2023 Volume 259 Issue 3 Pages 237-246
2023 Volume 259 Issue 3 Pages 237-246
Bone marrow mesenchymal stem cell (BMMSC) is reported to promote spinal cord injury (SCI) recovery via secreting exosomes to deliver RNAs, proteins, lipids, etc. The present study aimed to investigate the effect of microRNA 137 (miR-137)-overexpressing BMMSC exosomes on SCI rats. BMMSCs were extracted from Sprague–Dawley (SD) rat hind leg bone marrow, and then BMMSC-secreted exosomes were collected. MiR-137 mimic and negative control (NC) mimic were transfected into BMMSCs, and then the corresponding exosomes were collected. Subsequently, SD rats were treated with sham operation + phosphate-buffered saline (PBS), SCI operation + PBS, SCI operation + NC mimic BMMSC exosomes, or SCI operation + miR-137-overexpressing BMMSC exosomes. MiR-137 was downregulated in the spinal cord tissue of SCI rats compared to sham rats. Furthermore, BMMSC exosome injection elevated the Basso, Beattie, and Bresnahan (BBB) scores and neuronal viability and reduced tissue injury and proinflammatory cytokine expression in the spinal cord tissue of SCI rats compared to PBS treatment. Subsequently, miR-137-overexpressing BMMSC exosome injection improved the BBB score and neuron viability, and decreased tissue injury as well as proinflammatory cytokine expression in SCI rats compared to NC-overexpressing BMMSC exosomes. Additionally, miR-137-overexpressing BMMSC exosomes also diminished neuronal apoptosis in the spinal cord tissue of SCI rats compared to NC-overexpressing BMMSC exosomes. In conclusion, miR-137-overexpressing BMMSC exosomes reduce tissue injury and inflammation while improving locomotor capacity and neuronal viability in SCI rats. These findings suggest that miR-137-overexpressing BMMSC exosomes may serve as a treatment option for SCI recovery.
Spinal cord injury (SCI) is a traumatic disease in the spine that markedly impacts individuals’ life expectancy and quality, mostly due to constant pain, neuron dysfunction, disease-related disability, etc. (Ahuja et al. 2017; Ong et al. 2020). According to a recent integrated review, the incidence of SCI varies from 14.6 to 60.6 per million each year in China (Chen et al. 2022). SCI results in damage to the central or peripheral nervous system due to abnormal compression of the spinal cord, which is normally the most severe pathological consequence, along with other damage, such as vessel injury and subsequent harmful chemical release (Kjell and Olson 2016; Yokota et al. 2019). Currently, little progress has been made in clinical studies for SCI patient management; however, investigation of novel therapeutic tools or assisting biomarkers should be continued. Fortunately, thanks to the development of gene sequencing techniques, some dysregulated microRNAs (miRNAs) have been discovered in SCI, which has shed light on studies aimed to improve SCI patient prognosis by delving into potential biomarkers or therapeutic targets (Peng et al. 2020; Sun et al. 2020b; Wan et al. 2020).
MiR-137, a miRNA located on human chromosome 1p22, is intimately related to diseases of the nervous system, and its host gene is responsible for many psychological diseases (Bemis et al. 2008; Schizophrenia Psychiatric Genome-Wide Association Study (GWAS) Consortium 2011; Sun et al. 2011). Specifically, miR-137 can reduce inflammation, oxidative damage, and neuronal injury in ischemic stroke, and its overexpression can downregulate seizure activity and neuronal excitability in epilepsy (Wang et al. 2018; Tian et al. 2020). Interestingly, a previous study revealed a mechanistic role of miR-137 in SCI by modulating oxidative stress and inflammation, indicating that it might play a role in SCI pathology (Dai et al. 2018).
Previously, bone marrow mesenchymal stem cells (BMMSCs) were abundantly reported to play a medicinal role in SCI via exosomes, and more intriguingly, miR-137 could exert its roles in many diseases via delivery by exosomes (Jiang et al. 2019; Li et al. 2020b). However, whether miR-137 has an impact on SCI recovery via delivery by BMMSC exosomes is still largely unknown.
