2016 年 239 巻 3 号 p. 203-211
2016 年 239 巻 3 号 p. 203-211
G protein-coupled receptor 56 (GPR56) is an atypical G protein-coupled receptor, with the long extracellular N-terminus. GPR56 can trigger various downstream signaling responsible for cell survival, proliferation, adhesion, and migration. Expression of GPR56 is associated with cell malignant transformation and tumor cell metastasis in several carcinomas such as melanoma and glioma. Osteosarcoma is the most common malignant bone tumor in adolescents and young adults with high metastasis tendency. The overall survival of osteosarcoma is unsatisfied, partially due to the lacking of predictive markers for metastasis and overall prognosis. This study aimed at figuring out whether expression of the GPR56 was associated with clinicopathological features of osteosarcoma. Eighty-nine patients who received osteosarcoma operation between March 2004 and February 2011 in Linyi People’s Hospital were recruited. Immunohistochemical staining (IHC) was carried out to identify the expression of GPR56 in those osteosarcoma tissues, and our cohort was divided into higher-expression group and lower-expression group according to the cut-off of IHC score. Expression of GPR56 in osteosarcoma tissues was correlated with the TNM stage and overall survival. Univariate and multivariate analysis showed that GPR56 could act as an independent prognosis factor for osteosarcoma. Western blot results demonstrated that GPR56-siRNA down-regulated the expression of GTP-RhoA and Ki67. GTP-RhoA participates in the cell migration process, while Ki67 plays important roles in cell proliferation, indicating GPR56 may function in tumor development. Correspondingly, we show that GPR56 regulates the proliferation and invasion capacity of osteosarcoma cells. Our study has revealed the prognostic value of GPR56 expression in osteosarcoma.
Osteosarcoma, deriving from primitive bone-forming mesenchymal cells, is the most common malignant bone tumor in adolescents and young adults, which mainly affects the metaphysis of long bones (Picci 2007; Ottaviani and Jaffe 2009). It was reported that 40-80% of osteosarcoma patients showed pulmonary metastasis with poor prognosis (Bielack et al. 2002; Ohtani et al. 2008; Savage et al. 2013). The 5-year overall survival rate is only about 20% in patients with metastatic disease, although it is almost 65% in patients with localized disease (Bielack et al. 2008; Chen et al. 2013). The overall survival of osteosarcoma patients has been markedly improved over the past decades due to the development of combination treatment of neoadjuvant chemotherapy and radical surgery (Chou et al. 2008); however, nearly 80% of the osteosarcoma patients with metastasis will recur. Moreover, even for the patients without metastasis at the time of diagnosis, the 5-year disease-free survival is no more than 40% (Bacci et al. 2008). Consequently, it is of critical importance to characterize novel biomarkers to better predict the patient prognosis as well as exploring the molecular mechanisms involved in the osteosarcoma progression and metastasis.
G protein-coupled receptor (GPCR) is one of the largest families of membrane proteins, at least 30% of clinical drugs target GPCRs nowadays (Drews 2000). G protein-coupled receptor 56 (GPR56) belongs to the adhesion GPCRs, characterized with a long extracellular domain (ECD) and transmembrane domain (TM). It has been reported that GPR56 interacts with CD81 (Little et al. 2004), collagen III (Casey et al. 2012) and transglutaminase 2 (TG2) (Xu and Hynes 2007). GPR56 was highly expressed in neuronal progenitor cells of the cerebral cortical ventricular and played important roles in the migration of neuronal progenitor cells through RhoA signaling pathway (Piao et al. 2004; Iguchi et al. 2008). It was reported that GPR56 was down-regulated in highly metastatic human melanoma cells, and its overexpression can inhibit melanoma progression and invasion (Xu et al. 2006). In contrast, GPR56 can positively regulate tumor growth and metastasis of glioma (Shashidhar et al. 2005). Therefore, the expression and function of GPR56 may be distinct in different tumors.
In the current study, we aimed to explore the expression pattern of GPR56 in osteosarcoma and evaluate its prognostic value. The corresponding signaling pathways also needed to be figured out to verify the underlying molecular mechanism, this may be helpful to a better understanding of its distinct roles among different tumors.
