2020 Volume 8 Pages 58-69
2020 Volume 8 Pages 58-69
Pythium species are soil-borne pathogens which can cause serious economic loss worldwide and threatening agricultural production. Traditional management methods like chemical fungicides are effective against Pythium spp. But as people pay more attention to human health and environmental issues, alternative methods that are ecofriendly and harmless to health are urgently needed. Currently, various approaches had been made including using natural extract, regulating planting conditions, using plant growth-promoting rhizobacteria and screening disease tolerance plants. Hereby, we review the recent achievements, particularly chemical and physical methods, biocontrol and host plant defense which can be used to control Pythium disease.
Soil-borne pathogens such as Pythium spp. is basically difficult to control due to its highly contagious, wide range of host, and longevity, that will increase the cost of fungicide application. For instance, Pythium spp. can infect not only food crops (e.g. rice, wheat, and maize), but also vegetables and ornamental plants (e.g. cucumbers, sweet peppers, potatoes, tomatoes, peanut, and roses) (Johnstone et al., 2005; Li et al., 2007; Al-Mawaali et al., 2012; Okubara et al., 2014; Wheeler et al., 2016; Tabli et al., 2018). After the host is infected by Pythium, symptoms such as plant stunting, water-soak root rot, crown rot, and leaf blight could occur, resulting in host death (Kageyama et al., 2002; Higginbotham et al., 2004; Deng et al., 2005; Lin et al., 2018).
Pythium root rot can cause serious loss of agricultural production worldwide. In Kenya and Rwanda, Pythium root rot caused up to 70% yield losses in traditional local bean cultivars, while 35-54% and 12-17% loss at 18°C and 28°C in hydroponically grown lettuce (Stanghellini and Kronland, 1986; Nzungize et al., 2012). Besides, Pythium root rot also reduced ginger (Zingiber officinale Rosc.) production by 50% to 90% in severely affected areas (Rai et al., 2018). Interestingly, Johnstone et al. (2005) reported that the net carbon exchange rate had no difference between inoculated and noninoculated bell pepper on leaf area basis, while inoculated treatment showed significantly lower on whole-plant basis. Moreover, the daily carbon gain showed a similar result with the net carbon exchange rate. These results indicated that Pythium species do not influence photosynthesis directly, but to reduce the biomass of the plant. This finding suggested that the symptoms caused by Pythium spp. at the very beginning will be difficult to notice.
Pythium spp. have a wide range of hosts. Crop rotation cannot control Pythium efficiently due to its broad hosts. For example, four Pythium species isolated from symptomatic seedlings of corn and soybean showed equally pathogenic on both hosts (Matthiesen et al., 2015). In another experiment, Feng et al. (2019) detected four plant-pathogenic Pythium from different tissue of lettuce by loop-mediated isothermal amplification (LAMP). From the Poaceae weeds grown next to the rice field in Akita Prefecture in Japan, P. arrhenomanes and P. graminicola had been isolated (Toda et al., 2015). Furthermore, 11 Pythium spp. isolated from bell pepper in Florida also caused root rot in tomato (Chellemi et al., 2000). In addition, a single host can be attacked by multiple species of Pythium. Chamswarng and Cook (1985) recovered ten species and varieties of Pythium from both wheat root and the soil in eastern Washington and northern Idaho. On the other hand, 11 different distinct of Pythium from both ginger and the soil caused ginger soft rot in Queensland, Australia (Le et al., 2016).
The longevity of Pythium also makes some traditional management methods ineffective. The Pythium can survive in the soil as oospores which has thick wall without host. Hoppe (1966) reported that Pythium species survived after 12 years in air-dried muck soil. The sporangia of P. ultimum geminated as usual after 11 months under air-dried conditions compared with the field soil maintain moistly, and the population afterward remained stable (Stanghellini and Hancock, 1971). Garren (1971) also found that P. myriotylum still aggressive after 10 months at room temperature in a sealed plastic bag with low inoculum. Moreover, Ichitani and Goto (1982) isolated P. zingiberum successfully from the field after crop rotation for more than 6 years in Japan. On the other hand, Samejima and Ichitani (1988) reported that oospores of P. zingiberum showed no gemination after 70 days buried in autoclaved soil with autoclaved cucumber stem segments, but the gemination ability of P. butleri had no change. Even in polar regions, mycelia of all tested Pythium in host plants survived after 3 freeze-thaw cycles (Murakami et al., 2015).