Hence, the present study aimed to investigate the effect of miR-137-overexpressing BMMSC exosomes on SCI rats.
This study was approved by the Animal Care and Use Committee. All animal experimental procedures were carried out by strictly complying with the Care and Use of Laboratory Animals of the National Institutes of Health.
Culture and identification of BMMSCsFollowing a previous study (Li et al. 2020a), BMMSCs were extracted from Sprague‒Dawley (SD) rat hind leg bone marrow. The SD rats were homogeneous in body weight, and the mean body weight was 180 ± 20 g [mean ± standard deviation (S.D.)]. Briefly, the bone marrow was flushed out from the femurs and tibias. After centrifugation, the pellet was resuspended and cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Sigma, St. Louis, MO, USA) with 10% fetal bovine serum (FBS) (Sigma). The medium was replaced every other day. After isolation, the BMMSCs were identified by staining with PE-conjugated CD34 mouse mAb (1:50) (CST, Boston, MA, USA), PE-conjugated CD44 mouse mAb (1:50) (CST), FITC-conjugated CD45 mouse mAb (1:20) (CST) and FITC-conjugated CD90 mouse mAb (1:100) (BD, Franklin Lakes, NJ, USA).
Isolation and identification of exosomesThe exosomes were isolated from the culture medium of BMMSCs. The procedure was conducted following the HieffTM Quick exosome isolation kit (Yeasen Biotech, Shanghai, China) instructions. After isolation, the exosomes were quantified with a BCA Protein Assay Kit (Beyotime, Shanghai, China), and the expression levels of CD9, CD63 and CD81 in exosomes were evaluated. The culture medium cultured with BMMSCs was applied as a negative control (NC).
Western blotAfter isolating the protein with radioimmunoprecipitaion assay (RIPA) lysis buffer (Beyotime), separation with a 4-20% precast gel (Willget, Shanghai, China) was completed. The protein was then transferred to a nitrocellulose membrane (PALL, New York, NY, USA). Then, the sections were incubated with CD9 antibody (1:1,000, Santa Cruz, Santa Cruz City, CA, USA), CD63 antibody (1:500, Santa Cruz) and CD81 antibody (1:500, Santa Cruz) at 4℃ overnight. After washing, the membrane was incubated with goat anti-mouse IgG (H+L) secondary antibody (1:20,000, Santa Cruz) for 2 hours (h). Finally, the protein band was visualized with ECL Star (Beyotime).
BMMSC transfection and exosome isolationLipofectamine 2000 (Invitrogen, Waltham, MA, USA) (dose of 12 μl) was applied to transfect the miR-137 mimic and negative control (NC) mimic, which were obtained from Shanghai GenePharma Co., Ltd. (Shanghai, China), into BMMSCs to generate miR-mimic and NC-mimic BMMSCs. The BMMSCs without transfection were defined as control BMMSCs. MiR-137 expression in control, NC-mimic and miR-mimic BMMSCs was evaluated at 24 h after transfection. The medium of control, NC-mimic and miR-mimic BMMSCs was collected to isolate the exosomes at 48 h after transfection with methods described in “the isolation and identification of exosomes” subsection. Then, Exo-control, Exo-NC-mimic and Exo-miR-mimic were obtained, and exosomal miR-137 expression was assessed.
SCI modelTo generate the SCI rat, the SCI operation was performed (Gu et al. 2020). Briefly, 6- to 8-week-old SD rats (purchased from the Shanghai SLAC Laboratory Animal Co., Ltd., Shanghai, China) were anesthetized, the back skin and muscle were cut to expose the spine, and the T10 lamina was removed. Then, the completely exposed dorsal spinal cord surface was hit by a 10 g rod (with a diameter of 2.5 mm) from a height of 12.5 mm. Then, the muscle and skin were structured. The bladder was manually evacuated three times a day to avoid urinary retention until the rats’ autourination function recovered. The sham operation was defined as only performing the spinal laminectomy and not hitting the dorsal spinal cord surface with the rod.
