2020 Volume 8 Pages 70-88
2020 Volume 8 Pages 70-88
Sarcopenia is a degenerative phenomenon, common in elderly populations. As humans age, they are likely to experience skeletal muscle weakness and atrophy; however, unfortunately, there is still no effective treatment for sarcopenia. The health-promoting potential of plant-based foods is associated with the presence of bioactive components. This article reviews information on bioactive plant compounds which may affect skeletal muscle health, particularly with respect to therapeutic approaches to sarcopenia. In general, plant products can be categorized into two main groups, based on their general status in the human diet: inedible or edible. Investigations suggest that bioactive compounds from both groups show the potential to prevent the development of sarcopenia, in several ways, including anti-atrophy, prevention of oxidative damage, enhanced myogenesis, and anti-inflammatory activity. Each of these agents has been shown to suppress one or more of the signs of sarcopenia, and restore health to muscle, in the patient (in vivo) or in tissue culture (in vitro). The potential use of plant bioactive compounds as therapy for sarcopenia is worthy of further study.
Aging is thought to be a complex phenomenon since it involves many biological levels and signaling pathways in all living things, including humans. Even now, aging is a topic which attracts curiosity and generates unresolved questions, among researchers. There is no exact mechanism that would describe the emergence of aging in living things. Lopez-Otin et al. (2013) and Fedarko (2011) stated that aging is a progressive loss of physiological homeostasis which causes functional impairment and enhances the risk of pathological conditions. Several functional impairments are commonly found in the elderly, such as degenerative loss of cognitive, visual, and hearing function, a decline in stress management, sleep disorders, and a significant limitation to mobility (Brady et al., 2014). The onset and rate of aging of individuals are incredibly varied, dependent on a person’s homeodynamic maintenance and ability to maintain optimal biological function. These mechanisms of maintenance, such as DNA repair, the detection and clearance of defective biological macromolecules, damaged organelles and cells, regenerative capacity, and defensive capacity against pathogens, keep individual homeostasis on track and ensure optimal biological function (Fedarko, 2011).
On the other hand, frailty is a geriatric state which manifests by increased vulnerability to stressors and decreased physiological, defensive capacity to maintain homeostasis and to respond to internal and external stresses (Topinková, 2008). This geriatric syndrome is usually characterized by weakness, weight loss, low activity, and adverse health outcomes. There are at least five main criteria used in clinical screening for frailty: poor endurance, sudden weight loss of up to 10 lbs/year, slowed performance, weakness, and low physical activity (Fried et al., 2001). Recently, frailty has been recognized as one of the most problematic clinical conditions in an aged population, worldwide. As a consequence of multiple physiological impairments, related to aging, frail, elderly people have an increased risk of falls, disability, long-term hospitalization, and death. Clegg et al. (2013) stated that a quarter to a half of people over 85 years of age are estimated to be frail. Moreover, according to the WHO, the aged population worldwide is expected to rise rapidly from 600 million, in the year 2000, to around 2 billion by 2050. With this astonishing figure in mind, there is a growing interest to overcome the problem of frailty. Without proper management, people with frailty may became a burden, with high dependency, that affects not only individuals and family, but also the health care system and society (Buckinx et al., 2015).
Muscle strength is a strong predictor of severe limitation of mobility in older people. Besides an essential role in mobility, muscle mass also has a major role in human morbidity and mortality. To preserve skeletal muscle homeostasis, anabolic and catabolic activity need to be continuously maintained. As humans age, they are likely to experience skeletal muscle weakness and atrophy. From their 20s onward, humans can show a loss in skeletal muscle mass, strength and function, but this decline is more rapid from the age of 50 (Brooks and Myburgh, 2012). This degenerative phenomenon is commonly referred to as sarcopenia (Seene and Kaasik, 2012), and is considered a key component of frailty. Most elderly individuals with frailty will also exhibit sarcopenia, and sarcopenic patients are found to be frail (Cruz-Jentoft et al., 2010). Unfortunately, until now there have been no effective clinical strategies to overcome sarcopenia. Physical therapists use a resistance training approach to deal with sarcopenic patients. Although this method is relatively safe and proves effective in maintaining muscle strength and function, the degree of muscle response may vary among individuals. Early-detected sarcopenia and younger patients tend to respond better (Kosek et al., 2006). Pharmacological intervention, such as testosterone treatment, growth hormone treatment, dehydroepiandrosterone supplementation, and myostatin regulation also give promising results but there are potential adverse effects to be considered (Jones et al., 2009).
The impact of food on human health has recently attracted an unprecedented scale of scientific research. Many scientists have focused their research on the functional nutrition, innovation and mass production of foods. The role of food in decreasing the risk of illness and improving health status has highlighted a new class of foods, known as functional foods (Lordan et al., 2011). Fruits and vegetable are now considered as functional foods that have the capability to deliver health benefits and to fulfil biological needs (Vinha et al., 2012). The health promoting potential of fruits, vegetables and other plant-based foods are associated with the presence of bioactive components. The synergistic combination of their phytochemical extracts can offer various health benefits, beyond the basic nutritional value of a food product (Yaqub et al., 2016). Some have strong antioxidant and antiproliferative activities, to prevent the development of chronic disease (Liu, 2004). Therefore, although most bioactive compounds (such as tannins, flavones, triterpenoids, steroids, saponins, and alkaloids) are nonnutritive, they may have important roles in protection against various diseases (Zhang et al., 2015). Several studies relating botanical compounds to muscle health have shown promising results. Although few studies use humans as subjects to evaluate plant bioactive compounds on the welfare of skeletal muscle, recent in vivo and in vitro findings are worth consideration. The aim of this review is to investigate the roles of bioactive plant ingredients, from edible or inedible plant sources, on preventing the aging process of skeletal muscle, known as sarcopenia.