Given those above, Pythium can cause serious loss to many kinds of crops. Therefore, effective disease management is critically required. Using commercial fungicide could be a choice, however, people are anxious about the potential human health risk and the fungicide resistant ability of Pythium enhanced by chemical compound (Margni et al., 2002; Lookabaugh et al., 2015). Considering that Pythium root rot is more common during summer and high moisture conditions in some area, some researchers are focusing on controlling Pythium spp. by temperature and moisture (Matthiesen et al., 2015; Wheeler et al., 2017; Huzar-Novakowiski and Dorrance, 2018). Besides, biocontrol is also a hot topic because of less human health and environmental risk (Van Os and Van Ginkel, 2001; Mavrodi et al., 2012). Furthermore, with the development of molecular biology, it had been proved that many signal pathways are involved in plant defense against Pythium, but the current knowledge is still not enough to help us to breed disease resistant plants (Adie et al., 2007; De Vleesschauwer et al., 2012; Sánchez-Vallet et al., 2012). The management of Pythium is complicated, since the aggressiveness of Pythium, the effectiveness of fungicide, the applicability of biocontrol agents and the application of disease resistant plants can be influenced by many factors such as air temperature, water pH, even soil type. Hereby, we had focused on common management strategies that can be used to minimize the economic loss caused by Pythium.
Pythium species are widespread in soil and water (Senda et al., 2009; Toda et al., 2015; Uzuhashi et al., 2015). They can grow in a wide range of temperature, and the optimum temperature range from 13°C to 35°C depend on the species (Kageyama et al., 2002; Senda et al., 2009; Matthiesen et al., 2015; Huzar-Novakowiski and Dorrance, 2018). Plant root necrosis caused by P. myriotylum was highly correlated with nutrient solution temperature in hydroponic culture, while the lowest necrosis level at 15°C (Fortnum et al., 2000). Furthermore, other researchers also showed that low temperature could decrease the colonized and browned proportion in the root (Sutton et al., 2006). It is noteworthy that temperature not only influences the aggressiveness of Pythium spp., but also change the fungicide sensitivity of Pythium. Matthiesen et al. (2015) reported P. oopapillum and P. torulosum showed more aggressive under 18°C and 23°C, while P. sylvaticum showed more aggressive under 13°C. Interestingly, P. oopapillum and P. torulosum were more sensitive to fungicide at the same temperature range which they were more aggressive.
Apart from temperature, water content is also a critical factor that affects symptom severity. Gent and McAvoy (2011) showed that partial saturation reduced biomass but did not affect the rate of flower development or plant nutrient composition. For instance, in three of five experiments, plants showed less root rot symptoms, and even the recovery rate of Pythium on agar was lower than standard saturation in some individual experimental groups (Elmer et al., 2012). Furthermore, by irrigating and draining rapidly to simulated partial saturation, the biomass and stem height reduced 10-20%, while no symptoms occurred under partial saturation (Gent et al., 2011). On the other hand, by controlling the water content, relatively low precise substrate volumetric water content (0.2 m3/m3) showed lower root infection by P. aphanidermatum in Petunia × hybrida ‘Dreams Red’ compared with 0.4 m3/m3 and cyclic (0.18 to 0.43 m3/m3) treatment. However, the mortality proportion was lowest in cyclic treatment (Wheeler et al., 2017). Not only controlling water content, but also changing water property such as oxidation-reduction potential (ORP) inhibit Pythium root rot. For example, using chlorinated water eliminated Pythium zoospore in a few minutes (Lang et al., 2008). On the other hand, inoculated tomato grown under hydroponic conditions remained healthy with high oxygen concentration in solution (Chérif et al., 1997). Moreover, using filter and UV light are also considered as feasible way to control Pythium. However, it had been showed that filter only delayed root rot transmission in one or two week depending on the pore diameter, while UV light failed to relieve symptoms and reduced yield even if it could control zoospore population (Goldberg et al., 1992; Zhang and Tu, 2000).