Animal groups and assessmentThe SD rats were divided randomly into 4 groups: (1) Sham, the rats received sham operation and 500 μl phosphate-buffered saline (PBS) through tail intravenous injection; (2) SCI, the rats received SCI operation and 500 μl PBS through tail intravenous injection; (3) Exo-NC-mimic, the rats received SCI operation and 200 μg (in 500 μl PBS) Exo-NC-mimic exosomes through tail intravenous injection; (4) Exo-miR-mimic, the rats received SCI operation and 200 μg (in 500 μl PBS) Exo-miR-mimic exosomes through tail intravenous injection.
To determine the effect of miR-137 modification, 12 rats (3 rats for each group) were given tail intravenous injections at 1 h after the operation; then, the rats were euthanized to collect the spinal cord tissue at 1 day after the operation, and the expression of miR-137 was detected.
To determine the function assessment and assays, another 24 rats (6 rats for each group) were adopted, and tail intravenous injections were performed at 1 h, 7, 14 and 21 days after the operation. Then, the rats’ locomotor capacity was assessed with the Basso, Beattie, Bresnahan (BBB) locomotor rating scale according to a previous study (Basso et al. 1995) on the day of operation (before surgery) and days 1, 3, 7, 14, 21 and 28 after the operation. Furthermore, at day 28, all rats were euthanized, and the spinal cord tissues containing the injury center were collected, fixed, embedded and cut into 4 μm sections. Then, the sections were used for hematoxylin-eosin (HE), TdT-mediated dUTP nick end labeling (TUNEL), immunofluorescence (IF) and immunohistochemistry (IHC) staining analysis. The tissues stored at −80℃ were used for reverse transcription quantitative polymerase chain reaction (RT-qPCR) detection.
HE, TUNEL, IF and IHC stainingThe HE staining kit (Sangon, Shanghai, China) and Cell Apoptosis Detection Kit (Sangon) were adopted to conduct HE and TUNEL staining by strictly following the kits’ instructions. For the completion of IF and IHC staining, the sections were deparaffinated with xylene (Sangon) and rehydrated with gradient ethanol (Sangon). Then, the sections were subjected to antigen retrieval with the microwave method, and endogenous peroxidase was removed with H2O2 (Sangon). After blocking with bovine serum albumin (BSA) (Beyotime), the sections were incubated with primary antibodies at 4°C overnight. Subsequently, the sections were incubated with secondary antibodies. Then, for IHC staining, diaminobenzidine (DAB) (Sigma) and hematoxylin (Sigma) were used to stain the secondary antibody and nuclei; for IF staining, the nuclei were stained with 4',6-diamidino-2-phenylindole (DAPI) (Sigma). Finally, the sections were observed under an inverted fluorescence microscope (Nikon, Tokyo, Japan). The antibodies are listed in Table 1.

Antibodies for immunohistochemistry and immunofluorescence.
IL, interleukin; TNF, tumor necrosis factor; HRP, horse-radish peroxidase.
Total Exosome RNA & Protein Isolation Kit (Invitrogen) and TRIzol reagent (Invitrogen) were used to extract RNA from exosomes and tissues, respectively. Then, reverse transcription and qPCR were carried out with the PrimeScript™ RT Reagent Kit (Takara, Osaka, Japan) and TB Green qPCR Master Mix (Takara). The 2-ΔΔCt method was used to calculate the results. The primers are listed in Table 2.

Primers for PCR.
All data are expressed as the mean ± S.D. and were analyzed by GraphPad Prism 7.01 (GraphPad Software, San Diego, CA, USA). One-way ANOVA followed by Tukey’s multiple comparisons test was used to compare data among groups. The data from the experiments are presented as the mean ± S.D. from 6 samples from each group (n = 6 in each group). Statistical significance was defined as a p value < 0.05.