This study was approved by the Research Ethics Committee of Linyi People’s Hospital (Shandong, China). Written informed consent was obtained from all patients. All specimens were handled and made anonymous according to ethical and legal standards. A total of 89 patients diagnosed with primary osteosarcoma between March 2004 and February 2011 from Linyi People’s Hospital were selected for this retrospective analysis. All the patients enrolled in this study underwent the pre-operative biopsy for diagnosis, followed by pre-operative chemotherapy and primary tumor resection. Among them, 62 patients (69.7%) were treated with methotrexate, etoposide and ifosfamide; other patients received high-dose methotrexate and doxorubicin treatment. The following clinical parameters were collected: age, gender, tumor size, tumor location, histological type, lung metastasis, chemotherapy response, TNM stage, as well as the serum level of alkaline phosphatase and lactate dehydrogenase. The stage of the tumors was graded by the Enneking staging system (Enneking et al. 2003). Patients’ response to neoadjuvant chemotherapy was evaluated with the Huvos grading system, according to the necrosis sensitivity in the resected tumor specimen. Poor chemotherapy response showed < 90% tumor necrosis while good chemotherapy response suggested ≥ 90% necrosis in the resected tumors. The prognosis was evaluated as the months of overall survival from the tumor resection date up to February 2014 or the mortality of the patients.
Immunohistochemistry stainingAll the biopsy specimens were embedded in paraffin and cut into sections with de-waxed and rehydrated using a graded series of ethanol, followed by microwave antigen retrieval. After blocked with 0.3% hydrogen peroxidase, sections were incubated at 4°C overnight with GPR56 primary antibody (ab174697, abcam, Cambridge, MA, USA; 1:100). Immunostaining was conducted using the DAB kit. The sections were then followed with hematoxylin staining, dehydrated, cleared, and mounted. 5% fetal bovine serum (FBS) was used as the negative control.
Semi-quantitative analysis of IHC resultsThe staining results were evaluated by two independent pathologists. The GPR56 immunoreactivity was observed as cytoplasm and membrane staining. For the staining assessment, intensity was graded as score 0 (negative), 1 (weak, pale yellow), 2 (moderate, dark yellow), and 3 (strong, brown). The staining extent was scored by the percentage of the positive cells as 0 (0-5%), 1 (5-25%), 2 (26-50%) and 3 (51-100%). The final immunoreactivity score (IRS) was calculated by multiplying the intensity and percentage scores. The GPR56 staining were classified into low expression (IRS < 4) and high expression (IRS ≥ 4). The cut-off score was further tested by the receiver operating characteristics (ROC) curve analysis.
Isolation of primary osteosarcoma cells and cell cultureWe obtained the primary osteosarcoma cells from two patients (age 16 and 23, respectively) who underwent tumor resection without pre-operative chemotherapy. The informed consents from the two patients and their parents were collected. After washed with sterile D-Hanks for three times, the tumor tissues were cut into small pieces and digested with 0.25% trypsin containing 0.02% EDTA for 40 min. Then filtered the suspension with 40 mesh screen to obtain the separated cells. The MG63, U2OS (osteosarcoma cell lines) and hFOB 1.19 (osteoblast cell line) cells were purchased from ATCC (American Type Culture Collection). All the cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% FBS, penicillin and streptomycin at 37°C in 5% CO2.
siRNA transfectionTransient siRNA transfections of MG63 and U2OS cells were carried out following the manufacturer’s instructions. In brief, siRNAs were mixed with Lipofectamine 3000 and transfected into cells using Opti-MEM with reduced-serum for 4-6 h at approximately 50% confluence. All the siRNAs were ordered from Santa Cruze. The sequences of scrambled siRNA and GPR56 siRNA were as followed:
Scramble siRNA: 5′-UUCUCCGAACGUGUCACGU-3′
GPR56 siRNA: 5′’-AGAUUACAUCUUCUCUAUGGCAAGC-3′
Western blot analysisTo investigate the expression changes of the proteins in MG63, U2OS and hFOB 1.19 cells, the cells were lysed with NP-40 lysis buffer (Beyotime Biotechnology, Shanghai, China), and centrifuged at 12,000 rpm for 15 min at 4°C. The concentration of supernatant was quantified using Pierce BCA protein detection kit (Thermo Scientific, Hudson, NH, USA). Equal amount of protein (about 20 μg) was loaded in the SDS-PAGE gel, then transferred onto the PVDF membranes (Millipore Corp., Bedford, MA, USA). The PVDF membranes were then blocked with fresh prepared blocking buffer (0.5% BSA in TBST) at 25°C for 1 h. After incubation overnight at 4°C with primary antibodies (GPR56, ab174697, Abcam; Ki67, sc-7846, Santa Cruz Biotechnology; GTP-RhoA, #26904, NewEast Biosciences; total RhoA, sc-179, Santa Cruz Biotechnology; β-actin, sc-47778, Santa Cruz Biotechnology. All 1:1,000 dilution), PVDF membranes were incubated for another 45 min at 25°C with corresponding secondary antibodies. Identification of blotting immunoreactivities was performed by the chemiluminescence detection system (NEN Life Science Products, Boston, MA, USA) according to the manufacturer’s instructions.
