-
編集者のコメント
Wet granulation is one of the fundamental unit operations for manufacturing pharmaceutical solid dosage forms including tablets. This study investigated in detail the states of water incorporated in wet granules composed of different fillers. The key instrument to evaluate the state of water was a low-field benchtop 1H-NMR time-domain NMR (TD-NMR). This study successfully concluded that the state of water significantly affected the wet granulation process and the characteristics of the resultant granules. The findings can offer valuable knowledge on wet granulation process from the viewpoint of molecular mobility of water.
-
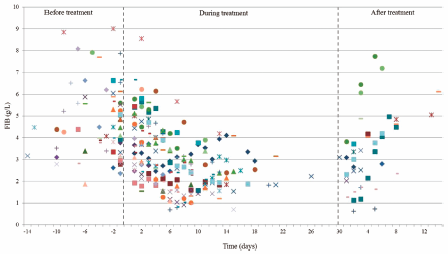
67 巻 (2019) 3 号 p. 258-264A Retrospective Analysis of the Effect of Tigecycline on Coagulation Function もっと読む編集者のコメント
A number of clinical trials demonstrated that tigecycline was effective and well tolerated in the treatment of patients with various bacterial infections, but few literatures had shown the coagulopathy induced by tigecycline. The retrospective analysis in this paper to assess the impact of tigecycline on coagulation parameters in 50 patients showed that the plasma fibrinogen (FIB) level decreased during treatment, which was statistically significant (P < 0.001). The mean values of activated partial thromboplastin time (aPTT) and prothrombin time (PT) were significantly increased, respectively. It is necessary for practitioners routinely monitor coagulation level in at-rick patient populations treated with tigecycline.
-
67 巻 (2019) 2 号 p. 130-134Salicylic Acid as a Photosensitizer for Thymidine Dimerization Induced by UV もっと読む編集者のコメント
When a neutral solution of a nucleoside mixture was irradiated with UV light having wavelength longer than 300 nm, addition of salicylic acid to the solution greatly accelerated the reaction of thymidine. The UV light irradiation of thymidine solution in the presence of salicylic acid generated isomers of cyclobutane thymidine dimers almost exclusively. UV irradiation with the longer wavelength of 350 nm induced almost no reaction. The results indicate that salicylic acid is a photosensitizer for thymidine dimerization excited by UV light of wavelength 300 to 350 nm.
-
編集者のコメント
“Chocolet” is an orally disintegrating tablet formulated with cocoa powder to mask the bitterness of drugs. The name “Chocolet” indicates that the formulation combines the good taste of chocolate with the ease of taking of an orally disintegrating tablet. Rebamipide chocolet has been prepared with excellent palatability properties, which could not be achieved using a sweetener alone, by using the combination of a sweetener and cocoa powder as an agent for masking bitterness. “Chocolet” can be used to provide patient-friendly tablets, thus helping to improve medication adherence.
-
67 巻 (2019) 1 号 p. 64-70Synthetic Studies of Isoschizogamine: Alternative Preparation of the Key Intermediate もっと読む編集者のコメント
Isoschizogamine is a member of the schizozygane family of indole alkaloids. Its highly fused hexacyclic structure containing an aminal adjacent to a quaternary stereocenter makes it a challenging synthetic target. While the authors reported the total synthesis of (–)-isoschizogamine in 2012, this paper describes an alternative preparation of their pivotal synthetic intermediate. The synthesis features a stereoselective construction of a quaternary carbon by the Claisen-Johnson rearrangement and a stereoselective rhodium-mediated 1,4-addition of arylboronic acid. The reliability of this synthetic route enables a sufficient supply of the intermediate for the total synthesis of isoschizogamine.
-
編集者のコメント
Assessment of repeatability in supercritical fluid chromatography with electrochemical detection (SFC-ECD) system is necessary to construct an SFC-ECD as a quantitative method with satisfactory precision. This article is the first report that a method for the assessment of repeatability in SFC-ECD has been proposed by means of the ISO 11843 part 7 which can theoretically provide detection limits and standard deviation (SD) through the stochastic properties of baseline noise without repetitive measurements of real samples. The present method is practically useful, and both experimental time and chemicals can be saved to estimate the repeatability in SFC-ECD.
