, that is, 10% to 15% of the total HCN in cassava. However, with this method, bound HCN, which constitutes 85–90 percent of total HCN, remains almost intact; being possible to eliminate the free HCN in more than 90 percent if the cassava is cooked for 15 minutes (Latif and Müller, 2015).
2.2 Sorghum (Sorghum spp.)Sorghum, a food destined for human consumption in approximately 40% worldwide, has positive effects against obesity, diabetes, hypertension and cancer due to its antioxidant potential (INTA, 2011a; Slima et al., 2018), with significant world production and trade (FAO, 2017); however, it is an important source of CN (Lo and Nicholson, 1998).
Sorghum contains the GCs derived from tyrosine, called dhurrin (Figure 2), which constitutes up to 6% of the dry weight of young sorghum plants. The high amount of dhurrin is synthesized after germination; after 4 days, it reaches maximum levels, then its catabolism prevails (Møller, 2010; Reddy et al., 2016). In dry conditions, forage sorghums accumulate dhurrin, which hydrolyzes during the chewing of animals that consume it, forming HCN, which can cause death. Dhurrin accumulates in young plants, in sprouts and in plants that have developed in adverse conditions, such as drought and frost. The danger is greater in young sprouts of old stems, although there is no risk once the foliage has been transformed into hay, the dry stems are also not toxic (INTA, 2011a). The foliage that is cut, chopped and aired for at least a week helps reduce the toxicity of sorghum; similarly, sorghum silage that has grown under favorable conditions when stored for several months, minimizes the risks of poisoning the cattle that consume it; even so, there have been cases of poisoning due to the ingestion of sorghum in horses, sheep and cattle in Australia, USA and Argentina; the signs are rapidly unleashed and include excitement, shortness of breath, foam at the mouth, seizures and finally death of the animal by suffocation; signs also include ataxia, urinary incontinence, and damage to the central nervous system (INTA, 2011b).
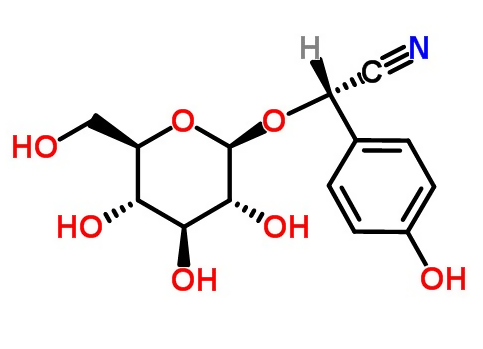
Figure 2: Structure of dhurrin, cyanogenic glycoside contained in sorghum (adapted of Chemspider, 2018)
The cyanide content of ungerminated sorghum seeds is around 22.5 mg/kg, can increase during germination to 1,376 mg/kg and 963 mg/kg (Tokpohozin et al., 2016); however, in various regions of Africa, bicolor sorghum sprouted malt is used as a raw material in beer making, whose free CN content can be between 3.5 to 11 ppm, exceeding the minimum necessary dose (1 ppm) on which forms ethyl carbamate, a carcinogenic substance (Tokpohozin et al., 2016). In an uncontrolled fermentation with the presence of oxygen, the CN can be oxidized to cyanate, which reacts with ethanol to form ethyl carbamate (Figure 3) (Lachenmeier et al., 2010), a carcinogenic substance.
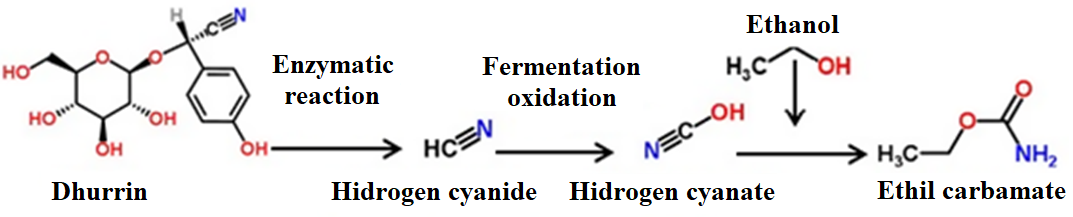
Figure 3: Formation of ethyl carbamate (carcinogen) from dhurrin
Adinsi et al. (2017) used seven varieties of bicolor sorghum malt, Sorghum bicolor (L.) Moench, to make a refreshing and energetic, non-alcoholic drink (Gowé); they found that the average total cyanide content was 12.8 mg/kg malt. Other studies evaluated the efficacy of the enzyme aryl-β-D-glucosidase in eliminating dhurrin in the production of sorghum beer, accompanied by the removal of shoots and roots, to minimize the formation of ethyl carbamate (Møller, 2010; Tokpohozin et al., 2016).
