2019 Volume 248 Issue 2 Pages 63-71
2019 Volume 248 Issue 2 Pages 63-71
Oxidative stress (OS) frequently contributes to the development of acute kidney injury (AKI). Iron can promote oxidative stress and tissue injury by catalyzing free reactive oxygen species (ROS) generation and increasing the steady-state concentration of these potent oxidants. The anticipated role of ferritin is to protect from OS by sequestering iron and limiting its involvement in reactions that generate ROS. In this prospective study, we aimed to investigate the association between serum ferritin levels and kidney function recovery among patients with AKI. Renal recovery was determined as a return of serum creatinine to less than 1.25 times the baseline value after 90 days of follow-up. One hundred twelve patients (72 males and 40 females, 63.68 ± 10.6 years old) were included in the final analysis. They were divided into AKI recovery (n = 76) and non-recovery groups (n = 36). Ferritin levels on admission were higher in AKI recovery group [284 (IQR 153-525) ng/mL] compared with the non-recovery group [127.4 (IQR 30-243) ng/mL], p < 0.001. Serum ferritin levels and the renal recovery significantly positively correlated (r = 0.72, p < 0.001). In multiple linear regression analysis, higher serum ferritin was associated with renal function recovery (OR 3.68, CI 2.02-3.97, p < 0.001). The optimal cut-off point of 240.5 ng/mL was determined for serum ferritin, which showed a sensitivity of 75.8% and a positive predictive value of 90%. In conclusion, serum ferritin levels on admission may be used as a prognostic marker for predicting renal recovery in AKI patients.
Acute kidney injury (AKI) is a frequent and severe complication in hospitalized patients and is associated with a prolonged hospital stay, increased morbidity, and mortality (Chawla and Kimmel 2012; Pannu et al. 2013; Nisula et al. 2013; Rewa and Bagshaw 2014). Therefore, AKI represents a significant public health problem as the reported incidence is 0.25% in the overall population and 18% in hospitalized patients (Palevsky et al. 2013). AKI survivors are at increased risk of chronic kidney disease (CKD) that may progress to end-stage renal disease (ESRD) (Chew et al. 2017). The AKI pathogenesis is deeply complex as a result of many insults, as well as the involvement of different individual and overlapping pathophysiological pathways. Accordingly, many different molecular mechanisms have been implicated in the pathogenesis of AKI, although kidney injury caused by reactive oxygen species (ROS) is recognized as one of the fundamental principles (Kasuno et al. 2014; Virzi et al. 2015; Pavlakou et al. 2017). ROS may injure the kidney in multiple ways. The generation of ROS activates mitogen-activated protein kinase (MAPK), JAK-STAT, or other pathways resulting in tubular epithelial cell damage (Aaronson and Horvath 2002; Kim and Choi 2010).
As a consequence, the fibrotic process is intensified by enhanced inflammation. Fibrosis and inflammation itself might feedback to the pathway and accordingly increase ROS generation, stimulating the production of cytokines and growth factors, provoking apoptosis, necrosis, and renal tubular cell death (Winterberg et al. 2013; Hosohata 2016). One of the key generators of reactive oxygen species in AKI is a free iron and various animal trials demonstrated that labile iron is involved in different types of kidney damage (Shah et al. 2011; Leaf et al. 2014), particularly in nephrotoxicity from ischemia-reperfusion (Baliga et al. 1993), aminoglycosides (Walker and Shah 1988), cisplatin (Baliga et al. 1998), rhabdomyolysis (Baliga et al. 1996), and hemoglobinuria (Paller 1988). Although the iron is crucial for normal cellular function and is a constituent of many essential proteins, excessive amounts of free iron can engage in various reactions that generate free radicals, through the iron-catalyzed Haber-Weiss and Fenton reaction. These reactions result in the formation of extremely reactive hydroxyl radicals that can damage DNA, lipids, and proteins, causing cellular oxidative damage (Shah et al. 2007; Haase et al. 2010). Despite conceivably toxic in its free labile form, the corresponding redox potential of iron makes it a crucial element for all of life. To maintain a delicate balance between homeostatic needs and to prevent possible harmful effects, several very synchronized regulatory mechanisms have emerged.
