Abstract
Osteoarthritis (OA) is a degenerative disorder that is characterized by articular cartilage destruction. The level of tenascin-C (TNC), an extracellular matrix protein, is increased in synovial fluid (SF) from OA patients, and TNC concentration in SF correlates with the severity of OA in the knee. Tenascin-X (TNX), another member of the tenascin family, has been identified as a causative protein of classical-like Ehlers-Danlos syndrome (clEDS). In this study, we investigated the correlation between TNX concentration and the degree of cartilage denaturation in OA patients. TNX concentrations in SF from patients with meniscus tears and from OA patients were lower than those of control sera. However, significant difference in TNX concentration in SF was not observed between patients with meniscus tears and OA patients. Furthermore, a significant correlation was not found between TNX concentration in SF and severity of OA in the knees of patients. These results suggest that TNX might not be as useful as TNC as a biomarker.
INTRODUCTION
Tenascins (TNs) form a family of related extracellular matrix (ECM) proteins. There are four tenascins: tenascin-C (TNC), tenascin-R (TNR), tenascin-X (TNX), and tenascin-W (TNW) [
1]. TNs consist of 4 distinct domains: heptad repeats, epidermal growth factor (EGF)-like repeats, fibronectin type III (FNIII)-like repeats, and a carboxy-terminal fibrinogen-like globe (FBG) [
1].
TNC is a multifunctional ECM protein and is associated with tissue injury and repair processes [
2]. While TNC expression is normally restricted in most adult tissues, it increases during acute inflammation [
3]. In OA and rheumatoid arthritis, the expression of TNC increases in both the cartilage and synovium [
4,
5].
On the other hand, TNX has crucial roles in the deposition of collagen fibrils [
6]. Furthermore, TNX has been identified as a causative protein of clEDS [
7]. Complete deficiency of TNX (clEDS) causes an autosomal recessive EDS that is characterized by joint hypermobility, hyperextensible skin without atrophic scarring, and easy bruising [
8]. We have already established a quantification method for serum form of TNX (sTNX) by using nano-liquid chromatography tandem mass spectrometry (LC/MS/MS) and reported that this method is useful for diagnosis of clEDS [
9].
OA is a degenerative disorder that is characterized by articular cartilage destruction. In clinical practice, the disease is generally assessed by using standard radiographic images of the joint [
10]. Several biochemical biomarkers for detecting the disease and determining the degree of severity of OA have also been examined. Although it was shown that TNC could be used as a biomarker for advanced OA, it does not seem to be sufficiently sensitive for early OA [
11].
Since TNX and TNC display reciprocal distributions during development [
12] and their abnormalities are associated with joint diseases [
8,
11], TNX levels in SF might be changed during the process of OA progression and TNX might be a more sensitive biomarker than TNC for OA. So far, only the tissue distribution of TNX in synovial samples of OA determined by analyses of the results of immunostaining experiments has been reported [
13].
In this study, we investigated the correlation between severity of OA in the knees and TNX concentrations in SF in OA patients.
MATERIALS AND METHODS
Patients and samples
SF samples were obtained at the time of primary total joint arthroplasty or arthroscopic surgery for 17 women and 2 men with OA of the knee who fulfilled with the American College of Rheumatology criteria [
14]. The knees of the OA patients were divided into four groups based on radiographic grading of the OA severity described by Kellgren and Lawrence [
10]. In the present study, we analyzed 5 OA patients with grade 3 severity, and 14 OA patients with grade 4 severity. There were no OA patients with grade 1 and grade 2 severities. On the other hand, SF samples from patients (2 women and 3 men) with knee joint pain caused by meniscus tears were used as control samples, since we could not obtain SF samples from healthy knee joints ethically. All of the patients were required to give informed consent to be included in this study. This study was approved by the ethics committees of Mie University and Shimane University. Commercially available human sera (Lonza) (Biowhittaker, Walkersville, MD, USA) were purchased and were used as control sera. Sample preparations were performed according to the method described previously except for hyaluronidase treatment of SF [
9]. To reduce the viscosity of SF, SF samples were treated with hyaluronidase [
15].
Nano-LC/MS/MS
To determine TNX concentrations in SF and serum, nano-LC/MS/MS analysis was carried out as described in our previous paper [
9]. Since the molecular mass of TNX in SF (138 kDa) was identical to that in serum (sTNX) [
9], TNX in SF was also designated as sTNX.
Statistical analysis
The Mann-Whitney U-test and Kruskal-Wallis test were used to analyze differences between the groups. Correlation was evaluated by Spearman’s rank correlation test. A p-value < 0.05 was considered to indicate a statistically significant difference.