The term “sarcopenia” comes from Greek roots: sarx which means “flesh” and penia which means “loss”. Rosenberg proposed this term to describe the phenomenon of age-dependent loss of skeletal muscle mass and strength (Rosenberg, 1997; Cruz-Jentoft et al., 2010). The operational definition of sarcopenia is important, either for clinical practice or research studies, in determining parameters for diagnosis of sarcopenia. The European Working Group on Sarcopenia in Older People (EWGSOP) defines sarcopenia as a degenerative syndrome, characterized by the presence of low muscle mass and low muscle function, both in strength and performance (Cruz-Jentoft et al., 2010). Based on this definition, EWGSOP also established specific parameters to identify sarcopenia and conceptual stages to measure its severity. There are three main variables to use as parameters for the assessment of sarcopenia syndrome: muscle mass, muscle strength, and physical performance. Furthermore, the detailed measurements of these three variables are used as guidelines to categorize each subject’s case as ‘presarcopenia’, ‘sarcopenia’, or ‘severe sarcopenia’ (Cruz-Jentoft et al., 2010; Santilli et al., 2014).

Figure 1. Molecular mechanisms of sarcopenia
Until now, the exact mechanism associated with muscle aging has been poorly understood. The aetiology of sarcopenia is rather complex, since it involves diverse factors, including physiological impairment, changes in physical activity, and nutritional factors. A general illustration of the molecular mechanisms of sarcopenia was shown in Figure 1. Several factors which might contribute to the development of sarcopenia was shown in Figure 2. The complexity of factors and their effects on molecular mechanisms are probable reasons why finding an effective treatment has proved illusive. Nevertheless, several possible mechanisms have been proposed to understand sarcopenia and to find the potential therapeutic avenues with the most promise.
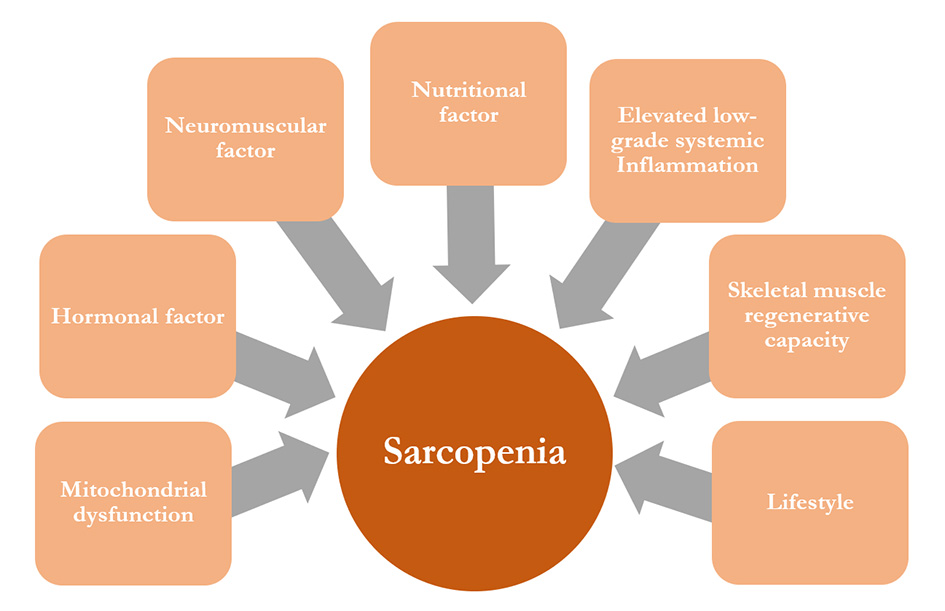
Figure 2. Sarcopenia pathophysiological factors
As organelles, mitochondria have a role in providing cellular ATP (McBride et al., 2006), maintaining redox stability (Marzetti et al., 2010), regulating cellular metabolism, organizing the cellular cycle (McBride et al., 2006), and integrating signaling pathways for cellular death (Wang and Youle, 2016). Notably, skeletal muscle and heart muscle contain two different mitochondrial subpopulations: subsarcolemmal mitochondria, which are located beneath the plasma membrane, and intermyofibrillar mitochondria, located between the myofibrils (Marzetti et al., 2010). These two subpopulations differ bioenergetically and structurally (Ferreira et al., 2010; Calvani et al., 2013). In addition, they might contribute differently to the pathogenesis of sarcopenia.
The aging of mitochondria has many consequences for cell function, especially in bioenergetic activity, to produce ATP. When mitochondria age, their capacity to synthesize ATP will decline, both in the resting state and at maximum respiration (Short et al., 2005). Coen et al. (2013) found there is a strong correlation between reduced mitochondrial capacity to synthesize ATP and low walking speed in older participants, one of the defining criteria of sarcopenia. Beside their age-dependent decline, mitochondria are also major producers of oxidant as well as the major target of oxidative damage. The mtDNA, an important mitochondrial component, is reported to be more vulnerable to oxidative stress, compared with nuclear DNA. Their proximity to oxidants, their lack of histones and introns, and relatively weak repair mechanisms make them prone to defects and dysfunction (Yakes and van Houten, 1997). The contribution of mtDNA damage to the progression of sarcopenia was demonstrated by Kujoth et al. (2005). They found that mice which express a defective version of the proofreading enzyme, mitochondrial polymerase-γ, accumulate a high load of mtDNA mutation. Mice also expressed a premature aging phenotype, including age-related loss of skeletal muscle. Regardless of their capability to generate macromolecular damage, oxidative stress from mitochondria also can regulate the expression of signaling molecules in catabolic pathways. Reactive oxygen species (ROS) activated several proteolytic enzymes (Smuder et al., 2010) or modified the elements to make them targets for proteolysis (Grune et al., 2003). In common with sarcopenia, this condition is aggravated by the suppression of muscle protein synthesis (Wu et al., 2010).