Ferritin is a distinct, highly conserved iron-binding protein which is mainly responsible for the preservation of iron homeostasis in cells. Ferritin has a substantial capacity for iron. Once converted to a mineral, iron becomes innocuous and cannot participate in redox reactions. That makes ferritin not only a valuable means to immediately store excess iron, but also an efficient way to detoxify the metal. Moreover, the synthesis of additional ferritin protein is stimulated by the influx of iron into the cell cytosol which is another feature of ferritin metabolism supportive of detoxification (Muckenthaler et al. 2008; Wang and Pantopoulos 2011). The existence of this vibrant and adaptive iron storage system emphasizes cells’ need to rapidly store and detoxify any incoming iron that is not immediately incorporated in the iron-containing proteins, aiming to prevent or minimize oxidative damage (Linder 2013). In other words, ferritin mitigates oxidative stress by sequestering iron and restricting its participation in chemical reactions that generate reactive oxygen species (Recalcati et al. 2008; Knovich et al. 2009). We, therefore, hypothesize that serum ferritin may alleviate elevated oxidative stress in AKI and thus contributes to the favorable renal outcome.
We conducted a prospective cohort study among the patients with the diagnosis of AKI admitted to the Clinical Center Nis, Serbia, during the two years period. Clinical Center Nis is a major teaching hospital and a specialist’s referral centers for the southeastern part of Serbia. The study protocol was approved by the Ethics Committee of Clinical Center Nis, Serbia. Associated clinical information was collected, including demographic data, comorbidity status, vital signs, baseline full blood count, biochemistry, and renal function tests. Further laboratory examinations were performed as indicated clinically thereafter.
Concerning the iron study, in addition to serum iron and serum ferritin levels, we measured plasma total iron binding capacity. The percentage of transferrin saturation was calculated as the ratio of total iron (in microgram per deciliter)/total iron binding capacity (TIBC) (in microgram per deciliter), multiplied by 100.
AKI was diagnosed referring to the patient’s serum creatinine (sCr) level, based on sCr increase of 26.5 µmol/L within 48 h, or a 1.5-fold increase compared to the baseline value, known or presumed to have occurred within the prior seven days. The severity of AKI was determined by the Kidney Disease: Improving Global Outcomes (KDIGO) staging criteria (KDIGO Acute Kidney Injury Work Group 2012). The KDIGO urine output criterion was not used to define AKI. The baseline sCr level was the latest stable measure obtained 1-3 months before admission with AKI. Lengths of the hospital stay were also recorded, and patients were followed during the hospital stay and for 90 days after the hospital discharge.
Patients with C-reactive protein (CRP) > 20 mg/L or leukocyte count > 10×109 were excluded from the present study with the intention to exclude subjects with infections, chronic inflammatory disorders or some hematological malignancies which might alter the analysis. Furthermore, we excluded patients with previous CKD diagnosis, hepatic insufficiency, oral iron supplement, cardiac shock and all the patients who had a history of receiving any blood or blood products between the beginning of their symptoms until admittance to the hospital.
If needed, renal replacement therapy (RRT) treatments were prescribed following internal procedures (intractable metabolic acidosis, persistent hyperkalemia, fluid overload not responding to diuretics, and anuria with increasing creatinine) with unfractionated or low molecular weight heparin as anticoagulation agents. All patients were on intermittent standard hemodialysis (HD).
The patients were divided into the renal recovery and the renal non-recovery group depending on the presence or absence of renal recovery, respectively.
Outcome measures were all-cause mortality from admission to 90-day follow-up, and renal function at three months [non-recovery was defined as sCr exceeded 25% of the baseline (preadmission value), development of ESRD or death]. Clinical management of the patients was defined by individual physicians.
Development of CKD in renal non-recovery patients was diagnosed according to the KDIGO guidelines (KDIGO CKD Work Group 2013).
Statistical analysisAll baseline demographic values for these two groups were compared using the Student’s t-test or Mann-Whitney rank sum test for continuous variables, and Fisher’s exact test for categorical variables, while correlations were made using the Pearsоn test. Linear regression analysis was performed to define independent parameters related to renal outcome. In the multiple regression model, we entered factors that were significant in univariate analysis. A receiver operating characteristic (ROC) analysis was performed to evaluate the predictive value of serum ferritin for renal recovery and determine the best cutoff value of serum ferritin. Results are presented as mean ± SD, or medians with interquartile ranges (IQRs) when appropriate. All statistics were performed using SPSS software, version 24 (SPSS Inc., Chicago, IL).