Cell proliferation assayBriefly, 3 × 103 cells were treated with different siRNA. On day 1, 2, 3 and 4, these cells were incubated with 10 μl 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (5 mg/ml) for 4 h. After DMSO dissolving, spectrophotometric absorbance was examined on the microplate reader at OD 490 nm.
Cell invasion assayInvasion of osteosarcoma cells was evaluated by Matrigel invasion assays. A total of 4 × 105 cells were seeded into the upper chamber side of an 8-μm Matrigel-coated transwells (BD Biosciences, San Jose, CA, USA). After cultured for 12 h, the invasive cells on the lower chamber were fixed with 4% paraformaldehyde, stained with 0.05% crystal violet, and counted using a microscope. Five fields were counted and photographed for each transwell chamber at 200× magnification. The experiment was performed in triplicate.
Statistical analysisSurvival curves were calculated using the Kaplan-Meier algorism with GraphPad Prism 6.01. The correlation between GPR56 expression and the clinicopathological parameters of osteosarcoma patients were evaluated by Chi-square test using SPSS 18.0. The significance was calculated using two-tailed Student’s t test. Univariate and multivariate statistics was performed with SPSS 18.0 using Kaplan-Meier survival analysis and Cox regression analysis, respectively. P value less than 0.05 was considered as statistically significant.
Overall, 51 males and 38 females with osteosarcoma were enrolled in our retrospective study. The mean age of those patients was 22.7 ± 6.5 years (range 10-58 years). The median survival time was 62 months. Median tumor size at diagnosis was 9 cm (range 2-18 cm). Histologic subtype classification indicated there were 63 cases of osteoblastic osteosarcoma, 14 cases of chondroblastic osteosarcoma and 6 cases of fibroblastic sarcoma. With regard to tumor location, 58 cases were located in the tibia or femur and 31 in other locations. Comparison of clinical characteristics according to GPR56 expression is shown in Table 1.

The correlations between GPR56 expression and clinical parameters.
#Chi-square test, *Statistically significant.
GPR56, G protein coupled receptor 56.
We performed the IHC staining to explore the expression of GPR56 in clinical osteosarcoma tissues. Through IHC results, we found that GPR56 was highly expressed in certain clinical osteosarcoma samples (35/89, 39.3%), mainly located in the cytoplasm and cell membrane (Fig. 1). In order to estimate the effects of GPR56 expression on clinical characteristics, we analyzed the relationships between GPR56 expression and clinical factors, including gender, age, tumor size, histologic subtype, tumor location, lung metastasis, chemotherapy response, TNM stage as well as the serum levels of alkaline phosphatase and lactate dehydrogenase. However, our findings suggested that there were no statistically correlations between GPR56 expression and the clinicopathological parameters except the lung metastasis (P = 0.039) and TNM stage (P = 0.043, Table 1).

Representative immunohistochemistry results of GPR56 in chemotherapy-naïve primary tumor samples.
(A) Negative GPR56 expression: both the staining intensity and percentage were scored as 0, so the final score was 0. (B) Low GPR56 expression: the staining intensity was scored as 1, the percentage was scored as 2, the final score was 2. (C) High GPR56 expression: both the intensity score and percentage score were 3, so the final score was 9.
Arrows: positive cells. Scale bar: 50 μm.
To evaluate the role of GPR56 expression in osteosarcoma, we analyzed the 5-year overall survival rate for the 89 osteosarcoma patients with the Kaplan-Meier survival analysis, and demonstrated that cases with higher GPR56 protein expression had poorer prognosis (P < 0.001) compared with those showing lower GPR56 expression (Table 2, Fig. 2F). Additionally, tumor size (P = 0.047), lung metastasis (P < 0.001), chemotherapy response (P = 0.022), and TNM stage (P < 0.001) were all associated with 5-year overall survival of osteosarcoma patients (Table 2).