In addition, any alteration in mitochondrial fusion and fission events may also contribute to mitochondrial dyshomeostasis, a major cause of muscle atrophy (Calvani et al., 2013). Yoon et al. (2006) stated that the highly interconnected networks found in aged muscle, with aberrant morphology and enlarged mitochondria, might be caused by an enhancement of fusion activity and or a significant decline in fission activity, along with low autophagic rates. Fusion is an event in which mitochondria can join and their contents become mixed. This event leads to an equal redistribution of metabolites and other important macromolecules, to equilibrate nuclear-encoded proteins in each mitochondrion (Ono et al., 2001). Fission events allow mitochondria to segregate their unnecessary or damaged components, so that autophagic mechanisms can remove them from the system (Twig et al., 2008).
Another mitochondria-related mechanism that may contribute to the sarcopenic condition is the altered function of mitochondrial renewal. Mitochondrial turnover is regulated by the balance between autophagy and biogenesis (Marzetti et al., 2010). During autophagic events, dysfunctional and redundant mitochondria is degraded, while replacement organelles are synthesized through biogenesis events. If these coordinated processes are hampered, specifically in muscle cells, mitochondrial function and myocyte homeostasis will also be disturbed. The potential link between mitochondrial turnover and muscle wasting is through peroxisome proliferator activated receptor-γ coactivator1-α (PGC-1α). Koltai et al. (2012) found that the expression of PGC-1α in old muscle of Wistar rats can indicate a decrease in mitochondriogenesis. In 2006, Sandri et al. published that the disruption of PGC-1α signaling escalated muscle protein degradation, via the ubiquitin-proteasome system, coordinated by FoxO3, causing severe muscular atrophy.
2.2 Elevated low-grade systemic inflammationChronic inflammation in the elderly has long been closely associated with aspects of frailty, including sarcopenia (Allen, 2017). The mechanism is clearly complex, since it involves a number of biochemical reactions and physiological functions that interact with each other. In general, the immune system has an important role in muscle growth and regeneration, in acute or chronic conditions (Sun et al. 2009; Tidball, 2017). During aging, this important role tends to become aberrant and eventually drives the development of degenerative dysfunction of skeletal muscle (Jo et al., 2012). As a result, negative outcomes, such as skeletal muscle regenerative failure (Mourkioti and Rosenthal, 2005), muscle protein turnover impairment (Toth et al., 2005), and apoptosis activation (Dirksa and Leeuwenburgh, 2006) are more likely to happen in the elderly. These three factors are also suggested to be the main mechanisms of inflammation-induced muscle wasting (Jo et al., 2012).
When a person experiences inflammaging, a chronic low-grade inflammation that develops with advanced age, then pro-inflammatory cytokines (IL-1β, IL-6), tissue necrosis factor (TNF-α), and C-reactive protein (CRP) will increase by 2-4 fold, while the serum levels of anti-inflammatory cytokines (IL-10, IL-1ra) are reduced (Wilson et al., 2017). Several studies have investigated the effects of excessive inflammaging. There is a strong correlation between high levels of IL-6 and CRP in serum and the loss of muscle strength (Schaap et al., 2006). IL-6 elevation can cause muscle degradation by activating lysosomal and non-lysosomal protease pathways in C2C12 cells (Ebisui et al., 1995), can induce ubiquitin expression in cachexia patients (Dejong et al., 2005), and promotes E3 ligase expression in a mouse cachexia model (White et al., 2012). Pro-inflammatory cytokines also upregulate the proteolytic pathway by activating the FoxO3a signaling pathway (Xia et al., 2017). In addition, chronically elevated IL-6 levels can influence glucose homeostasis, leading to insulin-stimulated glucose uptake impairment (Febbraio et al., 2004; Franckhauser et al., 2008). On the other hand, TNF-α can stimulate muscle wasting by increasing the ubiquitin/proteasome pathway, via NF-κB activation. To activate NF-κB, the TNF-α signal stimulates type 1 TNF-α receptors. Consequently, this step leads to increase ROS production, via mitochondrial electron transport. ROS then acts as a second messenger to activate NF-κB, directly or indirectly (Reid and Li, 2001). TNF-α, together with insulin-like growth factor I (IGF-1)/Akt, also controls the expression of two main factors in the proteolysis of skeletal muscle, Atrogin and MuRF1 (Clavel et al., 2006). The innate immune system was also found to contribute, indirectly, to the development of sarcopenia. This fact was considered after Leng et al. (2009) confirmed that high neutrophil and monocyte levels, in disabled older women, were associated with frailty status. Neutrophils, known for their chemotactic ability, lose their efficiency with aging. This may cause undesirable tissue defects and secondary inflammation. Neutrophil activity in injured muscle causes secondary damage to surrounding healthy muscle, such as myocyte apoptosis, muscle fiber degradation, and inflammatory enhancement (Wilson et al., 2017). Although it is still unclear how impairment of the innate immune system directly affects skeletal muscle health, the mechanisms outlined above may provide an explanation of the contribution of leukocytes to sarcopenia.
