-
72 巻 (2024) 6 号 p. 540-546Three neo-Clerodane Diterpenoids from Tinospora cordifolia Stems and Their Arginase Inhibitory Activities もっと読む編集者のコメント
[Highlighted Paper selected by Editor-in-Chief]
Arginases I and II are Mn(II)-dependent hydroxylases that convert L-arginine into L-ornithine and urea in the urea cycle, and has been proposed as a potential therapy target for various illnesses, such as cardiovascular, anti-inflammatory, autoimmune, oncological, and infectious diseases. Herein, authors report the isolations and structural elucidations of three neo-clerodane diterpenoids, including two new tinocordifoliols A and B and one known tinopanoid R from the Tinospora cordifolia stems as well as their inhibitory activities against human arginase I. The assay revealed that tinopanoid R was a natural arginase I inhibitor in a competitive manner with respect to L-arginine. -
72 巻 (2024) 6 号 p. 566-569Oxidative Coupling of Hydroquinone Derivatives with Olefins to Dihydrobenzofurans もっと読む編集者のコメント
The development of efficient synthesis of heterocyclic compounds is crucial important for drug discovery. Authors have newly developed the oxidative coupling of hydroquinones/4-aminophenols, bearing the electron-withdrawing groups, with various olefins (styrenes, enol ethers, and allyl silane) to provide dihydrobenzofurans as important heterocyclic skeletons for bioactive compounds and natural products. For example, the oxidation of 2-methoxycarbonylhydroquinone using 2,3-dichloro-5,6-dicyano-p-benzoquinone and the following coupling with styrene in the presence of Lewis acidic FeCl3 could procced under the mild reaction conditions to give the corresponding dihydrobenzofuran product. This method can easily yield various dihydrobenzofurans that can contribute to drug discovery.
-
編集者のコメント
The authors achieved the first total synthesis of silybin A, a hybrid natural polyphenol with attractive biological activities. The highlight of the study includes modified Julia-Kocienski olefination reaction and Sharpless dihydroxylation, which enabled the highly stereocontrolled synthesis. Additionally, the acid-promoted generation of two types of quinomethide intermediates led to the biomimetic construction of both the 1,4-benzodioxane neolignane and the flavanol lignan core skeletons within silybin A. The high generality of this methodology would allow for the synthesis of a diverse array of structurally related silybins, isosilybins, and other hybrid polyphenols.
-
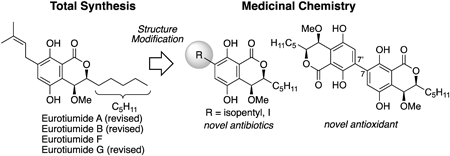
72 巻 (2024) 5 号 p. 422-431Studies on Comprehensive Total Synthesis of Natural and Pseudo-Natural Products for Drug Discovery もっと読む編集者のコメント
Natural products have played an important role in drug discovery. Recently, pseudo-natural products, whose structures have been modified based on natural products, have received much attention due to their unique biological properties that differ from the parent compounds. This review describes a series of total syntheses and structural elucidations of eurotiumides and developments of pseudo-natural products carried out by the author concerning the dihydroisocoumarin-type natural products eurotiumides with potent biological activities. One-point chemical structural modification and the dimerization strategy have led to the promising compounds, which are more active than the parent natural product.
-
編集者のコメント
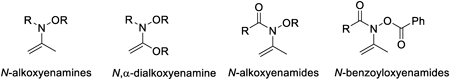
This review describes the development of new synthetic methodologies for polyfunctional compounds using enamines and enamides carrying an oxygen atom substituent on nitrogen atom, such as N-alkoxyenamines, N,2-dialkoxyenamines, N-alkoxyenamides, and N-(benzoyloxy) enamides that have not received much attention. The efficient synthetic reactions using N-alkoxy-enamines and enamides as substrates proceed in the presence of triarylaluminum reagent via cleavage of a relatively lower energy N–O bond, and formation of new stronger bonds to afford 2-arylketones, 2-arylcarboxylic acids, tert-alkylamies carrying aryl group, 1,2-disubstituted phenethylamines, and 2-amino-2-arylethanols that are useful as partial structures in biologically active compounds.