To further explore their independent predictive value, a multivariate Cox proportional-hazards model was performed within the all significant parameters detected by univariate analysis. The results showed that TNM stage (P = 0.043) was an independent prognostic factors in osteosarcoma patients, consistent with other reports. In addition, GPR56 expression level also appeared as a significant independent prognostic biomarker for the 5-year overall survival in osteosarcoma patients (Hazard ratio = 2.546, 95% Confidence Interval: 1.157-5.603, P = 0.020) (Table 3). However, tumor size, lung metastasis and chemotherapy response were not found to be independent prognostic indicators (Table 3).

Univariate analysis of overall survival in patients with osteosarcoma.
#Log-rank test, *Statistically significant.
GPR56, G protein coupled receptor 56; NR, not reached.
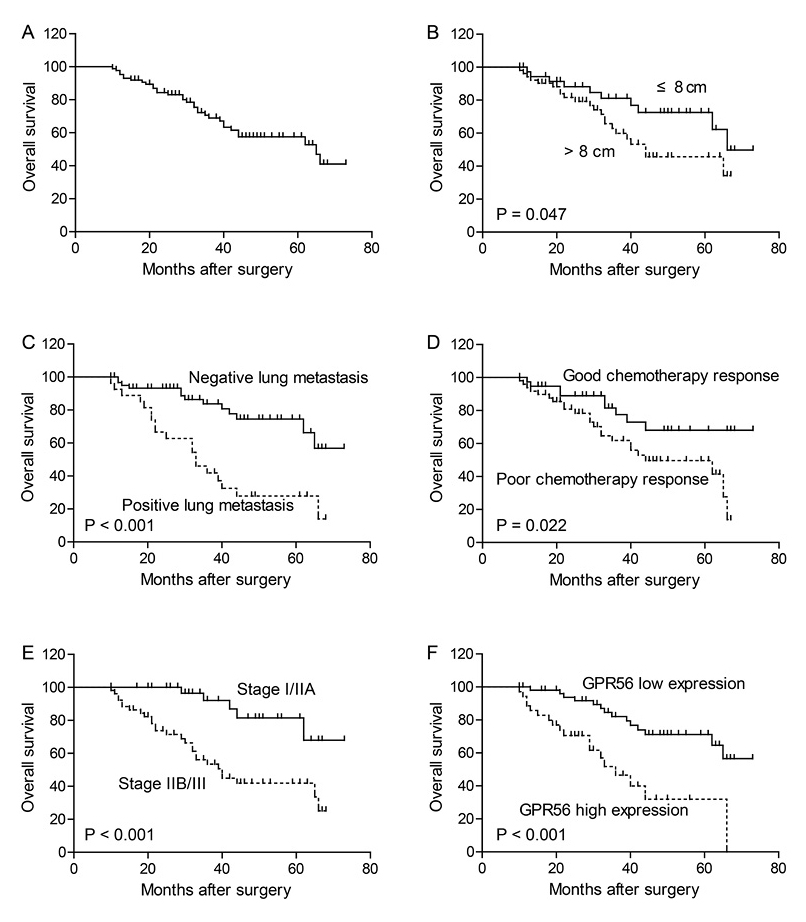
Kaplan-Meier analysis for the overall survival of osteosarcoma patients.
The 5-year overall survival rate of the patients was 57.5% (A). Patients with larger tumor size (B), positive lung metastasis (C), poor chemotherapy response (D), advanced TNM stage (E) and higher GPR56 expression (F) showed poorer overall survival than other patients.

Multivariate analysis of overall survival.
#Cox proportional hazards regression, *Statistically significant.
GPR56, G protein coupled receptor 56.