CD44-positive, CD90-positive, CD34-negative and CD45-negative cells were observed in isolated cells, which suggested that BMMSC isolation was successful (Fig. 1A). For exosomes, the expression of exosome marker proteins, including CD9, CD63 and CD81, was dramatically upregulated in the Exo group compared to the NC group, indicating that the isolation of exosomes from the culture medium of BMMSCs was also successful (Fig. 1B). Afterward, after transfection of BMMSCs, miR-137 expression was upregulated in the miR-mimic group compared to the NC-mimic group, suggesting that the miR-137 mimic was successfully transfected into BMMSCs (p < 0.001) (Fig. 2A). In addition, miR-137 was also increased in the Exo-miR-mimic group compared to the Exo-NC-mimic group, implying that the isolation of miR-137-overexpressing BMMSC exosomes was successful as well (p < 0.001) (Fig. 2B).

Bone marrow mesenchymal stem cells (BMMSCs) and exosome biomarker expression.
(A) The percentage of CD34-, CD44-, CD45- and CD90-positive cells in BMMSCs, and (B) the expression of CD9, CD63 and CD81 in the NC group and Exo group. BMMSCs, bone marrow mesenchymal stem cells; NC, negative control; Exo, exosome.

MiR-137 expression in bone marrow mesenchymal stem cells (BMMSCs) and exosomes.
(A) Relative miR-137 expression in the control group, NC-mimic group and miR-mimic group, and (B) relative miR-137 expression in the Exo-control group, Exo-NC-mimic group and Exo-miR-mimic group. ***p < 0.001. MiR, microRNA; BMMSCs, bone marrow mesenchymal stem cells; NC, negative control; Exo, exosome.
On day 1, miR-137 was downregulated in the SCI group compared to the sham group (p < 0.001), but was increased in the Exo-miR-mimic group compared to the Exo-NC-mimic group (p < 0.01) (Fig. 3A). At Day 28, miR-137 was reduced in the SCI group compared to the sham group (p < 0.001), but was elevated in the Exo-miR-mimic group compared to the Exo-NC-mimic group (p < 0.05) (Fig. 3B).

MiR-137 expression in rat models.
Relative miR-137 expression in the sham group, spinal cord injury (SCI) group, Exo-NC-mimic group and Exo-miR-mimic group at day 1 (A) and day 28 (B). *p < 0.05; **p < 0.01; ***p < 0.001. MiR, microRNA; Exo, exosome; NC, negative control.
Regarding the locomotor capacity in SCI rats, the BBB score was markedly decreased in the SCI group compared to the Sham group at 1, 3, 7, 14, 21 and 28 days (all p < 0.001); furthermore, the BBB score was increased in the Exo-miR-mimic group compared to the Exo-NC-mimic group at 1, 3, 7, 14, 21 and 28 days (all p < 0.05) (Fig. 4). These results indicated that miR-137-overexpressing BMMSC exosome injection improved the locomotor capacity of SCI rats.

The Basso, Beattie, Bresnahan (BBB) score in spinal cord injury (SCI) rat models.
BBB scores at days 1, 3, 7, 14, 21 and 28 in the sham group, SCI group, Exo-NC-mimic group and Exo-miR-mimic group. Exo, exosome; NC, negative control; miR, microRNA. Sham group vs. SCI group: ***p < 0.001; Exo-mimic-NC group vs. Exo-mimic group: #p < 0.05, ##p < 0.01, ###p < 0.001; Exo-mimic-NC group vs. SCI group: ^p < 0.05, ^^p < 0.01.
In the SCI group, spinal cord tissue injury was increased compared to that in the sham group, while injury was decreased in the Exo-miR-mimic group compared to that in the Exo-NC-mimic group postinjection (Fig. 5A). Regarding apoptosis in spinal cord tissue, the TUNEL-positive cell rate was elevated in the SCI group compared to the sham group; however, it was reduced in the Exo-miR-mimic group compared to the Exo-NC-mimic group (all p < 0.05) (Fig. 5B, C). In addition, the number of NeuN (+) cells/field was decreased in the SCI group compared to the sham group but was increased in the Exo-miR-mimic group compared to the Exo-NC-mimic group (all p < 0.01) (Fig. 6A, B). These data implied that miR-137-overexpressing BMMSC exosome injection diminished spinal cord injury and displayed a protective effect on neurons in SCI rats.