-
編集者のコメント
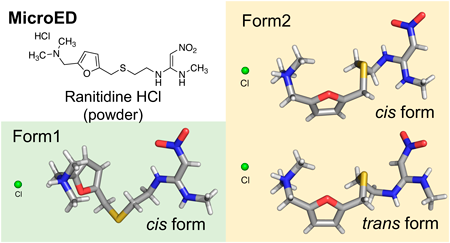
The solid-state properties of drug candidates are crucial for their selection process. Traditional crystallographic techniques for structural analysis have limitations and require high-quality single crystals. Microcrystal electron diffraction (microED) can overcome these challenges by analyzing difficult-to-crystallize or small-quantity samples. In this study, microED rapidly determined the configuration of two crystal forms of the active pharmaceutical ingredient ranitidine hydrochloride. The structures obtained through microED were consistent with those determined by X-ray crystallography, demonstrating that microED is a valuable tool for efficiently elucidating molecular structures in drug development and materials science
-
編集者のコメント
Authors have developed heterologous expression technique applicable to huge biosynthetic gene clusters (BGCs) which produce large molecular secondary metabolites. Authors targeted concanamycin BGC (~100 kb) in Streptomyces neyagawaensis IFO13477. Interestingly, heterologous expression of a BAC clone of which insert size was 211 kb involving the entire concanamycin resulted in the production of a new compound JBIR-157 in addition to concanamycin. INADEQUATE analysis revealed that JBIR-157 consists of an unusual new skeleton produced by the cryptic type-II polyketide synthases (PKS) BGC. In this study, authors reported the production, isolation, structure elucidation, and proposed biosynthetic mechanism of JBIR-157.
-
編集者のコメント
[Highlighted Paper selected by Editor-in-Chief]
Significant efforts have focused on developing cell-penetrating peptides (CPPs) for delivering nucleic acids into cells. In this study, authors tested seven peptides for their effectiveness in delivering plasmid DNA (pDNA). These peptides had previously been used for small interfering RNA (siRNA) delivery, with one peptide containing the dipropylglycine showing successful delivery with low cytotoxicity. Despite both being nucleic acids, pDNA and siRNA differ in size and function, potentially affecting optimal peptide sequences for delivery. The authors' results show that three peptides were effective in pDNA transfection, with only one also showing efficient siRNA delivery. These findings support our hypothesis and provide insights for designing CPPs for both pDNA and siRNA delivery. -
編集者のコメント
Preparation of drug metabolites at the milligram scale is essential for determining their structure and toxicity. However, their preparation using recombinant proteins and human liver microsomes is often difficult because of technical and ethical issues. In this study, authors found that bacteria isolated from “natto” can produce an unknown lidocaine metabolite, which is produced by human liver microsome. Then, they prepared a fraction containing the metabolite through mass cultivation of Bacillus subtilis, then identified the metabolite by NMR. Authors demonstrated that food microorganisms can be a tool to prepare drug metabolites at a low cost and without ethical issues.
-
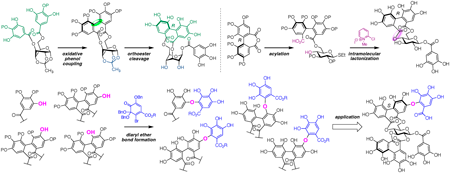
72 巻 (2024) 4 号 p. 349-359Development of Methodologies toward the Unified Synthesis of Ellagitannins もっと読む編集者のコメント
This review describes the development of methodologies toward the unified synthesis of ellagitannins, a class of polyphenols with divergent structures, as reported by the Yamada group at Kwansei Gakuin University during 2017–2023. Efficient methods for constructing 3,6-O-(aR)- and 4,6-O-(aR)-hexahydroxydiphenoyl-bridged glucose moieties, in addition to various C–O digallate structures, are disclosed. The total synthesis of corilagin, mallotusinin, neostrictinin, and rugosin C is also achieved via application of the established methods, which are expected to enable increase of the number of ellagitannins that can be chemically synthesized.