2.3 Hormonal factorsHormonal alterations that contribute to sarcopenia are usually represented by a resistance to hormonal factors for anabolic and catabolic activities. According to Kim et al. (2016), in general, steroid sex hormones have four main roles in skeletal muscle regulation: 1) regulate muscle cell metabolism, 2) promote muscle strength, 3) promote muscle growth, 4) promote muscle repairment. The effects of steroid sex hormones on the function of muscle cells are varied, dependent on gender, muscle cell types and sensitivity, and muscle position. Among the different types of sex hormone, androgens may be predominant, in relation to regulation of muscle homeostasis, in male and female (Carson and Manolagas, 2015). Testosterone, estrogens, and dehydroepiandrosterone are androgens, reported to decrease with aging, and to be related to skeletal muscle mass. Total testosterone levels were found to decrease by 1 % per year, whereas free testosterone and albumin-bound testosterone declined about 2 % per year in adult males from the age of 40 (Feldman et al., 2002). While in women, testosterone levels were found to decrease in adult females, aged 20 to 40 (Zumoff et al., 1995). Epidemiological studies show a significant relationship between low testosterone level and loss of muscle mass, strength, and function, in an elderly population (Rolland et al., 2008). According to Basualto-Alarcón et al. (2014), testosterone stimulated protein synthesis via short- and long-term mechanisms. Therefore, there is a possibility that testosterone impairment may also affect protein synthesis, although the exact mechanism remains under investigation. The most recognized molecular mechanism for testosterone, in controlling muscle protein synthesis, is through mTOR (mammalian target of rapamycin) activation.
The possible mechanism by which a decline in estrogen generates a loss in muscle mass may be associated with an escalated level of proinflammatory cytokines, such as TNF-α and IL-6 (Girasole et al., 1999; Kramer et al., 2004). There may also may be a more direct effect, since recent reports have shown that skeletal muscle cells have the estrogen β-receptor on their cell surface membrane, in the cytoplasm, and on their nuclear membrane (Brown, 2008).
Other than steroid sex hormones, several other hormones have been also reported to show an effect on skeletal muscle health, in aged people. Glucocorticoids were found to cause an increase in skeletal muscle proteolytic activity, specifically for type II or phasic muscle fibres (Ferrando et al., 1999). The possible mechanism by which glucocorticoid negatively affects skeletal muscle development may involve the upregulation of myostatin and glutamine synthetase promoters, two genes implicated in muscle degradation (Carballo-Jane et al., 2004). In addition, glucocorticoid was also shown to increase muscle proteolytic expression (atrogin-1, MuRF1, poly-Ub, metallothionein, and cathepsin L) in vitro (Sacheck et al., 2004). In contrast, insulin and IGF-I are known to have a positive effect on skeletal muscle growth. Insulin stimulated mitochondrial protein synthesis in skeletal muscle (Boirie et al., 2001). Unfortunately, this response is impaired in elderly humans, due to an alteration in signaling systems (Guillet et al., 2004). Other hormones, such as growth hormone, also coordinate the postnatal growth of multiple target tissues, including skeletal muscle (Florini et al., 1996). Growth hormone also decreased in elderly men, and could be considered as a potential contributor to sarcopenia (Martin et al., 1997). The exact mechanism of growth hormone action is not fully understood, but evidence in vitro shows that it may induce IGF-I mRNA expression, phosphorylation of JAK2 and Stat5 pathways, and induce the expression of SOCS-2 (Sadowski et al., 2001). Vitamin D is also considered as a hormone whose deficiency may be implicated in geriatric sarcopenia. Worldwide, almost 1 billion elderly people are Vitamin D deficient, including approximately 50 % of men 65 years or older, (Sakuma and Yamaguchi, 2012). Recent examination suggests that muscle tissue from subjects with Vitamin D deficiency shows increasing interfibrillar space, intramuscular adipose tissue infiltrates (myosteatosis), and fibrosis (Russell, 1994). Vitamin D deficiency also causes abnormalities of mitochondria and sarcoplasmic reticulum, in muscle cells, and impairs Ca homeostasis at the levels of the genome and of the membrane (Boland, 1986). In addition, it induced muscle protein turnover to become imbalanced, by a reduction in the secretion of insulin (Wassner et al., 1983).
Effective methods of treatment and therapy for sarcopenia remain unclear. Researchers are still trying to find the best medication to halt, or at least delay, the progression of sarcopenia. Although further research is rapidly building upon existing knowledge, every therapeutic option, thus far, seems to show a varied outcome, case by case, indicating that complex factors and mechanisms may be involved. The utilization of plant bioactive compounds as an alternative therapeutic option is often ruled out. Although they may have beneficial effects, the issues of accessibility and availability are always a challenge, which leaves them underused. Compared with conventional medical treatments, natural compounds show lower physiological and psychological addiction among patients, with relatively few side effects (Rondanelli et al., 2016). Therefore, the potential of plant bioactive compounds, to prevent and treat sarcopenia, is an important topic for the study.
In the next section, the positive effects of natural bioactive compounds on skeletal muscle health, particularly in therapeutic approaches to sarcopenia, will be considered. To simplify their classification, we categorized substances according to their general status in the human diet: either as an inedible or an edible source. “Inedible source” means that the plant is not commonly consumed in the diet, though some people may use it as an herbal medicine. Edible sources are plants which are often eaten as a part of everyday diets.