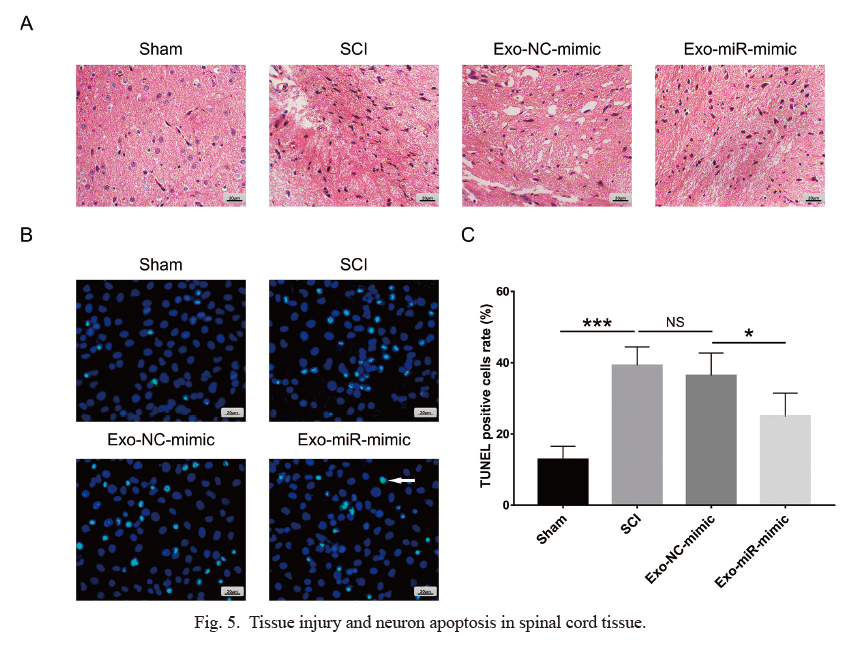
Tissue injury and neuron apoptosis in spinal cord tissue.
(A) Tissue injury by hematoxylin-eosin (HE) staining, (B) cell apoptosis images by TUNEL assay, and (C) TUNEL-positive cell rate in spinal cord tissue in the sham group, SCI group, Exo-NC-mimic group and Exo-miR-mimic group. *p < 0.05; ***p < 0.001. TUNEL, TdT-mediated dUTP nick end labeling; SCI, spinal cord injury; Exo, exosome; NC, negative control; miR, microRNA.

Neuronal viability in spinal cord tissue.
Images of immunofluorescence (IF) staining of NeuN (+) cells (A) and the NeuN (+) cells/field (B) in the Sham group, SCI group, Exo-NC-mimic group and Exo-miR-mimic group. *p < 0.05; **p < 0.01; ***p < 0.001. Green dots represent NeuN-positive cells, and blue dots represent the cell nucleus. SCI, spinal cord injury; Exo, exosome; NC, negative control; miR, microRNA.
Fig. 7 shows relative mRNA expression levels of interleukin-1 beta (IL-1β) (Fig. 7A), interleukin-6 (IL-6) (Fig. 7B) and tumor necrosis factor-alpha (TNF-α) (Fig. 7C) and IHC images of IL-1β, IL-6 and TNF-α protein expression (Fig. 7D).
In terms of inflammatory cytokine expression in spinal cord tissue post injection, IL-1β mRNA and protein were upregulated in the SCI group compared to the sham group (p < 0.01) but were downregulated in the Exo-miR-mimic group compared to the Exo-NC-mimic group (p < 0.05) (Fig. 7A, D). The mRNA and protein levels of IL-6 were increased in the SCI group compared to the sham group (p < 0.001); however, they were decreased in the Exo-miR-mimic group compared to the Exo-NC-mimic group (p < 0.05) (Fig. 7B, D). In addition, TNF-α mRNA and protein were elevated in the SCI group compared to the sham group (p < 0.001) but were reduced in the Exo-miR-mimic group compared to the Exo-NC-mimic group (p < 0.05) (Fig. 7C, D). These data indicated that miR-137-overexpressing BMMSC exosome injection alleviated inflammation in the spinal cord tissue of SCI rats.