The study flow-chart is summarized in Fig. 1. After considering the exclusion criteria, 112 patients (72 males and 40 females, 63.68 ± 10.6 years old) were included in the final analysis.
Patients’ demographics, clinical parameters, laboratory findings and comorbidities at the time of enrollment are included in Table 1. The mean baseline serum creatinine level was 93.2 ± 18.3 µmmol/L and mean estimated glomerular filtration rate (eGFR) was 69.1 ± 6.7 mL/min/1.73 m2. Among the patients, 14 (12.5%) had AKI stage I, 26 (23.2%) had AKI stage 2, and 72 (64.3%) developed AKI stage 3.
The most common cause of AKI in the patients was nephrotoxicity (32.14%), followed by hypovolemia (26.7%; volume depletion or hypotension), urinary tract obstruction (21.43%), and cardiorenal syndrome (19.64%; acute coronary syndrome or acute heart failure). Out of 112 patients, 46 required acute dialysis support.
The patients were divided into the renal recovery and renal non-recovery groups depending on the presence or absence of renal recovery, respectively. The non-recovery group included patients that died and patients that developed the ESRD or CKD grade III-V. During the 90-day follow-up period, 76 patients completely recovered kidney function. Among 36 patients without renal function recovery, 8 (22.2%) patients died, and 10 (27.7%) patients reached ESRD and required RRT, while the rest of the patents in this group progressed to CKD stage III-V. Table 2 shows the demographic, clinical, and laboratory data of renal recovery and renal non-recovery group.
As shown in Table 2, the percentage of sex and the laboratory findings on admission, except sCr, ferritin, TIBC, and transferrin saturation, were similar between the recovery group and the non-recovery group. Likewise, there was no significant difference found in the baseline sCr or eGFR between the two groups. Those with full renal recovery were younger and more likely not to have diabetes mellitus, hypertension, and chronic heart failure. Also, renal recovery patients had lower admission serum creatinine, but higher eGFR and serum ferritin level. Diuresis was helpful in predicting renal recovery: more than two-thirds (79.2%) of patients with preserved diuresis recovered renal function compared to 35.7% of anuric patients and 47.6% of those with oliguria.
Although patients with a recovered renal function had fewer hospitalization days, these results were not significant (p = 0.062). In addition, no significant association was observed between the renal recovery and the renal non-recovery group regarding acute dialysis treatment.
However, among all above-mentioned admission parameters, serum ferritin levels showed the most significant difference between patients with renal recovery and renal non-recovery (284 ± 78.1 ng/mL vs. 127.4 ± 54.2 ng/mL; p < 0.001) (Fig. 2).
Serum ferritin significantly correlated with renal recovery (r = 0.72, p < 0.001), transferrin saturation (r = −0.54, p < 0.001), and TIBC (r = −0.34, p < 0.001), and more weakly with the eGFR (r = −0.26, p = 0.04), serum iron (r = −0.23, p = 0.03) and hemoglobin (r = −0.25, p = 0.05). No significant correlation was found between serum ferritin and the CRP (r = 0.16, p = 0.089), age (r = 0.22, p = 0.077) or RRT (r = 0.11, p = 0.18).
Seven variables that were significant in univariate analysis were entered into a multivariate linear regression analysis to determine the significant predictors of renal recovery. The results show that kidney recovery was associated with younger age, absence of diabetes and hypertension, lower serum creatinine on admission, preserved diuresis (OR 8.44, 95% CI 3.15-14.3, p < 0.001), and higher serum ferritin (OR 3.68, 95% CI 2.02-3.97, p < 0.001) (Table 3).
A receiver operating characteristic (ROC) curve analysis was used to investigate the performance of serum ferritin level in distinguishing renal recovery from renal non-recovery after AKI. In our study, the area under the curve was 0.92 (95% CI: 0.87-0.97, p < 0.001) (Fig. 3). The optimum cut-off point of serum ferritin was 240.5 ng/mL, with a sensitivity of 75.8% and a positive predictive value of 90%.

Flow chart of patient exclusion.
Out of 348 AKI patients, 112 were included in the final analysis.