RESULTS AND DISCUSSION
To examine the correlation between severity of OA in the knees and sTNX concentrations in SF from patients with OA, we measured sTNX concentrations in SF samples from nineteen OA patients and five patients with meniscus tears as control samples by using nano-LC/MS/MS. There was no difference between the two groups in age (p = 0.630), weight (p = 0.153), BMI (p = 0.851) or gender (p = 0.138) as shown in
Table 1.
sTNX concentration in control serum was 107 ng/mL. This value was almost the same as the previous reported concentration (144 ng/mL) [
9]. The sTNX concentrations in SF samples from patients with meniscus tears (control) and from OA patients (grade 3 and grade 4 groups) were 33 ng/mL (median, control), 34 ng/mL (median, grade 3 group), and 34 ng/mL (median, grade 4 group), respectively (
Fig. 1 and
Table 1). sTNX concentrations in SF samples from both patients with meniscus tears and OA patients were about one third of that in the control serum. However, there was no significant difference in sTNX levels in SF samples between patients with meniscus tears and OA patients (grade 3 and grade 4) (p = 0.897) (
Fig. 1 and
Table 1).
Furthermore, Spearman’s rank correlation test indicated that there was no significant correlation (r = 0.319, p = 0.698) between the severity of OA in knees and sTNX concentrations in OA patients.
Li et al. [
13] investigated the tissue distributions of TNX in synovial samples from OA patients and control traumatic knees by immunostaining using a polyclonal antibody against the EGF-like repeats of murine TNX. This antibody recognizes the mature form of TNX as a ~500-kDa protein [
13] but not sTNX, a 138-kDa protein that consists of the 23rd to 33rd FNIII repeats (hu23-hu33) followed by an FBG domain. Li [
13] reported that the staining intensity for TNX in synovial samples from OA patients was significantly increased compared with that in synovial samples from control traumatic knees. However, the localization of TNX in OA is restricted to the inner synovial membrane. Therefore, Li et al. [
13] suggested that TNX is an essential component of the normal synovial membrane and that TNX increases locally in the synovial membrane of OA patients.
In contrast, we quantified sTNX in SF samples by using the peptide AVAVSGLDPAR in the 26th FNIII repeat (hu26) in sTNX with nano-LC/MS/MS [
9]. The levels of sTNX in SF samples were not different between patients with meniscus tears and OA patients (
Fig. 1). Therefore, the differences in the results for TNX in the present study by Li et al. [
13] and our study might be caused by the difference in the analytical method.
In conclusion, sTNX concentrations in SF samples from both patients with meniscus tears and OA patients were about one third of that in the control serum. Significant difference in the sTNX concentration in SF was not observed between patients with meniscus tears and OA patients and a significant correlation was not found between sTNX concentration in SF and severity of OA in the knees of patients. Therefore, TNX might not be as useful as TNC as a biomarker for OA. However, since this study was performed with the small sample size, further analyses with larger sample size are needed to study the precise relationship between severity of OA in the knees and sTNX concentrations in OA patients.
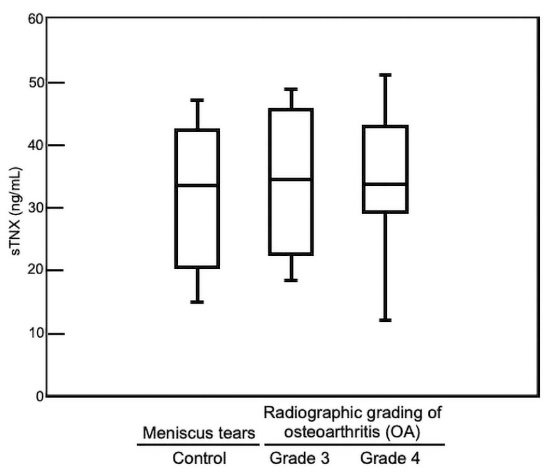
Table 1.
Characteristics and sTNX concentrations of OA patients*
|
Control
n = 5
|
OA
n = 19
|
p-value
|
|
Grade 3
n = 5
|
Grade 4
n = 14
|
| Age (years) |
74.0 (55-81) |
79.0 (65-87) |
72.5 (58-82) |
0.630 |
| Weight (kg) |
72.0 (58-84) |
62.0 (37-65) |
59.0 (49-94) |
0.153 |
| BMI (kg/m2)
|
26.8 (23.8-29.0) |
24.8 (20.0-28.7) |
27.1 (22.5-39.1) |
0.851 |
| Percentage of women |
40 |
100 |
86 |
0.138 |
| sTNX (ng/mL) |
33 (15-47) |
34 (18-49) |
34 (12-51) |
0.897 |
*Except for the percentage of women, values are medians (range).