There are many commercial chemical fungicides that are effective against Pythium spp. such as metalaxyl, azoxystrobin, fosetyl-Al, pyraclostrobin, and trifloxystrobin (Taylor et al., 2002; Stiles et al., 2005; Lookabaugh et al., 2015; Matthiesen et al., 2015). However, fungicides showed different abilities among different plants, even among different Pythium spp. (Múnera and Hausbeck, 2015). Based upon environmental temperature, the EC50 value could increase more than 100 times in some fungicides (Matthiesen et al., 2015). Instead of chemical fungicides, some people set their sight on chemical agents which can enhance plant defense. For example, pre-treatment by Acibenzolar-S-methyl, a functional analogue of salicylic acid to turmeric (Curcuma longa L.) induce the activities of peroxidases and protease inhibitors, resulting in decreasing cell death after inoculation with P. aphanidermatum (Radhakrishnan et al., 2011). Apart from fungicide, Zhao et al. (2000) reported that the silver ion dissolved from silver-coated cloth reduced the root rot symptoms significantly. Surprisingly, nonionic surfactants completely controlled Pythium zoospores spread under hydroponic conditions (Stanghellini et al., 1996).
In addition to chemical products, some natural products such as Brassica juncea seed meal suppressed P. abappressorium constantly at least 12 weeks on apple seedlings (Weerakoon et al., 2012). Moreover, Gent and Elmer (2017) reported that combining with silicon, the disease symptoms of poinsettias (Euphorbia pulcherrima) significantly reduced under partial saturation ebb and flow system after inoculation with P. aphanidermatum. Meanwhile, polymer sodium silicate aqueous solution suppressed Pythium in planta, but not in vitro (Mohsen et al., 2015). Natural extract such as Vitex agnus-castus methanolic extract not only showed total antifungal ability against P. ultimum in vitro, but also induced certain pathogenesis-related proteins once inoculated with Pythium to enhance the plant defense ability, which showed unharmful to tomato seedlings (Švecová et al., 2013).
In addition to chemical fungicides, biocontrol is also considered as a very promising way to control disease. By using the antagonism between different microorganisms, many biocontrol agents had been reported. For instance, Enterobacter cloacae and Erwinia herbicola were known to control preemergence damping-off effectively, and significantly suppressed Pythium colonization on cotton seed at 15°C as effective as fungicide (Nelson, 1988). Misk and Franco (2011) reported that all 11 isolated endophytic actinobacteria from different plants showed antimicrobial ability against P. irregulare. Furthermore, strains isolated from irrigation well also showed Pythium damping-off controlling ability in pea (Tabli et al., 2018). Biocontrol agents used to manage Pythium spp. have been provided in Table 1.
In addition, some biocontrol agents can even promote plant growth while relieving symptoms (Suwannarach et al., 2015). For example, six strains of Pseudomonas spp. were ideal biocontrol bacteria that reduced disease symptoms of both Rhizoctonia solani AG-8 and P. ultimum (Mavrodi et al., 2012). Besides, two strains of them even increased wheat seedling shoot length and root weight while suppressing pathogens (Mavrodi et al., 2012). Kipngeno et al. (2015) also reported that the dry mass of tomato seedling was significantly increased by coating Bacillus subtilis and Trichoderma asperellum to the seeds in the presence of fertilizer. Meanwhile, the post-emergence damping-off proportions were 10.87% and 15.3%, respectively, when compared that to the control (63.9%). On the other hand, some biocontrol agents can reduce symptoms through inducing plant defense systems. For example, Pseudomonas corrugata strain 13 and Pseudomonas aureofaciens strain 63-28 produced salicylic acid, and induced endogenous salicylic acid accumulation after inoculation with biocontrol agent for 24 hours in cucumber root (Chen et al., 1999). Furthermore, applying these two plant growth-promoting rhizobacteria suppressed cucumber root rot caused by P. aphanidermatum through stimulating the phenylalanine ammonia-lyase (PAL) activity, while Pseudomonas corrugata strain 13 also stimulated peroxidase (PO) and polyphenol oxidase (PPO) activities (Chen et al., 2000). In addition, by increasing benzyl isothiocyanate and its precursor glucotropaeolin in the root of Brassicaceae plant Lepidium sativum after inoculation with two non-pathogenic Fusarium strains, the resistance ability of host plant was enhanced against P. ultimum (Ishimoto et al., 2004). By microscopy, Benhamou and Brodeur (2001) found that a mycoparasite, Verticillium lecanii (Zimm.) not only inhibited the colonization of P. ultimum, but also induced host plant defense, to restrict the pathogen penetration in the epidermis and the outer cortex.