By itself, dhurrin is not harmful; however, due to the action of the enzymes of the intestinal microbiota, it generates HCN, responsible for various harmful effects within the body. When HCN reacts with sulfur (S), it generates thiocyanate, which affects the availability of iodine and causes goiter and cretinism, in addition to other toxic and nutritional diseases such as konzo or tropical ataxic neuropathy (Bradbury et al., 2011; Tivana et al., 2014; Banea et al., 2015; Nzwalo and Cliff, 2011).
It has been established that the most important factors governing the concentration of cyanide in raw sorghum (in decreasing order) are the age of the plant, the irrigation system and the type of tissue (Gleadow et al., 2016). Thus, future research could focus on obtaining a simple method to assess cyanide levels in the same agricultural area and develop new varieties with less cyanide content.
2.3 Almonds (Prunus amygdalus), apricot (Prunus armeniaca), peach (Prunus pérsica), cherries (Prunus cerasus) and apples (Malus domestica)In the seeds of fruits and berries such as apples, apricots, cherries, plums, papaya, peaches, and particularly in almonds, the cyanogenic glycoside called amygdaline is synthesized (Bolarinwa et al., 2014; Blaheta et al., 2016).
According to the IUPAC, amygdalin has the following nomenclature: [(6-O-β-D-glucopyranosyl-β-D-glucopyranosyl) oxy] (phenyl) acetonitrile; its molecular formula is C20H27NO11 (Figure 4) and it hydrolyzes by enzymatic action (Figure 5) when the seeds or fruits mentioned above are macerated, crushed or chewed (Barceloux, 2009; Bolarinwa et al., 2016).
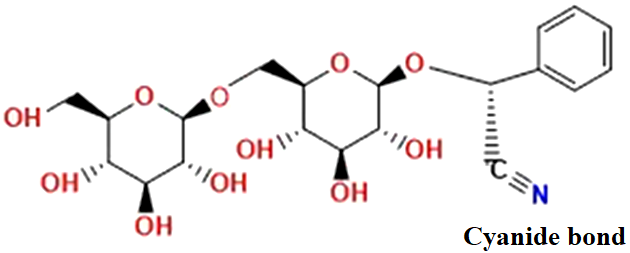
Figure 4: Structure of amygdalin, cyanogenic glucoside contained in almonds (adapted from Chemspider, 2018)
In the first reaction, amygdalin is converted to prunasin and glucose by the enzyme β-glucosidase amygdalin hydrolase (Bolarinwa et al., 2014). In the second reaction, the hydrolysis of prunasin to mandelonitrile and glucose is carried out, with the participation of the enzyme prunasin β-D-glucohydrolase (Milazzo et al., 2015). The final hydrolysis reaction is the decomposition of mandelonitrile to benzaldehyde and HCN by hydroxynitrile lyase (Tomescu et al., 2020); this last phase is rapid and occurs at pH 10. The hydrolysis of amygdalin is catalyzed by the enzyme emulsin, a β-glucosidase (Bhalla et al., 2017), and by the bacterial flora; its enzymatic decomposition occurs rapidly under alkaline conditions (COT, 2006; Bolarinwa et al., 2014) and accelerates in the presence of heat. The clinical dose of amygdalin should not exceed 680 mg/kg (Jaswal et al., 2018).