3.1 Inedible plant extractWe found at least ten inedible plants with the potential to overcome sarcopenia (Table 1). These plants demonstrate three main activities in maintaining skeletal muscle health and preventing muscle aging: anti-atrophy, prevention of oxidative damage, and enhanced myogenesis.
3.1.1 Anti-atrophyOne of the main, general effects of plant bioactive compounds, in regulating skeletal muscle health, is a capacity to maintain skeletal muscle turnover, eventually slowing atrophy. Skeletal muscle mass depends on the overall balance between muscle protein synthesis and muscle protein breakdown, called muscle protein turnover. Therefore, muscle atrophy may result either from a downregulated level of muscle protein synthesis or an upregulated level of muscle protein breakdown (Attaix et al., 2005). These alterations are multi-factorial, and several have already been discussed. Various bioactive plant ingredients have been found to be effective in preventing the wasting of skeletal muscle, or muscle atrophy, either in vitro or in vivo. Dihydromyricetin (DM), a flavanol from Ampelopsis grossedentata (moyeam), showed a positive effect in maintaining skeletal muscle mass and function. Huang et al. (2018) found that a 14-day supplementation of DM extract could restore skeletal muscle weight in cases of dexamethasone-induced muscle atrophy and contributed to the restoration of muscle strength. In addition, DM extract prevented dexamethasone-induced atrophy in L6 skeletal muscle cells by suppressing ubiquitin E3 ligase activity, a major factor in muscle protein degradation. By inhibiting the catabolic pathway in skeletal muscle cells, canadine from Corydalis turtschaninovii also suppressed the activity of MAFbx and Murf1, two muscle-specific E3 ubiquitin ligases. This result, in vitro, indicates the potential of this extract to protect against cancer-induced muscle wasting (Lee et al., 2017b). In addition to DM and canadine, root extract of evening primrose (EVP), Oenothera odorata, also prevented skeletal muscle wasting in old male C57/BL6 mice. Intraperitoneal injection of EVP delayed the loss of skeletal muscle volume. According to Lee et al. (2016a), EVP restored the level of ceramide, a molecule which plays a key role in muscle protein synthesis. This extract appeared to maintain skeletal muscle turnover, in old mice. Other than that, an extract from the leaves, twigs and fruit of the Korean mistletoe (KME), Viscum album, also showed some anti-atrophy activity. A 4-week supplementation of KME can improve skeletal muscle weight and strength, in denervation-induced atrophy mice. The expression of the skeletal muscle protein degradation genes (Murf1 and Atrogin1) were downregulated by KME treatment, hinting at a possible mechanism in preventing skeletal muscle wasting (Jeong et al., 2017).
| Plant Species | Compounds | Chemical Structures | Model | Functions | References | |
| 1. | Ampelopsis grossedentata | dihydromyricetin |  |
Adult male sprague-dawley rats | ·Maintained muscle mass and strength ·Prevented mitochondrial oxidative damage ·Restored mitochondrial function |
Huang, et al., 2018 |
| Rat L6 skeletal muscle cell | ·Prevented dexamethasone induced muscle atrophy ·Enhanced muscle protein metabolism ·Restored mitochondrial function |
|||||
| 2. | Broussonetia kazinoki | kazinol-P |  |
Murine C2C12 skeletal muscle cell | ·Enhanced myogenesis ·Enhanced myogenic conversion of mouse embryonic fibroblasts through trans-differentiation activity |
Hwang, et al., 2015 |
| 3. | Coptis japonica | magnoflorine |  |
Murine C2C12 skeletal muscle cell | ·Enhanced myogenesis | Lee, et al., 2017a |
| 4. | Corydalis turtschaninovii | dehydrocorydaline | 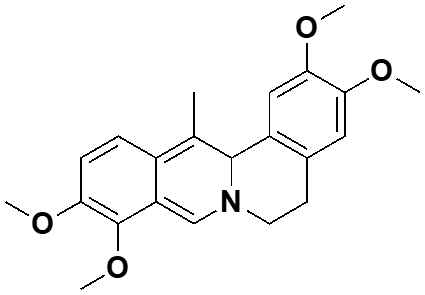 |
Murine C2C12 skeletal muscle cell | ·Enhanced myogenesis ·Restored the expression of muscle-specific proteins and myotube formation ability in defective myoblast to similar level of normal cells |
Yoo, et al., 2016 |
| canadine |  |
Murine C2C12 skeletal muscle cell | ·Enhanced myogenesis ·Downregulated the expression of MAFbx and Murf1 in CM-induced muscle wasting cells |
Lee, et al., 2017b | ||
| tetrahydropalmatine |  |
Murine C2C12 skeletal muscle cell | ·Enhanced myogenesis ·Enhanced myogenic conversion of mouse embryonic fibroblasts through trans-differentiation activity |
Lee, et al., 2014 | ||
| 5. | Gynostemma longipens | 12,23-dione dammarane triterpenes |
 |
Murine C2C12 skeletal muscle cell | ·Enhanced cell proliferation ·Enhanced DNA synthesis ·Enhanced glucose uptake and ATP synthesis |
Ha, et al., 2019 |
| 6. | Oenothera odorata | roots extract | 4 week-old male C57/BL6 mice | ·Prevented sciatic denervation induced muscle atrophy | Lee, et al., 2016a | |
| Murine C2C12 skeletal muscle cell | ·Prevented H2O2-induced muscle atrophy ·Prevented H2O2-induced apoptosis ·Prevented H2O2-induced oxidative stress and damage |
|||||
| 7. | Poncirus trifoliate | tangeretin (5,6,7,8,40-penta-methoxyflavone) |  |
Murine C2C12 skeletal muscle cell | ·Enhanced the activation of AMPK pathway ·Enhanced glucose uptake activity |
Kim, et al., 2012a |
| 4 week-old male C57BL/6 J mice | ·Enhanced the activation of AMPK pathway ·Reduced fat accumulation and insulin resistance ·Ameliorated inflammatory cytokine serum levels |
|||||
| 8. | Psolarea corylifolia | bakuchiol |  |