Keeping the population of the biocontrol agent is the key to ensure the effectiveness of biocontrol. It was found that the biocontrol agent Pseudomonas chlororaphis Tx-1 will keep relative stable density after the sweet pepper was inoculated with P. aphanidermatum and P. dissotocum, but rapidly declined in the non-inoculated root (Chatterton et al., 2004). In another study, Postma et al. (2009) reported that combining with chitosan, the suppress ability of Lysobacter enzymogenes 3.1T8 against P. aphanidermatum in cucumber was enhanced and the bacterial population increased.
Except by using microorganisms directly, using compost is also an effective way to control Pythium. It was found that commercial compost strongly suppressed Pythium wilt disease on cucumber plants by the presence (0.056-0.36%) of fungal Cystobasidiomycetes and the presence (0.011-0.018%) of Acidobacteria Gp14 (Yu et al., 2015). On the other hand, the soil with higher organic matter content and lower sand and clay showed less disease index by comparing andosols and ferralsols in Cameroon (Adiobo et al., 2007). Moreover, the suppressiveness of andosols was significantly reduced by pasteurization, applying fungicide and bactericide. Similarly, using autoclaved rockwool increased disease incidence significantly compared with used or new rockwool due to more fungal population was present in used and recolonized rockwool (Postma et al., 2000). In addition, Van Os and Van Ginkel (2001) reported that Pythium growth rate was highest in sterilized soil, and lowest in sterilized soil with compost, suggested soil microflora can suppress Pythium.
| Pathogens | Biocontrol agents | References |
| Bacteria | ||
| P. aphanidermatum | Pseudomonas spp. | Chen et al. (1999); Chen et al. (2000); Gravel et al. (2005) |
| Pseudomonas chlororaphis | Chatterton et al. (2004) | |
| Actinoplanes campanulatus | El‐Tarabily et al. (2009) | |
| Micromonospora chalcea | ||
| Streptomyces spiralis | ||
| Lysobacter enzymogenes | Postma et al. (2009); Zhao et al. (2017) | |
| Bacillus subtilis | Kipngeno et al. (2015) | |
| Pseudomonas sp. | Tabli et al. (2018) | |
| Serratia sp. | ||
| P. coloratum | Actinomadura rubra | El-Tarabily et al. (1997) |
| Actinoplanes philippinensis | ||
| Bacillus subtilis | ||
| Micromonospora carbonaceae | ||
| Pseudomonas fluorescens | ||
| Streptomyces spp. | ||
| Streptosporangium albidum | ||
| Streptoverticillium netropsis | ||
| P. dissotocum | Pseudomonas chlororaphis | Chatterton et al. (2004) |
| P. irregulare | Microbispora sp. | Misk and Franco (2011) |
| Streptomyces sp. | ||
| Pseudomonas spp. | Mavrodi et al. (2012) | |
| P. ultimum | Enterobacter cloacae | Nelson (1988) |
| Erwinia herbicola | ||
| Stenotrophomonas maltophilia | Dunne et al. (1997) | |
| Pseudomonas spp. | Gravel et al. (2005); Mavrodi et al. (2012) | |
| Paenibacillus alvei | Fatouros et al. (2018) | |
Fungi |
||
| P. aphanidermatum | Trichoderma asperellum | Kipngeno et al. (2015) |
| P. ultimum | Gliocladium virens | Lumsden and Locke (1989) |
| Trichoderma spp. | Cliquet and Scheffer (1996) | |
| Trichoderma | Naseby et al. (2000) | |
| Verticillium lecanii | Benhamou and Brodeur (2001) | |
| Fusarium | Ishimoto et al. (2004) | |
| Aspergillus sp. | Kerkeni et al. (2007) | |
| Trichoderma viride |
There is evidence that by expressing defense signaling pathways such as jasmonic acid (JA) signaling, mitogen-activated protein kinase (MAPK) signaling and wall-associated kinases (WAKs) inhibit root necrosis effectively after inoculation (Zhu et al., 2019). Furthermore, it had been reported that salicylic acid and gibberellic acid signal pathways are critical for rice (Oryza sativa) to defense against P. graminicola, while JA, abscisic acid, and ethylene signal pathway are involved in Arabidopsis defense against P. irregulare (Adie et al., 2007; De Vleesschauwer et al., 2012; Sánchez-Vallet et al., 2012). Except for the critical signal pathway, Castro et al. (2016) found that constitutive expression of pathogenesis-related-10 gene in moss tissue increased resistance against P. irregulare. In another study, reducing availability of sugar in the rhizosphere due to a putative sugar transporter SWEET2 activity contributed to resistance to P. irregulare (Chen et al., 2015). Recently, Nair and Thomas (2013) found one potential resistant gene ZzR1 against Pythium in wild ginger relative viz., Zingiber zerumbet L. Smith.