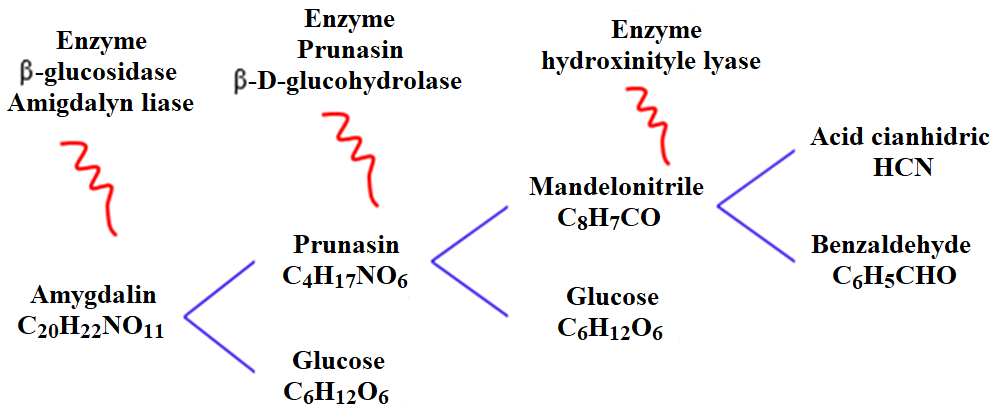
Figure 5: Enzymatic hydrolysis of amygdalin and generation of HCN
Cherry, peach, apricot seeds can contain up to 6% amygdalin on a fresh weight basis, while the GC content of the pulp ranges from about 0.001% to 0.01%, consequently canned stone fruits can contain small amounts up to 4 mg HCN/kg. Apricot, peach, and apple kernels may contain HCN in amounts of 0.05 to 4 mg/g, 0.4 to 2.6 mg/g, and 0.6 mg/g, respectively (Barceloux, 2009). Table 1 shows the amygdalin contents of some fruit seeds and processed fruits, with a marked difference between their GC contents.
Accidental poisoning has been reported from eating cherries whose pulp contained amygdalin, with CN levels ranging from 4.7 to 15 mg/kg; this is the case of a 56-year-old woman in Italy, who after recovery from coma, the patient showed signs similar to Parkinson's disease, retrobulbar neuritis and sensorimotor neuropathy (WHO, 2004a). For their part, Jaswal et al. (2018) describe that when the cherry temperature is increased (up to 65 °C), it hydrolyzes benzaldehyde due to the accelerated action of amylase hydrolase, prunasin lyase, β-glucosidase, water and mandelonitrile lyase.
Chaouali et al. (2013) found that the average levels of HCN that can be generated in sweet almonds, bitter almonds and the apricot kernel are in the order of 25.2 ± 8.24 mg/kg, 1062 ± 148.70 mg/kg and 851.04 ± 303.28 mg/kg of dry matter, respectively. According to COT (2006), the CN concentration in the apricot kernel can reach 2000 mg/kg of dry matter. The WHO (2012a) notes that consuming 50 bitter almonds can be fatal for adults, but for young children a dose of 5 to 10 almonds is fatal.
Amygdalin is also known as "laetrile". The National Cancer Institute (2017) reports that laetrile is a purified form of amygdalin that has been used in some countries for cancer treatment; initially used as an alternative cancer therapy in Russia and then in the United States, around the 1920s (Blaheta et al., 2016; Jaswal et al., 2018). Makarevic et al. (2016) determined a remarkable antineoplastic activity of amygdalin, in cells of metastasis from cancer to the prostate. Similarly, Li et al. (2018) refers that the interaction between amygdalin and β-glucosidase combined with a monoclonal antibody, produces a positive lysis of cancer cells and points out that said interaction produces HCN, which is the agent that destroys cancer cells.
| Amygdalin content (mg/g) | |
| Fruit seeds | |
| Apricot | 14.37 ± 0.28 |
| Cherry (Black) | 2.68 ± 0.02 |
| Cherry (Red) | 3.89 ± 0.31 |
| Peach | 6.81 ± 0.02 |
| Plum (Green) | 17.49 ± 0.26 |
| Plum (Black) | 10.00 ± 0.14 |
| Plum (Red) | 0.44 ± 0.04 |
| Apple | 2.96 ± 0.02 |
| Processed fruit | |
| Toasted almond | 0.12 ± 0.06 |
| Almond milk (toasted) | 0.05 ± 0.01 |
| Almond cocoa dessert | 0.04 ± 0.02 |
| Almond flour | 0.03 ± 0.01 |
| Apple juice (pressed) | 0.09 ± 0.03 |
| UHT apple juice | 0.004 ± 0.01 |
| Apple puree | 0.02 ± 0.01 |
| Apricot slices | 0.05 ± 0.07 |
| Peach drink | 0.04 ± 0.05 |
| Peach slices | 0.06 ± 0.01 |
Milazzo et al. (2015) conclude that there is no reliable evidence of the effectiveness of laetrile for cancer treatment and its risk-benefit balance is negative; but it leaves for consideration new well-designed and controlled clinical trials to evaluate laetrile or amygdalin. Similarly, Jaswal et al. (2018) conclude that various effects of amygdalin and its efficacy have not yet been investigated and those that have been tested (in vivo and in vitro) are highly controversial, so its use as a therapeutic agent is dangerous; However, he argues that amygdalin causes toxicity in oral consumption and not intravenous administration, but its mode of action and toxicity caused by the dose have not yet been confirmed, but suggests that the unproven and contradictory data give way to a broad avenue of research and that amygdalin could be the next step in cancer therapy. Also, Blaheta et al. (2016) conclude that there is no convincing evidence that the amygdalin produces a rapid tumor regression; on the contrary, it states that there is evidence that purified amygdalin, administered in therapeutic doses, causes toxicity. They clarify that there is a need for additional research to assess its actual therapeutic potential. More recent studies reveal that the toxicity of amygdalin is caused by a poisonous decomposition product of benzaldehyde and HCN and can be avoided with an oral dose ranging from 0.6 to 1 g per day (He et al., 2020).