AKI, acute kidney injury; CKD, chronic kidney disease; sCr, serum creatinine; CRP, c reactive protein; WBC, white blood cells.

Baseline characteristics of the 112 patients.
Data are mean ± standard deviation or number (%).
BMI, body mass index; BP, blood pressure; eGFR, estimated glomerular filtration rate; CRP, C reactive protein; TIBC, total iron binding capacity.

Demographic and laboratory data of AKI patients with and without the recovery of renal function.
CKD, chronic kidney disease; ESRD, end-stage renal disease; BMI, body mass index; BP, blood pressure; eGFR, estimated glomerular filtration rate; CRP, C reactive protein; TIBC, total iron binding capacity; RRR, renal replacement therapy.

Serum ferritin in AKI patients with and without renal recovery.
Serum ferritin is significantly higher in patients with renal recovery.
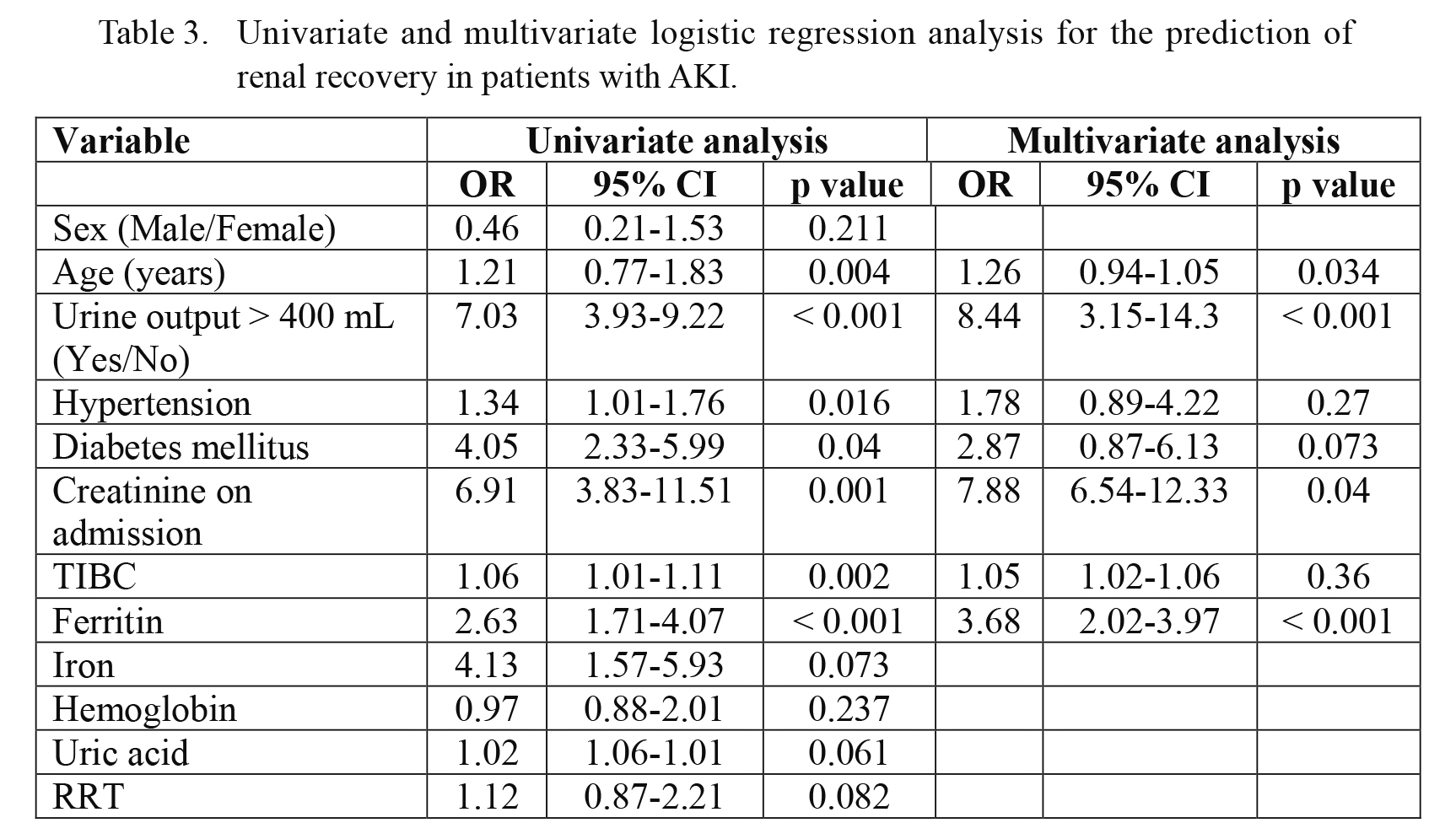
Univariate and multivariate logistic regression analysis for the prediction of renal recovery in patients with AKI.