Murine C2C12 skeletal muscle cell | ·Enhanced myogenesis ·Restored the expression of muscle-specific proteins and myotube formation ability in defective myoblast to similar level of normal cells ·Enhanced myogenic conversion of mouse embryonic fibroblasts and rhabdomyosarcoma cells |
Lee, et al., 2016b |
| 9. | Rhodiola rosea | rosavin (1), rosarin (2), rosin (3), and salidroside (4) |
 (1)  (2)  (3)  (4) |
Murine C2C12 skeletal muscle cell | ·Restored cell viability and prevented stress oxidative induced by H2O2 | Hernández-Santana, et al., 2013 |
| 10 | Viscum album | leaves, twigs, and fruits extract | Murine C2C12 skeletal muscle cell | ·Promoted muscle hypertrophy | Jeong, et al., 2017 | |
| 5 week-old male ICR mice | ·Prevented sciatic denervation induced muscle atrophy ·Enhanced muscle mass, muscle area, muscle diameter, and muscle strengths |
Mitochondrial malfunction is one possible cause of muscle atrophy, which leads to sarcopenia. Mitochondria malfunction generates ROS and increases proteolysis in skeletal muscle. Several bioactive plant ingredients exhibit potential as deterrents to oxidative damage. This role is usually indicated by the activation of the AMPK pathway and the downregulation of oxidant. Besides its role in anti-atrophy, DM also presented a potential role in preventing oxidative damage, through the restoration of mitochondrial function (Huang et al., 2018). DM treatment could attenuate mitochondrial swelling, the disarray of cristae, and fragmentation caused by dexamethasone. In terms of mitochondrial function, DM also improved mitochondrial membrane potential and respiratory activity. DM enhanced mitochondrial biogenesis through the activation of PGC-1α signaling pathway, leading to the rebalancing of mitochondrial dynamics. Lee et al. (2016) showed that the main role of EVP in overcoming sarcopenia was by prevention of oxidative damage. EVP protects myoblasts, both in vitro and in vivo, from oxidative-stress-induced cell death by regulating apoptotic factors (Bax, Bcl-2, and caspase 3) and enhancing the expression of HSP70. Later, this effect inhibited the emergence of apoptosis which may lead to skeletal muscle atrophy. Rhodiola rosea root extract also possessed the same mechanism of action. Treatment with Rhodiola rosea root extract can restore the viability of murine C2C12 cells, after H2O2-induced oxidative stress. The extract upregulated the expression of HSP70, protein, which has a central role in preventing chemically active groups from reacting with harmful substances which might lead to cellular malfunction (Hernández-Santana et al., 2013). Gynostemma longipens extract and tangeretin in Poncirus trifoliate extract inhibited oxidative damage with a slightly different mechanism. These two compounds promote the activation of the AMPK pathway, a central regulator of cellular energy metabolism and antioxidative mechanisms. AMPK regulates the homeostasis of energy requirements and nutrient usage via protein and lipid metabolism (Lin et al., 2017). Dammarane triterpenes from Gynostemma longipens significantly activated AMPK in murine C2C12 cells, leading to the escalation of glucose uptake and mitochondrial ATP synthesis. This condition was accompanied by the enhancement of myoblast proliferation, one potential mechanism to intensify muscle regeneration (Ha et al., 2019). Tangeretin extract, from Poncirus trifoliate, also led to AMPK signal activation which affected anti-inflammatory activity in vitro and in vivo. Beside supporting glucose uptake in muscle cells, AMPK activation eventually leads to the downregulation of several adipocytokines. Tangeretin extract also showed potential as an anti-diabetic compound, though further studies are needed (Kim et al., 2012).
3.1.3 Enhanced myogenesisMost bioactive compounds from inedible plant have a potential role in promoting skeletal muscle regeneration (promyogenic agents). Extracts from Broussonetia kazinoki, Coptis japonica, Corydalis turtschaninovi, and Psolarea corylifolia can activate p38 MAPK pathways and enhance the expression of myogenic factors. By using murine C2C12 skeletal muscle cells as a model, each plant extracts showed a positive effect in generating differentiation in skeletal muscle. Isoquinoline alkaloids from the root extract of Coptis japonica (CJ) induced the upregulation of MyoD transcriptional activity and MHC expression, with a treatment of just 10 nM. Further investigation suggested that CJ extract intensifies the activation of p38 MAPK and Akt pathways, thus upregulating myogenic factors and stimulating myoblast differentiation (Lee et al., 2017a). Beside enhanced myogenesis and escalated MyoD activity through the activation of the p38 MAPK pathway, dehydrocorydaline extract from Corydalis turtschaninovii and bakuhicol extract from Psolarea corylifolia also restored the expression of myogenic factors and myotube formation in defective myoblasts. Dehydrocorydaline and bakuhicol rescued the myogenic differentiation defect in Cdo depletion-induced C2C12 cells, by inducing the activation of the p38 MAPK pathway (Yoo et al., 2016; Lee et al., 2016b). Furthermore, kazinol P-extract from Broussonetia kazinoki, tetrahydropalmatine from Corydalis turtschaninovii, and bakuhicol also showed the ability to induce trans-differentiation. These extracts upregulated the activation of P38 MAPK, promoting the trans-differentiation of fibroblast cells into myoblasts (Hwang et al., 2015; Lee et al., 2014; Lee et al., 2016b).