It is highly likely that cassava or other species processing factories mentioned above, or processes that use cyanide as input, may be emitting or filtering cyanide into water bodies; so, it is important to know the toxicity of cyanide. Fish, invertebrates, and higher organisms have been used as toxicity indicator organisms (Table 2), it is noteworthy, when sodium cyanide (NaCN) and potassium cyanide (KCN) were used, the toxic concentrations are lower.
The lethal dose of CN for mammals is in a range less than 0.5 mg/kg of body weight and the oral lethal dose of HCN for humans is 0.5 to 3.5 mg/kg of body weight (COT, 2006; Chaouali et al., 2013; WHO, 2012a). Logsdon et al. (2001) indicate that the lethal dose in humans by ingestion or inhalation of free CN varies between 1 to 3 mg/kg of body weight and the lethal dose by dermal absorption is around 100 mg/kg of body weight.
Abraham et al. (2016) argue that when consuming plant foods with GCs, lower levels of bioavailable cyanide would be expected at a certain time, compared to ingesting the same equivalent dose of free CN, due to its low adsorption rate, which leads to low levels of cyanide concentration in the blood and body tissues. This concentration varies for each type of food eaten that contains a certain GC; possibly due to the delayed and incomplete release of cyanide that require a greater mechanical destruction of the plant tissue structure and enzymatic degradation by the specific β-glucosidase, as well as by the pH of the stomach, bacterial flora of the intestine and even the same influence of the plant matrix.
| Organism | Form of cyanide | Parameter/exposure time | Concentration |
| Fish | |||
| Oncorhynchus mykiss | K3Co(CN)6 | LC50/96 h | 112.9 mg/L |
| Cyprinus carpio | NaCN | LC50/96 h | 1.0 mg/L |
| Carassius auratus | NaCN | LC50/96 h | 318 μg/L |
| Pimephales promelas | NaCN | LC50/8 days | 114 μg/L |
| Invertebrate | |||
| Daphnia magna | NaCN | LC50/24 h | 0.171 mg/L |
| LC50/48 h | 0.12 mg/L | ||
| LC50/72 h | 0.07 mg/L | ||
| LC50/96 h | 0.019 mg/L | ||
| Daphnia magna | K3Co(CN)6 | LC50/96 h | 0.502 mg/L |
| Higher organisms | |||
| Mouse | KCN | LD50/24 h | 8.4 mg/kg |
| Mouse | KCN | LD50/24 h | 8.87 mg/kg |
| Rat | CH3CN | LD50/24 h | >500 mg/kg |
| CH2CHCN | LD50/24 h | 95.1 mg/kg | |
| CH2(CN)2 | LD50/24 h | 66.4 mg/kg | |
| CH3CH2CN | LD50/24 h | 83.6 mg/kg | |
| C2H4(CN)2 | LD50/24 h | 378.5 mg/kg | |
Adapted from Jaszczak et al. (2017)
LD50 (Lethal dose 50): Amount of a given substance (mg/100 g or mg/kg of body weight) that causes the death of 50% of a group of animals under test (Dermal or oral)
LC50 (Lethal concentration 50): Concentration of a chemical in the air or in water (mg/L), which kills 50% of the test animals, in each time (usually 4 hours) (CCSSO, 2019)
Treatment of poisoning from ingestion of cyanogenic sources is similar to treatment for CN poisoning (Barceloux, 2009; Houzé et al., 2018). Clinical signs of acute CN poisoning in humans may include vomiting, epigastric pain, diarrhea, rapid breathing, decreased blood pressure, rapid pulse, dizziness, headache, mental confusion, stupor, cyanosis with spasms, seizures, and respiratory paralysis (Bradberry and Vale, 2016; Satpute et al., 2019). Death from CN poisoning can occur when the cyanide level exceeds the limit that an individual is able to detoxify (Onojah and Odin, 2015).