-
編集者のコメント
A series of 8-methoxy or 8-methylquinolones bearing novel 3-aminooctahydrocyclopenta[c]pyrrole derivatives at the C-7 position was synthesized, and the pharmacological, physicochemical, and toxicological properties of the individual compounds were evaluated. Novel 7-[(1R,5S)-1-amino-5-fluoro-3-azabicyclo[3.3.0]octan-3-yl]-6-fluoro-8-methylquinolone 7 exhibited potent and better activity than LVFX and MFLX against streptococci, staphylococci, enterococci, E. coli, A. baumannii, and anaerobes. Compound 7 also demonstrated favorable pharmacokinetic and pharmacodynamic properties and an acceptably safe toxicological profile. Consequently, compound 7 was selected as a clinical candidate for further evaluation as a new-generation, broad-spectrum quinolone antibiotic. Compound 7 is expected to become an option for antibacterial therapy against multidrug-resistant A. baumannii.
-
編集者のコメント
Polygenetic and complex diseases are great burden and challenge of human, such as ischemic cerebrovascular disease (ICD) and cardiovascular diseases (CVD). Drug repositioning, is one important approach to reexamine the new indications of marketed drugs, especially drugs with multi-targets. Based on the interplay among diseases, genes (targets) and drugs, new method can be applied to dissect the association information. A multi-database, in silico target identification, gene function enrichment, and network pharmacology analysis integrated methods were proposed to investigate the approved CVD drugs repurposing for ICD. It provides promising alternative to inferring novel disease indications for existing safe and effective drugs.
-
編集者のコメント
Oxiranyl anions are very unstable and uncommon nucleophiles while epoxies are widely used as electrophiles in organic chemistry. A sulfonyl-stabilized oxiranyl anion reacts efficiently with a triflate to afford an alkylated product in high yield. The high synthetic potential of the oxiranyl anion chemistry was demonstrated by the total synthesis of gymnocin-A, a cytotoxic polycyclic ether marine natural product produced by the red tide organism Karenia mikimotoi.
-
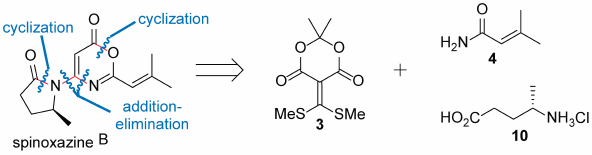
編集者のコメント
This paper describes a short total synthesis of (+)-spinoxazine B, which inhibits NO production in BV-2 microgrial cells. The synthesis features a double cyclization to rapidly construct the bicyclic skeleton of spinoxazine B. Spinoxazine B is the first example of a natural alkaloid containing an oxazinone-pyrrolidone nucleus. Because new ring system is considered to be a new resource for drug discovery, spinoxazine B is expected to serve as a novel drug lead compound as well as a drug discovery scaffold.
-
編集者のコメント
A TLC-based simple and convenient method using UV-sensitive constituents as markers to identify the crude drug Polygala Root (the root of Polygala tenuifolia Willdenow; Japanese name “Onji”) was investigated. Twenty-three aromatic compounds including two new compounds, polygalaonjisides A and B, were characterized. Based on the phytochemical results obtained, a TLC method focusing on three marker spots with Rf values of approximately 0.4-0.5 due to tenuifolisides A and B and 3,6′-di-O-sinapoylsucrose was proposed as a simple and convenient test to identify Polygala Root and its single-extract products on the market. The data presented in this paper could be useful in stipulating a confirmation test to identify Polygala Root.
-
66 巻 (2018) 12 号 p. 1153-1164Microflow Fluorinations of Benzynes: Efficient Synthesis of Fluoroaromatic Compounds もっと読む編集者のコメント
Continuous flow synthesis has drawn increasing attention in current organic synthesis. In this paper, the authors demonstrate a new entry for the beneficial application of a microflow reactor to the synthesis of aromatic fluorinated compounds via domino benzyne generation/nucleophilic fluorination. In particular, the high mixing ability of the flow reactor significantly reduced the reaction times to ~10 s and improved the product yields in comparison to their previously reported method under ordinary batch conditions. In some cases, aryl fluorides were obtained only under microflow conditions. Thus, the flow chemistry is the method not only for continuous chemical production but also for achieving transformations that are otherwise inaccessible by the conventional batch method.
-
66 巻 (2018) 12 号 p. 1104-1113Dibutyltin(IV) Complexes Derived from L-DOPA: Synthesis, Molecular Docking, Cytotoxic and Antifungal Activity もっと読む編集者のコメント
A novel series of pentacoordinated organotin(IV) complexes derived from L-DOPA were designed; the synthesis was performed by a one-pot strategy. The biological evaluation revealed that organotin complexes were substantially more cytotoxic than cisplatin and significantly more effective than Topotecan in inhibiting the growth of leukemia, breast and lung cancer cell lines. The cytotoxicity depended on the nature of the substituent bonded to the aromatic ring. The brine shrimp lethality assay was also used to determine the toxicity. Molecular docking revealed that organotin (IV) complexes bind to the active site of topoisomerase I. Antifungal activity was tested against species of Candida.