3.2 Edible plant extractsOne advantage of edible plant extracts as therapeutic agents for sarcopenia is the relative practicality for daily use. These plants are commonly consumed, either as main dishes or as a spice. Therefore, these products are, potentially, part of a healthy diet for maintaining muscle health. Table 2 shows plants possessing various activities in maintaining skeletal muscle health and preventing muscle aging. We defined four different roles for edible plant compounds, to potentially overcome sarcopenia: prevention of oxidative damage, promotion of skeletal muscle regeneration, anti-inflammation, and anti-atrophy.
| Plant Species | Compounds | Chemical Structures | Model | Functions | References | |
| 1. | Aegle marmelos | polyphenols | BALB/c mice | ·Prevented early fatigue effect ·Promoted antioxidant activity |
Nallamuthu, et al., 2014 | |
| 2. | Castanea sativa | Tocopherols (1), sphingolipids extract |  (1) (1) |
Murine C2C12 skeletal muscle cell | ·Enhanced myogenesis ·Suppressed muscle protein degradation |
Frati, et al., 2014 |
| 3. | Coffea sp. | chlorogenic acid (1), anhydrous caffeine (2), polyphenols |  (1)  (2) |
27-month male C57BL/6 mice | ·Increased muscle weight and strength ·Accelerated skeletal muscle regenerative capacity ·Suppressed the levels of pro-inflammatory cytokine |
Guo, et al., 2014 |
| 4. | Curcuma longa | curcumin |  |
32-month male F344xBN rats | ·Maintained skeletal muscle mass and functional response ·Prevented oxidative damage |
Receno, et al., 2019 |
| 5. | Camellia sinensis | epigallocatechin-3-gallate |  |
Murine C2C12 skeletal muscle cell | ·Suppressed muscle protein degradation and induced muscle protein synthesis | Mirza, et al., 2014 |
| 6. | Citrus aurantium | flavonoids (hesperidin (1), nobiletin (2), and naringin (3)) |  (1)  (2)  (3) |
Rat L6 skeletal muscle cell | ·Ameliorated inflammatory cytokine level ·Prevented oxidative damage |
Kim, et al., 2012 |
| 7. | Eriobotrya japonica | EtOH extract of leaf | 5 and 18-19- month male Sprague‑Dawley rats | ·Maintained muscle mass and strength ·Abrogated the age‑associated muscle atrophy |
Sung, et al., 2015 | |
| EtOH extract of leaf | Murine C2C12 skeletal muscle cell | ·Enhanced myogenesis ·Enhanced muscle growth and survival |
||||
| 8. | Myristica fragrance | EtOH extract of seeds | 80-week male Wistar rats | ·Maintained muscle mass ·Enhanced myogenesis and prevented muscle autophagy |
Pratiwi, et al., 2018 | |
| 9. | Vitis vinifera | resveratrol |  |
Murine C2C12 skeletal muscle cell | ·Maintained muscle mass ·Suppressed muscle protein degradation and induced muscle protein synthesis |
Wang, et al., 2014 |
| 10. | Schizandra chinensis | EtOH extracts of dried fruits | Adult SPF/VAF outbred CrljOri:CD1 (ICR) mice | ·Maintained muscle mass, strength, and weight ·Prevented sciatic denervation induced muscle atrophy ·Prevented oxidative stress and damage |
Kim, et al., 2018 | |
| 11. | Glycine max | isoflavones (genistein (1) and daidzein (2)) |  (1) (1) (2) |
Murine C2C12 skeletal muscle cell | ·Prevented TNF-α induced muscle atrophy ·Suppressed muscle protein degradation |
Hirasaka, et al., 2013 |
Polyphenols from Aegle marmelos (Nallamuthu et al., 2014), curcumin from Curcuma longa (Receno et al., 2019), and extract of Schizandra chinensis (Kim et al., 2016) have protective effects that can prevent oxidative damage in skeletal muscle. Aegle marmelos has antioxidant properties and induces the upregulation of endogenous antioxidant enzyme in BALB/c mice. Therefore, supplementation of polyphenols from Aegle marmelos could reduce peroxidation and over-expressed Heat Shock Protein (HSP) in mouse skeletal muscle tissue (Nallamuthu et al., 2014). Despite the fact that curcuminoids have not shown any significant antioxidant activity, Receno et al. (2019) found that the expression of Nrf2 protein is upregulated due to curcumin supplementation. Nrf2 protein can modulate the expression of genes related to antioxidant expression (Baldelli et al., 2013). Receno et al. (2019) stated that this may explain the lower oxidative damage marker in their curcumin-treated group, without curcumin itself acting as a direct quencher of ROS. In addition, dried fruit extract of Schizandra chinensis reduced the oxidative stress levels of adult SPF/VAF mice, beside promoting the expression of endogenous antioxidant.