Pro-inflammatory cytokine expression in spinal cord tissue.
Relative IL-1β (A), IL-6 (B) and TNF-α (C) mRNA expression and images of IL-1β, IL-6 and TNF-α protein expression by immunohistochemistry (IHC) staining (D) in the Sham group, SCI group, Exo-NC-mimic group, and Exo-miR-mimic group. *p < 0.05; **p < 0.01; ***p < 0.001. IL-1β, interleukin-1 beta; IL-6, interleukin-6; TNF-α, tumor necrosis factor-alpha; SCI, spinal cord injury; Exo, exosome; NC, negative control; miR, microRNA.
SCI brings about many disastrous consequences to the affected patients, being harmful to many aspects of the patients’ quality of life, including social, psychiatric, financial and vocational status (National Spinal Cord Injury Satistical Center 2014). Moreover, the mortality of SCI patients is not increasing; it has been reported that the hospitalized mortality ranges from 4% to 17%, and the mortality post-hospitalization remains at approximately 3% (Krause et al. 1997; Azarhomayoun et al. 2018). To improve the quality of life and reduce the mortality rate among SCI patients, multiple efforts have been made to explore potential cell therapies for SCI. For the same reason, we investigated the regulatory role of miR-137-overexpressing BMMSC exosomes in SCI rats. Then, we discovered that miR-137-overexpressing BMMSC exosomes decreased inflammatory cytokine expression in the spinal cord tissue of SCI rats. In addition, miR-137 expression in the Exo-miR-mimic group at day 28 was numerically lower than that at day 1. This could be because exosomes were injected at 1 h, 7, 14 and 21 days after the operation, and miR-137 expression at day 1 lasted one day post injection, while miR-137 expression at day 28 lasted 7 days post injection, so the gap in the last day led to a decrease in miR-137.
MiR-137 is a miRNA crucial to the development of many diseases of the nervous system; however, its investigation in SCI is very limited. A previous study elucidated that miR-137 overexpression ameliorates cognitive dysfunction induced by propofol by modulating the pleiotrophin/protein tyrosine phosphatase receptor type Z (PTN/PTPRZ) signaling pathway in a manner that downregulates PTN (Yang et al. 2020). Another study revealed that miR-137 could diminish TNF-α, IL-1β and IL-6 expression, oxygen-glucose deprivation (OGD)/R-induced cell damage, and apoptosis while enhancing cell proliferation in rats with cerebral ischemic injury (Zhang et al. 2020). In terms of BMMSC exosomes, a prior study reported that BMMSC exosomes are predominantly located at the spinal cord injury site and reduce SCI-induced complement activation in rats. In addition, they also downregulate the SCI-activated nuclear factor-kappa B (NF-κB) signaling pathway in rats (Zhao et al. 2019). Another study showed that exosomes secreted from BMMSCs overexpressing G protein-coupled receptor kinase 2 interacting protein 1 improve the rehabilitation of functional behaviors in SCI rats (Luo et al. 2021). In the present study, miR-137 was downregulated in SCI rats compared to sham rats, and in SCI rats, miR-137-overexpressing BMMSC exosomes improved locomotor capacity, reduced tissue injury and neuronal apoptosis and elevated neuronal viability. We presumed that these effects may be achieved because both miR-137 and BMMSC exosomes exert protective roles in SCI rats in our study according to previous findings, such as the function of miR-137 in reducing injury in diseases of the nervous system and the medicinal effect of BMMSC exosomes in SCI (Zhao et al. 2019; Yang et al. 2020; Zhang et al. 2020; Luo et al. 2021).