OR, odds ratio; CI, confidence interval; TIBC, total iron binding capacity; RRT, renal replacement therapy.

Schematic of the ROC curve for renal recovery in AKI by serum ferritin.
ROC indicates the receiver operating characteristic curve. The AUC of ROC for serum ferritin was 0.92 with an optimal sensitivity of 75.8% and specificity of 90%.
In this prospective cohort study, we evaluated the predictive value of serum ferritin levels on the renal recovery among patients with AKI. After eliminating all potential confounders which could be a source of free iron and elevated ferritin, we show that higher serum ferritin levels on admission are associated with improved renal recovery among patients with AKI.
In our study group, 67.8% of patients have fully recovered renal function to baseline levels. This high percentage of recovery is probably due to the fact that we excluded critically ill, septic patients and patients with a severe infection that may contribute to increased mortality and poor renal outcome. Since ferritin is an acute phase reactant and is generally increased under inflammatory conditions, we studied only patients with normal CRP and normal white blood cells count to eliminate elevated ferritin caused by acute phase inflammatory reactions. In the light of previous studies, as pre-existing CKD might influence the prognosis of patients with AKI (Groeneveld et al. 1991; Mehta et al. 2002; Khosla et al. 2009), we also excluded such patients.
We did not analyze the influence of the sex on the data. The menopause may have certain effects on iron metabolism in women. Male body iron storage rises progressively with an equivalent increase in serum ferritin, whereas female serum ferritin levels are consistently lower during the reproductive period. The ferritin levels are, however, increased by two- to three-fold after menopause, compared with the period before menopause (Jian et al. 2009). In the present study, ferritin levels were similar between male and female patients presumably because we included postmenopausal women.
Subsequently, increasing age seems to be a risk factor for poor renal recovery from AKI, as higher percentage of aged patients with AKI develop CKD or ESRD. In our cohort, we noticed statistical significance in age between renal recovery and non-recovery patients; however, serum ferritin levels were not correlated with age.
Patients with RRT-requiring AKI are particularly predisposed to worse long-term renal outcomes, including end-stage renal disease. An increasing number of studies have linked dialysis-dependant AKI to development and acceleration of CKD and literature data have been summarized in a meta-analysis (Coca et al. 2012). Still, we did not observe a difference in RRT requirement between our two study groups. Hemodialysis treatment, particularly via temporary HD catheter, may be associated with inflammation (Borazan et al. 2004) and the consequent elevation of serum ferritin. However, no correlation was found between RRT and serum ferritin levels in our study group.
As previously stated, several studies reported an association between higher concentrations of plasma free iron and a higher risk of incident AKI (Leaf et al. 2014, 2015). Nevertheless, regardless of convincing data based on animal studies and indirect data in humans regarding iron and AKI, rare studies have directly evaluated the relationship between serum ferritin levels and AKI in humans. Mavromatidis et al. (1998) noticed that patients with acute renal failure had markedly increased ferritin levels which is further confirmed by Gulcelik and Kayatas (2002). Vaugier et al. (2017) showed that the increase in the serum ferritin level was associated with improved graft function in kidney transplant patients.
Similar results were obtained in a large prospective study conducted by Choi et al. (2019), where low intraoperative serum ferritin was independently associated with the development of postoperative AKI, indicating a weakened capacity to handle free iron released during cardiopulmonary bypass rapidly. Whereas Leaf et al. (2014, 2015) demonstrated the direct relationship between AKI and plasma iron, a recent large study from the same authors has shown that serum ferritin levels were associated with increased mortality in AKI patients (Leaf et al. 2019). However, thier study cohort comprised of critically ill patients with sepsis, and the most likely explanation is that serum ferritin was elevated nonspecifically, reflecting its role as an acute-phase reactant.