Mild to moderate intakes of cyanide usually require only certain care, such as the application of oxygen, gastric lavage, or simply skin lavage if the contact was external (WHO, 2012b). The CN- ion can be rapidly adsorbed through wet skin, the mucous membrane of the respiratory tract and the gastrointestinal tract; our body counteracts its toxic effect, converting it to thiocyanate by the action of the enzyme rhodanase (thiosulfate sulfurtransferase), located in the mitochondria. This process requires sulfur (S) donors from protein amino acids, mainly cystine and methionine, or by administering sodium thiosulfate (Figure 6); as a product of this reaction, thiocyanate ions are formed that are approximately 200 times less toxic than cyanide (Jaszczak et al., 2017). The thiocyanate formed is excreted in the urine (Jaswal et al, 2018; Onojah and Odin, 2015).
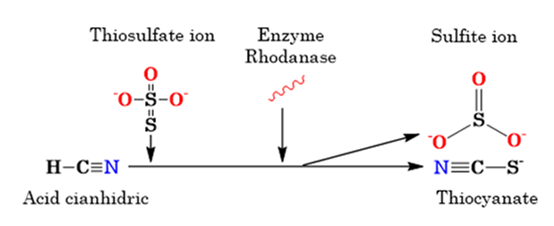
Figure 6: Formation of thiocyanate by action of the enzyme rhodanase
By injecting sodium nitrite, the concentration of methemoglobin (Hb-Fe3+) in the blood is increased due to the oxidation of iron in the hemoglobin molecule (Eq.1), changing from its ferrous to ferric form, thus losing its oxygen transport capacity (Franco et al., 2004).
| (1) |
| (2) |
Indications for the use of an antidote such as nitrites, thiosulfate, or hydroxycobalamin (vitamin B-12), include altered mental status, severe acidosis, continuous seizures, and resistant hypotension (Barceloux, 2009).
According to WHO (2004b) (Table 3), CN poisoning can be treated with sodium nitrite followed by sodium thiosulfate.
| Description | Sodium nitrite | Sodium thiosulfate |
| Injection | 30 mg/ml, ampoule 10 ml | 250 mg/ml, ampoule 50 ml |
| Indications | Along with sodium thiosulfate | Along with sodium nitrite |
| Dosage | By intravenous injection for 5–20 minutes. Adults 300 mg (followed by sodium thiosulfate); if symptoms reappear, another 150 mg dose, after 30 minutes. Children 4–10 mg/kg (initially lower dose) | By slow intravenous injection over 10 minutes. Adults 12.5 g; if symptoms reappear, another 6.25 g dose after 30 minutes. Children 400 mg/kg |
In animals, treatment for CN poisoning is accomplished by intravenous injection of a mixture of sodium nitrite and sodium thiosulfate; the dose is 3 g of sodium nitrite and 15 g of sodium thiosulfate in 200 g of water for cattle and 1 g of sodium nitrate with 2.5 g of sodium thiosulfate in 50 g of water, for sheep. The treatment can be repeated, due to the additional release of HCN (Onojah and Odin, 2015).
Cassava, sorghum, almonds, cherries, peaches, apples and apricots can generate significant amounts of cyanide; If they are not processed properly, GCs do not completely release the CN contained in their structure and when consumed they can cause poisoning and even death. Particularly in African countries, monotonous and poorly processed cassava diets cause epidemic diseases such as konzo; it is possible to decrease the rate of this disease in these regions, if food aid programs are implemented that contain doses of sulfur in their structure. Intake of foods with different GC, but with the same cyanide content, generate different amounts of bioavailable cyanide at a certain time. It is important to disseminate documents and treatises on food sources that can generate CN, including the toxicological implications on the organisms that consume it; in this way, the necessary precautions are taken to reduce the levels of CN that can be generated by foods with GCs. Although amygdalin has been used for cancer treatment, more research is still needed to evaluate its potential therapeutic use.