Furthermore, miR-137 is also involved in the regulation of inflammation. For instance, a previous study showed that miR-137 is downregulated in cartilage tissues of osteoarthritis (OA) rats, and miR-137 overexpression represses the activation of inflammatory cytokines, including TNF-α, IL-1β and IL-6, as well as the activation of the AMP-activated protein kinase/nuclear factor-kappa B (AMPK/NF-κB) signaling pathway in OA rats (Wang et al. 2020). Additionally, a study illustrated that miR-137 upregulation in rheumatoid arthritis fibroblast-like synoviocytes reduces cell proliferation, migration, invasion and the expression of proinflammatory cytokines (Du et al. 2018). Most importantly, miR-137 is reported to regulate inflammation in SCI as well, although the reports are very rare. For example, miR-137 is decreased while MAPK-activated protein kinase 2 (MK2) is increased in the serum of SCI patients and hydrogen peroxide-treated C8-D1A and C8-B4 cells (SCI cell model); furthermore, miR-137 overexpression suppresses proinflammatory cytokine expression and cell apoptosis in hydrogen peroxide-treated C8-D1A and C8-B4 cells by targeting MK2 (Gao et al. 2018). Moreover, miR-137 inactivates the NF-κB pathway in a TNFAIP1-dependent manner to reduce Aβ-induced neurotoxicity (He et al. 2017); meanwhile, its overexpressed exosomes modify oxidation resistance 1 (OXR1), a neuroprotective protein to repress oxidative stress (Jiang et al. 2019). Regarding the functional role of BMMSC exosomes in inflammation, a study illustrated that lipopolysaccharide (LPS)-stimulated BMMSC exosomes downregulate proinflammatory cytokine expression and oxidative stress induced by H2O2 in cardiomyocytes (Liu et al. 2020). In addition, another study revealed that heme oxygenase-1-modified BMMSC exosomes overexpressing miR-200b ameliorate inflammatory injury induced by TNF-α and lymphocyte treatment in intestinal epithelial cells by targeting high mobility group box 3 (Sun et al. 2020a). These previous findings show that miR-137 and BMMSC exosomes play a role in the inhibition of inflammation in SCI (or in other diseases with inflammation). In our study, miR-137-overexpressing BMMSC exosomes downregulated the expression of proinflammatory cytokines, including IL-1β, IL-6 and TNF-α, in the tissues of SCI rats. This result added to the evidence that miR-137 also plays a protective role in SCI by reducing inflammation, which was in accordance with previous studies (Gao et al. 2018; Lv et al. 2019). However, unlike previous similar studies, our study showed that the protective effect of miR-137 in SCI was achieved through delivery by exosomes secreted by BMMSCs.
In addition, a limitation of our study should be discussed: The exosome purity was not assessed post isolation. However, the method used for exosome isolation in our study was efficient and applied in another similar study (Ogata-Kawata et al. 2014). In addition, there was an issue that we would like to address: Tail intravenous injection of exosomes was chosen in our study. The delivery of drugs directly to the spinal cord via intrathecal injection was more invasive, which imposed toxicity on uninjured/injured spinal cord tissue, and it was difficult to perform intrathecal injection in a nonhospital setting (Kartha et al. 2020). Therefore, intravenous injection was chosen in our study.
Collectively, miR-137-overexpressing BMMSC exosomes improve locomotor capacity and enhance neuron viability while reducing tissue injury and diminishing tissue inflammation in SCI rats. These findings suggest that miR-137-overexpressing BMMSC exosomes may serve as a treatment option for SCI recovery.
This study was supported by the Scientific Research Project of Wuxi Municipal Health Committee (ZYZL201801; Q201945; No. Z202006), Medical and Health Guidance Project of Wuxi Science and Technology Bureau (2020-19), the High-level Talent Training Project of Wuxi Taihu Talent Plan (BJ2020066; HB2020064), 2020 Jiangsu Province Traditional Chinese Medicine Science and Technology Development Plan Project (YB2020041; YB2020042) and the National Natural Science Foundation of China (82174400).
M.W. and J.W. conceived, designed, and supervised the study and revised the manuscript. Y.S. and Q.W. performed the experiments and provided technical support. L.L. analyzed the data and edited the manuscript. All authors have read, revised and approved the final version of the manuscript.
The authors declare no conflict of interest.