In addition to elevated serum ferritin levels, we observed a decrease in the TIBC in our renal recovery group. Lower TIBC could increase the amount of non-transferrin-bound iron and, consequently the amount of catalytic iron in the circulation as a result of reduced iron-carrying capacity. Accordingly, ferritin translation becomes notably induced when the iron is abundant.
Since the serum ferritin increased in our AKI recovery compared to non-recovery AKI patients, we postulated that this was renoprotective response to reduce oxidative stress, free radical damage, and renal injury mediated via the mechanisms of intracellular sequestration of iron.
There is a general agreement that multiple pathways contribute to necrotic cell death in a highly regulated fashion. Several studies have shown that an additional pathway of iron-mediated cell death may account for the iron-related cell injury involved in several common and clinically relevant forms of AKI. Ferroptosis is a recently identified caspase-independent form of regulated cell death characterized by the accumulation of lethal lipid ROS, generated through iron-dependent lipid peroxidation (Sancho-Martínez et al. 2015). This form of iron‐dependent cell death is notably different from other types of regulated cell death, including apoptosis, unregulated necrosis, and necroptosis in terms of morphology, biochemistry, and genetics. A kidney damaged by ferroptosis responds by immediate induction of its own antioxidant machinery prompting proximal tubules to express H-ferritin, consequently enabling safe sequestration of iron in the ferritin shell (Zarjou et al. 2013). When bound to ferritin iron loses its oxidative capacity, and iron chelators were among the first-ever compounds that reversed renal tubular injury caused by different means (Zager and Foerder 1992; Zager et al. 1993, 1995). This observed elevation in serum ferritin with lower transferrin saturation in some AKI patients may reveal an enhanced capacity to sequester iron to alleviate the effects of catalytic iron release during initiation of AKI, and thereby enable better renal recovery.
On the contrary with the AKI patients, it was reported that serum ferritin is a strong predictor of morbidity and mortality in HD and peritoneal dialysis patients (Kalantar-Zadeh et al. 2001; Hasuike et al. 2010; Hur et al. 2014). Notably, elevated serum ferritin is a frequent finding among patients with ESRD who require renal replacement therapy. Such high serum ferritin levels do not always reflect the iron status of these patients. It is well known that hemodialysis treatment is associated with increased inflammatory biomarkers, leading to oxidative stress and inflammation (Danielski et al. 2003; Libetta et al. 2011). It is therefore expected that there is a connection between serum ferritin and inflammation. Kalantar-Zadeh and colleagues (2005) first reported the inter-relationship among serum ferritin, inflammation, and mortality risk in a historical cohort study of 58,058 maintenance HD patients. These findings are supported by the work of Karaboyas et al. (2018) who reported that a high ferritin level was commonly associated with high mortality. While both inflammation and anemia management practices influenced ferritin levels, the relationship between high ferritin and mortality was attenuated more by adjustment for markers of inflammation than by hemoglobin levels, intravenous iron, and erythropoiesis-stimulating agents doses. The recent paper also encourages the view that the association between serum ferritin and all-cause mortality in HD patients is modified by inflammation (Shoji et al. 2017).
Our study has certain limitations. First, this is a single center study with a relatively small sample size. Patients outcome is affected by our clinical practice which may differ from other centers. Second, the definition of AKI in our analysis was based on sCr levels, as we did not use the urine output criteria because of incomplete data. Additionally, the serum ferritin level was measured only once on admission.
In conclusion, elevated serum ferritin levels on admission were associated with a favorable renal outcome among patients with acute kidney injury. Serum ferritin, in combination with clinical predictors (younger age, preserved diuresis, and absence of comorbidities), can help with recovery prediction and risk stratification in patients with AKI. Mechanisms by which ferritin could mediate the renal recovery in AKI are complex and possibly include multiple pathways.
Z.D. contributed to the conception of the study, data collection, data analysis, and interpretation, and drafted the manuscript. S.S.M. and R.J. contributed to data collection and interpretation and revised the manuscript critically for important intellectual content. B.M. contributed to interpretation and revised the manuscript critically for important intellectual content.
The authors declare no conflict of interest.