-
66 巻 (2018) 12 号 p. 1091-1103i-Motif-Binding Ligands and Their Effects on the Structure and Biological Functions of i-Motif もっと読む編集者のコメント
The i-motif is a high-order DNA structure, forming in cytosine-rich sequences of gene promoters and telomeric regions. Due to the limited stability of this structure under the physiological conditions, the i-motif DNA was unknown for several years. Recently, the biological functions of this DNA and its application have been discovered by applying i-motif interacting agents which is showing the importance of these ligands in uncovering the distinctive features of i-motif.
-
編集者のコメント
Quercetin-pivaloxymethyl conjugate (Q-POM) potentiated the activity of ampicillin, cefepime, and vancomycin against S. aureus and Enterococcus (including highly resistant strains such as hVISA, VISA, and VRE), by decreasing the MICs of these antibiotics by 4-128 folds. Q-POM was found to be partially synergistic with ampicillin and cefepime against S. aureus and Enterococcus, while it was strongly synergistic with vancomycin. Q-POM at 5 mg/L inhibited the formation of biofilms of S. aureus by 24-83% and VRE by 70%. Additionally, Q-POM inhibited the hemolytic activity of S. aureus in a dose-dependent manner.
-
編集者のコメント
Metal carbenoid species are known to insert into a C−H bond, C=X double bonds (X = C, N, O), and Y−H bonds (Y = N, O, Si, P, S, etc), however, a carbenoid insertion into a urea C−N bond has not yet been reported. In the article, the first urea insertion reaction of carbenoid species is described. The urea insertion reaction proceeded smoothly using Rh2(NHPiv)4, a rhodium catalyst previously designed by the authors’ group, to produce highly functionalized bridged molecules with three adjacent stereocenters.
-
66 巻 (2018) 11 号 p. 1048-1056Scale-Up Procedure for Primary Drying Process in Lyophilizer by Using the Vial Heat Transfer and the Drying Resistance もっと読む編集者のコメント
Primary drying conditions for the commercial manufacturing were designed based on the vial heat transfer coefficient of the production lyophilizer and the drying resistance (Rp) calculated from manufacture with the pilot lyophilizer under dust-free condition. The production scale-verification study confirmed that the Rp obtained using pilot lyophilizer under dust-free condition could be available for the production lyophilizer. This scale-up theory, which bridges the gap between the laboratory scale and the production scale, is useful for the development of an efficient and robust process at production scale.
-
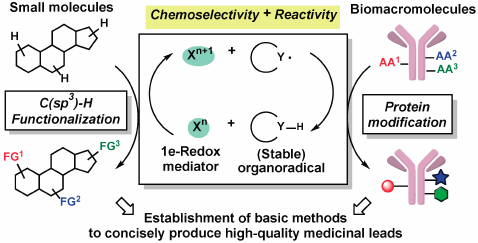
66 巻 (2018) 10 号 p. 907-919Development of Highly Chemoselective Oxidative Transformations by Designing Organoradicals もっと読む編集者のコメント
For organic synthesis in the field of pharmaceutical sciences, methodologies that can easily and quickly supply compounds with high drug-likeness is highly desirable. Based on the original catalyst design concept "Radical-Conjugated Redox Catalysis (RCRC)" established during author's research, various C(sp3)-H functionalizations and protein modifications have been developed, taking advantage of high reactivity and chemoselectivity of single-electron transfer process. This review will focus on the research concept and efforts over eight years of the author and his collaborators.
-
編集者のコメント
Allylic fluoride is a useful synthetic intermediate for the preparation of various organofluorine compounds. The authors demonstrated a highly enantioselective fluorination of cyclic tetrasubstituted alkenes with a pendant amide group using their dianionic phase-transfer catalyst. The deprotonative fluorination mainly proceeded in preference to the intramolecular nucleophilic attack of the amide group, and the corresponding allylic fluorides with a chiral tetrasubstituted carbon center were obtained with up to 97% ee.
-
編集者のコメント
The amlodipine dissolution from orally disintegrating tablets (ODTs) in vivo in the human oral cavity was examined. Various amlodipine ODTs with different levels of physical masking effectiveness were manufactured. The present results are the first to show that drug dissolution from ODT is dependent on time in the oral cavity and coating amount. The mimicking of the inside of the human oral cavity is accurate with a testing time of 30 s, while the Tricorptester method was the most preferable of all in vitro short dissolution test methods investigated in this study.