3.2.2 Promote skeletal muscle regenerationSeveral plant substances have a substantial effect on the regenerative capacity of skeletal muscle. A coffee dilution was found to escalate the regenerative capacity of C57BL/6 mice skeletal muscle and to induce isolated satellite cells to proliferate (Guo et al., 2014). Although not directly involved in myogenesis, Eriobotrya japonica (loquat) leaf extract and Myristica fragrans seed (nutmeg) extract also affected muscle differentiation. According to Sung et al. (2015), loquat leaf extract could enhance the expression of creatine kinase and promote myogenesis through the stimulation of myogenic regulatory factor, in murine C2C12 cells. Loquat leaf extract has also been shown to affect signaling pathways which regulate muscle growth, proliferation, survival, and myogenic differentiation. In line with these two findings, supplementation by a seed extract of Myristica fragrans was proved to increase the expression of the myogenesis gene in old Wistar rats. Pratiwi et al. (2018) surmise that nutmeg seed extract may maintain skeletal muscle mass by enhancing satellite cell myogenesis, in aged rats.
3.2.3 Anti-inflammationCoffee (Coffea sp.) and Korean citrus (Citrus aurantium) have significant potential to prevent muscle damage, as anti-inflammatory substances. Beside its positive effect on stimulating satellite cell proliferation, coffee supplementation also suppressed the levels of interleukins IL-1α, IL-6, and TNF-α in C57BL/6 mice. Guo et al. (2014) stated that coffee contains many bioactive components and some of them have an immunomodulatory effect, such as kahweol (Kim et al., 2006). Coffee may partly suppress inflammatory activity, by its antioxidative mechanism, since it is also found to be a good source of antioxidants, and oxidative stress is one of the causes of inflammation. Similarly, Citrus aurantium also hampers the expression of inflammatory cytokines in LPS-induced L6 skeletal muscle cells (Kim et al., 2012). Flavonoids extract, from Korean citrus, significantly inhibited mRNA and protein expression of iNOS, COX-2, TNF-α, IL-6, and NF-κβ. iNOS and COX-2 are inducible enzymes, expressed during inflammation. Furthermore, flavonoid from Korean citrus (hesperidin, nobiletin, and naringin) regulated MAPK pathways, which mediate the inflammatory response. This extract also inhibited phosphorylation and induced the degradation of I-kB, thereby blocking NF-kB activation.
3.2.4 Anti-atrophyEpigallocatechin-3-gallate (EGCg) from Camellia sinensis (tea) attenuated the alteration of muscle protein turnover, in C2C12 skeletal muscle. Mirza et al. (2014) found that EGCg restored muscle protein synthesis after depression induced by TNF-α. This extract also restored the over-expressed proteasome to basal level, which caused the suppression of degradation of muscle protein. At high concentration, EGCg affected PI3K/Akt and FoxO signaling pathways, well known in their regulation of muscle protein turnover. All these effects led to the reduction of atrophy gene expression in the EGCg-treated group. Beside its pronounced effect on myogenesis, in vitro, Sung et al. (2015) also found that a leaf extract of Eriobotrya japonica could maintained muscle mass in old Sprague‑Dawley rats by increasing the activity of creatine kinase. This loquat extract also helped restore the cross-sectional muscle area and decreased the amount of connective tissue, in aged rats. The results exhibited the anti-atrophy activity. Resveratrol from Vitis vinifera showed anti-atrophy activity in muscle mass, in vitro By affecting the Akt/mTOR/FoxO1 signaling pathway, 100 µM of resveratrol extract prevented C2C12 atrophy induced by TNF-α. Resveratrol extract was also depressed the expression of ubiquitin ligase genes: MAFbx and MuRF1. These results indicated that resveratrol inhibits atrophy responses as well as inducing hypertrophy (Wang et al., 2014). Beside regulating the balance between antioxidant and oxidative stress levels, the dried fruit extract of Schizandra chinensis also showed convincing activity as an anti-atrophy compound. This extract preserved skeletal muscle mass and strength in adult SPF/VAF mice. Such a profound effect is likely to be due to intensified expression of genes related to muscle protein synthesis (Akt1 and PI3K) and muscle growth (A1R, TRPV4), while downregulating the expression of genes for muscle protein degradation (atrogin-1, MuRF1) and muscle growth inhibitor (myostatin) (Kim et al., 2018). Other polyphenols, which have shown a positive effect in maintaining muscle mass, are isoflavones extracted from Glycine max (soya bean). Hirasaka et al. (2013) found that 100 µM of isoflavone extract could prevent muscle atrophy in C2C12 mouse myoblasts, induced by TNF-α. Specifically, isoflavone counteracts the effect of TNF-α by increasing the expression of PGC-1α and SIRT1.
Recently, sarcopenia has become the most problematic geriatric condition in the rapidly-expanding elderly populations of many countries. In the search for an effective medication for sarcopenic patients, the utilization of bioactive plant compounds, as alternative therapeutic options, are often ruled out. In fact, compared with conventional medicine, natural compounds show less physiological and psychological addiction among patients, with relatively few side effects. Several studies investigating the effect of botanical compounds on muscle health have showed promise. This review is focused on the potential effects of phytochemical compounds, in relation to sarcopenia. In general, plant products can be categorized into two main groups, based on their general status in the human diet: from an inedible or edible plant source. We suggest that bioactive compounds from both groups have several potential benefits in the prevention of sarcopenia. Some of them are similar, although several distinct roles are found, in each group. Biochemical compounds from both groups had three main roles in maintaining skeletal muscle health: anti-atrophy, preventing oxidative damage, and enhancing myogenesis. We also found anti-inflammatory activity from several, edible plant extracts. Although the results discussed in this review paper show promise, further intensive studies are still needed to support all these findings. Much of the research has used animals and cell cultures as models for evaluating the effectiveness of plant bioactive compounds. Research that involves humans, as a main model, is relatively rare. Therefore, there is still a long way to go to find effective, low-risk phytochemical compounds, for use in the effective treatment of sarcopenia.