-
編集者のコメント
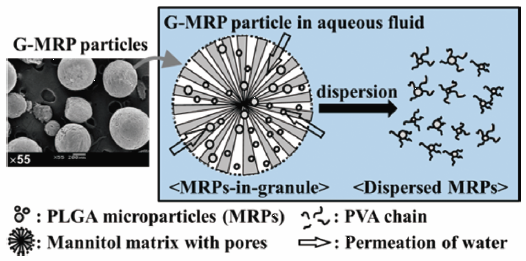
In this article, injectable products composed of biocompatible and biodegradable poly-lactic glycolic acid copolymer (PLGA) microparticles are described. In order to improve the dispersibility of MRPs in injection solutions, MRPs were subjected to a secondary processing, i.e., the drop freeze-drying technique. The suspension with MRPs and water-soluble additives were rapidly dripped into liquid nitrogen in the form of droplets. The resultant frozen particles were freeze-dried to prepare spherical granules containing multiple MRPs (Granulated-MRPs). When the G-MRPs were placed in water for injection, the porous matrix base was immediately dissolved, and each embedded MRPs were individually released, thus inducing monodispersion and significantly improved dispersibility. The granular products could be automatically filled into the pre-filled syringes and may give rise to the excellent passage of PLGA MRPs through needles for use in depot injected formulation as a ready-to-use.
-
編集者のコメント
In this paper, the authors used the charge-transfer complexes between iodine and tertiary amines for the initiation of atom-transfer radical reactions. The visible light irradiation of charge-transfer complex in the ground state gives the excited state complex, in which the single electron transfer from the donor amine to the acceptor iodine proceeds effectively to give an iodine radical.
-
編集者のコメント
In this study, the authors focused on two permeation enhancers: nerolidol and levulinic acid to clarify the mechanism of action of permeation enhancers on lipids structures. Synchrotron X-ray diffraction revealed that nerolidol strongly affected the orthorhombic and hexagonal structures, whereas levulinic acid had little effect on the lateral packing structures.
-
編集者のコメント
In this paper, the authors used a panel of fluorogenic substrates to search for altered enzyme activities in bronchoalveolar lavage fluid (BALF) from a mouse model of lung inflammation and found that acylamino acid releasing enzyme (APEH) activity was highly elevated, apparently reflecting the increased population of immune cells in the inflamed lung.
-
編集者のコメント
In the review, the authors summarize recent reports on direct translocation of arginine-rich cell-penetrating peptides (CPPs) through cell membranes, including counteranion-assisted delivery method, and discuss its mechanism, which would provide insight into highly efficient intracellular delivery.
-
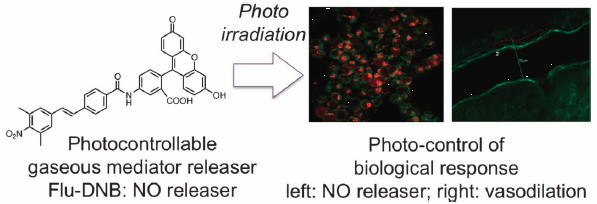
64 巻 (2016) 9 号 p. 1249-1255Photocontrol of NO, H2S, and HNO Release in Biological Systems by Using Specific Caged Compounds もっと読む編集者のコメント
This review summarizes the authors’ recent work on photocontrolable releases, or caged compounds, which can release specific biological mediators such as NO, HNO, and H2S, upon photoirradiation, and a wide range of colors (wavelengths), from ultraviolet to near infrared, are available depending on the properties of the compounds. These photocontrollable compounds realized the spatiotemporal control of the release, and the manipulation of biological responses specific for the mediators. One of our photocontrollable NO releasers with the near infrared two photon excitation succeeded to control vasodilation in the anesthetized mouse brain with spatiotemporal resolution, and the ex vivo rat aorta relaxation was also achieved with another controllable NO releaser by the visible light irradiation.