We carried out knock-down experiments to elucidate the molecular mechanisms of GPR56 in regulating the tumor progression of osteosarcoma patients. We found similar expression level of GPR56 in primary osteosarcoma cells compared with MG63 and U2OS osteosarcoma cell lines, all higher than that in the osteoblast hFOB 1.19 cells (Fig. 3A, B). Moreover, the expression level of GTP-RhoA and Ki67 were reduced in response to GPR56 knock-down in both MG63 cells (Fig. 3C-E) and U2OS cells (Fig. 3F-H), compared to the corresponding controls. This indicated that GPR56 may regulate the signaling through GTP-RhoA and Ki67 pathways in osteosarcoma cells. To evaluate the biological functions of GPR56 in osteosarcoma cells, we further performed cell proliferation assay as well as the Tranwell assay. The results showed that knock-down of GPR56 expression can significantly inhibit the cell proliferation as well as cell invasion capacity in both MG63 and U2OS cells (Fig. 4).

Expression and downstream signaling of GPR56 in osteosarcoma cells.
Through Western Blot analysis, we verified that GPR56 was highly expressed in primary osteosarcoma cells (POC #1 and POC #2, represent different primary cell lines from two patients) as well as MG63 and U2OS osteosarcoma cell lines, compared with normal osteoblast cells (A). The semi-quantification of the Western Blot results was analyzed by Image J software and showed in (B). GPR56-siRNA experiment was performed and Western Blot results demonstrated that GPR56 knock-down can down-regulate the GTP-RhoA and Ki67 protein level in MG63 (C) and U2OS cells (F) without changing the total RhoA expression. The semi-quantification of the Western Blot results was showed in (D, E) and (G, H), respectively. Comparisons of results from the control and tested groups were performed by Student t test. Error bars are standard deviation of the mean (SD) calculated from three experiments performed in parallel. *P < 0.05. POC, primary osteosarcoma cells.

GPR56 regulates the biological characteristics of osteosarcoma cells.
MTT assays showed that GPR56 knock-down can inhibit the cell proliferation of MG63 (A) and U2OS cells (B), indicating the oncogenetic role of GPR56 in tumor development. Transwell assays demonstrated that silencing of GPR56 can significantly inhibit the invasion capacity of osteosarcoma cells (C), consistent with the clinical results that GPR56 was correlated with lung metastasis and TNM stage. Statistical comparisons of results from different groups were evaluated by Student t test. Error bars are standard deviation of the mean (SD) calculated from three experiments performed in parallel. *P < 0.05.
Despite the substantial progress in the diagnosis and treatment of osteosarcoma has been made, the overall survival of osteosarcoma is still unsatisfied due to the fast cell proliferation and metastasis. More and more concentration has been focused on figuring out specific prognostic biomarkers that can predict osteosarcoma metastasis and the overall prognosis. GPR56 belongs to the adhesion GPCRs and participate in the various cell functions such as cell proliferation, adhesion and migration. It has been proved that GPR56 expression was negatively correlated with the metastatic capacity of melanoma cells (Zendman et al. 1999; Xu et al. 2006). However, there were several reports indicating GPR56 may be positively involved in the tumorigenesis of glioma and digestive cancers (Shashidhar et al. 2005; Ke et al. 2007). Our study aimed to investigate the expression and role of GPR56 in osteosarcoma, which will be helpful to develop novel biomarkers for osteosarcoma and also enrich the knowledge of GPR56.
In the current study, we identified the membrane and cytoplasm expression of GPR56 in clinical osteosarcoma tissues with IHC staining. The expression of GPR56 was correlated with TNM stage and lung metastasis of osteosarcoma. In addition, we validated GPR56 expression as an independent prognostic marker for the 5-year overall survival from two independent osteosarcoma cohorts.
We verified the higher expression of GPR56 in primary osteosarcoma cells from patients’ material, compared with that in the osteoblast hFOB 1.19 cells. Similar results were also found in the MG63 and U2OS osteosarcoma cell lines. To further investigate the mechanisms of GPR56 in the regulation of osteosarcoma development, we performed the siRNA knock-down experiments and found that knock-down of GPR56 can down-regulate the Ki67 expression level, reflecting the critical role of GPR56 in tumor progression. Besides, the active RhoA-GTP protein level was also decreased with GPR56 knock-down. RhoA-GTP can promote the cell invasion and metastasis through Rho-associated coiled coil-containing protein kinase (ROCK) and MAP kinase signaling pathways. Thus, we carried out the in vitro experiments to see whether GPR56 can regulate the biological phenotype of osteosarcoma cells, showing that GPR56 can positively regulate the proliferation and invasion capacity of osteosarcoma cells.
In conclusion, we have demonstrated the prognostic value of GPR56 in osteosarcoma.
The authors declare no conflict of interest.