Besides investigating the molecular aspect of plants, some researchers focus on breeding and screening. By examining the level of tolerance to Pythium root rot in main wheat product varieties, ‘KS93U161’, ‘OH708’, and ‘Sunco’ genotypes showed most tolerant to the disease determined by the number of root tips and total root length (Higginbotham et al., 2004). Besides, ornamental plants such as Caladium cultivars had also been screened and 4 of 19 cultivars showed partial resistance to Pythium root rot (Deng et al., 2005). Furthermore, by conducting somatic hybridization with potato ‘Aminca-Cardinal’ and ‘Cardinal-Nicola’, Nouri-Ellouz et al. (2006) obtained some hybrid lines which showed improved resistance ability against P. aphanidermatum, one line even noted as complete resistance. Among all the plants which showed tolerance against Pythium, phenolic compounds were identified as active ingredients. Temgo and Boyomo (2002) observed that the content of antifungal phenolic compounds determined the resistance ability of cocoyam clone. Li (2006) reported that Rosa multiflora ‘Matsushima No. 3 showed great resistance ability against P. helicoides (Table 2). Zhuang et al. (2012) further observed that the phenolic substances extracted from R. ‘Matsushima No. 3’ inhibited the germ tube elongation of P. helicoides in vitro (Figure 1). Disease symptom of resistant and susceptible cultivars are showed in Figure 2.
| Cultivars | Relative root rot severitya | Infection rateb (%) |
| R. multiflora ‘Matsushima No. 3’ | 0.26 | 10.3 |
| R. multiflora var. carnea | 0.27 | 17.8 |
| R. ‘Veilchenblau’ | 0.31 | 15.6 |
| R. multiflora var. cathayensis | 0.34 | 17.0 |
| R. ‘Dance de Feu’ | 0.38 | 46.4 |
| R. ‘The Garland’ | 0.44 | 22.5 |
| R. ‘Tapis Volant’ | 0.46 | 33.6 |
| R. multiflora | 0.47 | 31.8 |
| R. ‘Cecile Brunner’ | 0.49 | 19.6 |
| R. ‘Apple Blossom’ | 0.52 | 24.9 |
| R. ‘Rush’ | 0.53 | 34.7 |
| R. ‘Margo Koster’ | 0.57 | 27.8 |
| R. ‘Ghislaine de Feligonde’ | 0.71 | 55.6 |
| R. ‘Seagull’ | 0.72 | 38.1 |
| R. ‘Miss Edith Cavell’ | 0.75 | 31.9 |
| R. ‘Kew Rambler’ | 0.85 | 38.9 |
| R. ‘Rambling Rector’ | 0.86 | 40.4 |
| R. ‘Nathalie Nypels’ | 0.90 | 35.1 |
| R. ‘Cameo’ | 0.94 | 45.9 |
| R. ‘Nakashima 91’ | 1.00 | 47.8 |
| R. ‘Leonie Lamesch’ | 1.01 | 62.4 |
| R. ‘Russelliana’ | 1.08 | 68.9 |
| R. ‘Coral Clustar’ | 1.19 | 69.7 |
| R. ‘Blush Rambler’ | 1.41 | 52.5 |
| R. ‘Claire Jacqueir’ | 1.45 | 44.6 |
| R. ‘Goldfinch’ | 1.52 | 76.4 |
a The calculation methods were described by Li et al. (2007).
b Infection rate (%) = (Total number of plants with disease symptoms over 1)/(Total number of plants) ×100.