Acknowledgments
This work was supported by the Japan Society for the Promotion of Science KAKENHI grant number 26462296 from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Conflict of interest
All authors declare no conflict of interests.
References
- 1)
Adams
JC
,
Chiquet-Ehrismann
R
,
Tucker
RP
.
The evolution of tenascins and fibronectin.
Cell Adh Migr
2015;
9:
22-
33. doi:
10.4161/19336918.2014.970030.
- 2)
Midwood
KS
,
Orend
G
.
The role of tenascin-C in tissue injury and tumorigenesis.
J Cell Commun Signal
2009;
3:
287-
310. doi:
10.1007/s12079-009-0075-1.
- 3)
Chiquet-Ehrismann
R
,
Chiquet
M
.
Tenascins: regulation and putative functions during pathological stress.
J Pathol
2003;
200:
488-
99. doi:
10.1002/path.1415.
- 4)
Chevalier
X
,
Groult
N
,
Larget-Piet
B
,
Zardi
L
,
Hornebeck
W
.
Tenascin distribution in articular cartilage from normal subjects and from patients with osteoarthritis and rheumatoid arthritis.
Arthritis Rheum
1994;
37:
1013-
22. doi:
10.1002/art.1780370706.
- 5)
Salter
DM
.
Tenascin is increased in cartilage and synovium from arthritic knees.
Br J Rheumatol
1993;
32:
780-
6. doi:
10.1093/rheumatology/32.9.780.
- 6)
Bristow
J
,
Carey
W
,
Egging
D
,
Schalkwijk
J
.
Tenascin-X, collagen, elastin, and the Ehlers-Danlos syndrome.
Am J Med Genet C Semin Med Genet
2005;139C:
24-
30. doi:
10.1002/ajmg.c.30071.
- 7)
Burch
GH
,
Gong
Y
,
Liu
W
,
et al.
Tenascin-X deficiency is associated with Ehlers-Danlos syndrome.
Nat Genet
1997;
17:
104-
8. doi:
10.1038/ng0997-104.
- 8)
Malfait
F
,
Francomano
C
,
Byers
P
,
et al.
The 2017 international classification of the Ehlers-Danlos syndromes.
Am J Med Genet C Semin Med Genet
2017;
175:
8-
26. doi:
10.1002/ajmg.c.31552.
- 9)
Yamada
K
,
Watanabe
A
,
Takeshita
H
,
Matsumoto
K
.
A method for quantification of serum tenascin-X by nano-LC/MS/MS.
Clin Chem Acta
2016;
459:
94-
100. doi:
10.1016/j.cca.2016.05.022.
- 10)
Kellgren
JH
,
Lawrence
JS
.
Radiological assessment of osteoarthritis.
Ann Rheum Dis
1957;
16:
494-
501. doi:
10.1136/ard.16.4.494.
- 11)
Hasegawa
M
,
Hirata
H
,
Sudo
A
,
et al. Tenascin-C concentration in synovial fluid correlates with radiographic progression of knee osteoarthritis
.
J Rheumatol
2004;
31:
2021-
6.
- 12)
Matsumoto
K
,
Saga
Y
,
Ikemura
T
,
Sakakura
T
,
Chiquet-Ehrismann
R
.
The distribution of tenascin-X is distinct and often reciprocal to that of tenascin-C.
J Cell Biol
1994;
125:
483-
93. doi:
10.1083/jcb.125.2.483.
- 13)
Li
TF
,
Warris
V
,
Ma
J
,
et al.
Distribution of tenascin-X in different synovial samples and synovial membrane-like interface tissue from aseptic loosening of total hip replacement.
Rheumatol Int
2000;
19:
177-
83. doi:
10.1007/s002960000044.
- 14)
Altman
R
,
Asch
E
,
Bloch
D
,
et al.
Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee.
Arthritis Rheum
1986;
29:
1039-
49. doi:
10.1002/art.1780290816.
- 15)
Liao
H
,
Wu
J
,
Kuhn
E
,
et al.
Use of mass spectrometry to identify protein biomarkers of disease severity in the synovial fluid and serum of patients with rheumatoid arthritis.
Arthritis Rheum
2004;
50:
3792-
803. doi:
10.1002/art.20720.