-
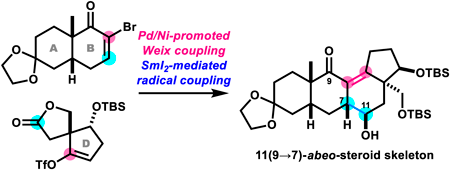
72 巻 (2024) 4 号 p. 360-364Unexpected Formation of 11(9→7)-abeo-Steroid Skeleton in Synthetic Studies toward Batrachotoxin もっと読む
-
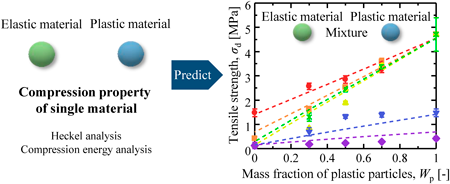
72 巻 (2024) 4 号 p. 374-380A Method for the Tensile Strength Prediction of Tablets with Differing Powder Plasticities もっと読む編集者のコメント
his study provided the prediction equation of tablet strength of binary powder mixture with different plastic deformability. The five materials from the general pharmaceutical powders were selected based on their plastic deformability, and their compression properties were evaluated by the Heckel analysis and the compression energy analysis. The plastic deformability of the powder mixture was evaluated to estimate the tablet strength using the compression properties of powder mixture. This finding indicated that the ideal mass fraction of plastic powders to form tablets with sufficient tablet strength could be predicted from the compression properties of single material.
-
72 巻 (2024) 4 号 p. 413-420P(III)-Mediated Formal Reductive N–H Bond Insertion Reaction of Hydrazones to α-Keto Esters もっと読む編集者のコメント
[Highlighted Paper selected by Editor-in-Chief]
N-H bond insertion reaction is one of the useful transformations, wherein C1 units such as carbenes, carbenoids, and their surrogates can be incorporated into N-H bond to form N-C-H bond. To achieve successful this reaction, the use of diazo compounds as well as transition metal catalysts, thermal conditions, or blue LED irradiation were often required. The authors report a diazo- and metal-free N-H bond insertion reaction of a hydrazone with alpha-keto ester, demonstrating a broad substrate scope, a good functional group tolerance, and a late-stage functionalization of pharmaceuticals. -
編集者のコメント
Codeine, is used worldwide and abused as a recreational drug. The authors developed a system to selectively and sensitively detect codeine from over-the-counter medications containing interfering drug components by electrochemiluminescence (ECL) combined with potential modulated technique (PM). The sensitivity for detection of codeine by PM-ECL was more than one order of magnitude larger than that obtained in conventional potential sweep mode. The established technique was applied to codeine determination in over-the-counter drugs and medicines and was not affected by the presence of structurally similar chemicals. The proposed method expected to apply as a sensitive on-site analytical method for a wide range of detection, especially clinical and forensic analysis.
-
編集者のコメント
Reliable data on the compatibility and chemoselectivity of functional groups are essential for assessing the usefulness of chemical reactions. Authors systematically evaluated the functional group tolerance of carbene-mediated reactions as a core project of the Grant-in-Aid for Transformative Research Area A “Digitalization-driven Transformative Organic Synthesis”. In the course of this study, unexpected C-H functionalization of a naphthol derivative used as an additive was observed. Authors believe that collecting dependable information, including negative experimental results, plays a crucial role in developing organic synthesis.
-
72 巻 (2024) 3 号 p. 311-312Effect of Two-Photon Excitation to 8-Azacoumarin Derivatives as Photolabile Protecting Groups もっと読む編集者のコメント
Photolabile protecting groups (PPGs) have been utilized in many research fields such as organic synthesis and chemical biology because their fast and selective photocleavage proceeds under mild conditions. The authors previously reported the design and synthesis of 8-azacoumarin-type PPGs based on the alkene-to-amide replacement of the 6-bromo-7-hydroxy-coumarin-4-ylmethyl (Bhc) group. The characteristic feature of these PPGs is their aqueous solubility, which is remarkably higher than that of Bhc. The authors found that 8-azacoumarin-type PPGs can also be used as two-photon excitation sources because the photolytic efficiency for two-photon excitation showed preferable physicochemical values for applications in cells and tissues.