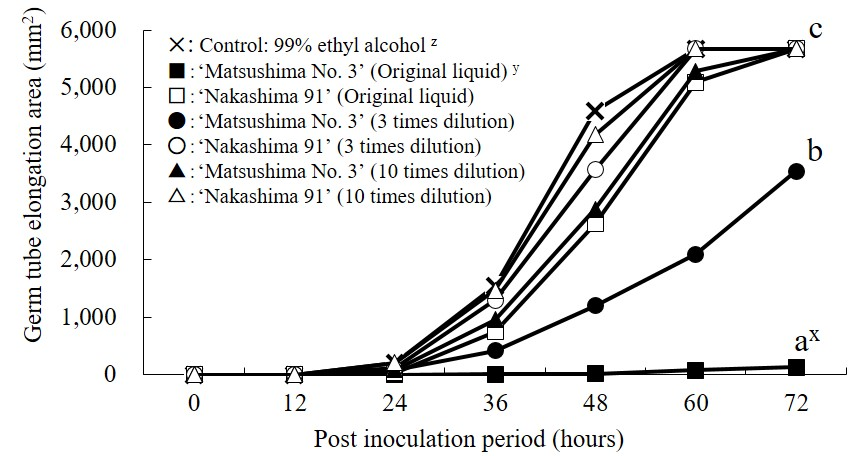
Figure 1: Tolerant effects of bound phenolic substances from R. multiflora ‘Matsushima No. 3’ and R. ‘Nakashima 91’ on germ tube elongation. (modified from Zhuang et al., 2012)
x) Values with different superscripts are significant at p=0.05 by Fisher’s LSD test (n=3).
y) Bound phenolic substances (original liquid) were detected in root extracts of 5 g (fresh weight) root・mL-1.
z) 5 μL extracted phenolic substances (or 99% ethyl alcohol) was added at 4 corners in a petri dish which a 5 mm P. helicoides B-5 inoculum was placed in the middle, and the germ tube elongation area was measured every 12 h

Figure 2: Roots of R. multiflora ‘Matsushima No. 3’ and R. ‘Nakashima 91’ one week after inoculation with P. helicoides and noninoculation.
Inducing the plant defense with exogenous stimulation is a hot topic during these decades. Many materials can be used to induce plant defense response. For instance, silicon can induce resistance in cucumber against P. ultimum by forming electron-dense layers along primary and secondary cell walls, and pit membranes of xylem vessels, as well as significantly increased the percentage of cells filled with phenolic-like material (Chérif et al., 1992). On the other hand, pre-treated with salicylic acid increased the activity of peroxidases and protease inhibitors, resulting in cell death reduction after inoculation with P. aphanidermatum (Radhakrishnan and Balasubramanian, 2009).
A classic example of using host plant defense is grafting, which is widely used in the world to manage soil-borne disease. By grafting onto cucurbit hybrid rootstock ‘Titan’ and ‘Hercules’, no symptoms occurred on cucumber, and they also increased the vegetative growth and fruit product and quality comparing with self-grafted cucumber (Al-Mawaali et al., 2012).
Pythium spp. cause serious economic loss around the world. As understanding of Pythium become wider and deeper, more feasible disease management strategies have been provided. Until now, the most widely used strategy is chemical fungicides, because it is easy to use and efficient. Except for fungicides, controlling water content, adjusting temperature and using compost can bring benefits to Pythium management, however, these methods can only delay the development of the disease instead of eliminating the pathogen due to destroy optimal growth conditions of Pythium. As biocontrol is emerging issues in these decades, many biocontrol agents have been discovered and researched. Recently, the plant growth-promoting rhizobacteria attract more attention compared to antagonistic microorganisms. So far, it seems that the biocontrol in the lab is a great success. Unfortunately, compared to the efficiency in the lab, the field test is another story. There are still some problems remaining like keeping the population of the biocontrol agents, and the potential risks to other plants and microorganisms. Plant response to Pythium has been intensively studied these years. Many important signal pathways in the plant had been proved to involve disease resistance. However, even though tolerant individuals were found among many plants, the complete resistant has not been reported. Despite disease management, Wheeler et al. (2016) reported that linear relationship was found between average pod rot in the field and the sampling units with pod rot at low disease incidences in peanut plantation, and they believed this research will help farmers to manage the field more scientifically. There is no doubt that controlling Pythium effectively will require more than one method. As the research further develops, more tolerant plant will be found, and more effective biocontrol agents and less harmful fungicide will be discovered.
We are gratefully acknowledged Mr. Htay Win and Dr. Liujie Wu for the valuable comments to improve the manuscript.