-
編集者のコメント
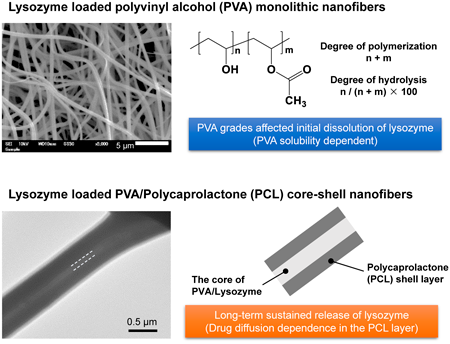
[Highlighted Paper selected by Editor-in-Chief]
The authors investigated the use of electrospun polyvinyl alcohol (PVA) nanofibers for the drug delivery of lysozyme (LZM), focusing on how different PVA grades affect drug release characteristics. PVA nanofibers with a 50% LZM content achieved efficient encapsulation and quick release within 30 minutes. Using fully hydrolyzed PVA led to more controlled release due to its reduced water solubility. Notably, the study highlighted coaxial electrospinning to create PVA/LZM nanofibers coated with polycaprolactone, facilitating extended drug release. This approach clarified the relationship between the characteristics of PVA in nanofibers and drug release properties, offering promising insights for pharmaceutical nanofibers. -
72 巻 (2024) 3 号 p. 336-339Synthesis of 2-Substituted Indoles via Migration Reaction of 3-Substituted Indoles with Triflic Acid もっと読む編集者のコメント
Indole is a crucial heterocycle found in various biologically active natural products and medicinal compounds. Therefore, developing convenient methodologies for modifying the indole structure is an interesting topic for the discovery of pharmaceuticals and functional materials. In this context, the authors report trifluoromethanesulfonic acid (2.1 equiv) efficiently facilitates the 1,2-migration reaction of the substituent from C3- to C2- position of the indole structure. Alkyl, aryl groups such as benzyl, isopropyl, phenyl were tolerated for this reaction (up to 98% yield).
-
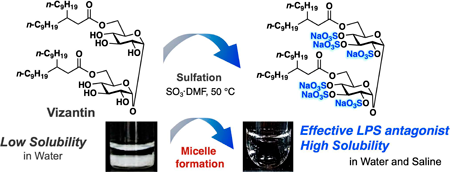
72 巻 (2024) 2 号 p. 226-233Development of a Water Soluble Self-assembling Analogue of Vizantin もっと読む編集者のコメント
Vizantin is a TLR-4 antagonist developed in the author's laboratory. In this article, to improve the water solubility of vizantin, the authors designed a new vizantin derivative in which all hydroxyl groups of the sugar unit were sulfated. It has been confirmed that the synthesized vizantin derivative spontaneously forms string-like micelles and dissolves in water. The authors also report that string-like micelles of more uniform size are formed in physiological saline than in distilled water, making it possible to prepare an ideal injection solution.
-
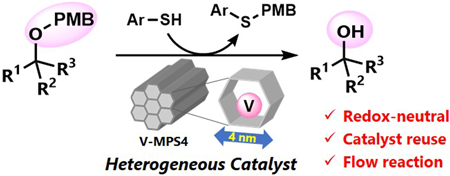
72 巻 (2024) 2 号 p. 213-219Nucleophilic Deprotection of p-Methoxybenzyl Ethers Using Heterogeneous Oxovanadium Catalyst もっと読む編集者のコメント
Heterogeneous catalysis has gained increasing interest in the growing demand of sustainable manufacturing of pharmaceutically relevant compounds. The authors report herein a mesoporous silica-supported oxovanadium (V-MPS4)-catalyzed nucleophilic removal of the p-methoxybenzyl (PMB) protective group on alcohols under mild and redox-neutral conditions. The method has a wide reaction scope, including primary, secondary, and tertiary alcohols with various functional groups. The catalyst was reused six times without a significant loss in the conversion. The advantages of using the heterogeneous catalyst were further demonstrated by conducting the deprotection reaction in a flow process, which showed significantly higher turnover frequency compared to the batch reactions.