2013 年 36 巻 4 号 p. 515-521
2013 年 36 巻 4 号 p. 515-521
Astragaloside IV (AS-IV) is one of the main active constituents of Astragalus membranaceus, which has various actions on the cardiovascular system. However, its electrophysiological mechanisms are not clear. In the present study, we investigated the effects of AS-IV on action potentials and membrane currents using the whole-cell patch clamp technique in isolated guinea-pig ventricular myocytes. AS-IV prolonged the action potential duration (APD) at all three tested concentrations. The peak effect was achieved with 1×10−6 m, at which concentration AS-IV significantly prolonged the APD at 95% repolarization from 313.1±38.9 to 785.3±83.7 ms. AS-IV at 1×10−6 m also enhanced the inward rectifier K+ currents (IK1) and inhibited the delayed rectifier K+ currents (IK). AS-IV (1×10−6 m) strongly depressed the peak of voltage-dependent Ca2+ channel current (ICaL) from −607.3±37.5 to −321.1±38.3 pA. However, AS-IV was not found to affect the Na+ currents. Taken together, AS-IV prolonged APD of guinea-pig ventricular myocytes, which might be explained by its inhibition of IK. AS-IV also influences Ca2+ signaling through suppressing ICaL.
Astragaloside IV (AS-IV) is one of the main active ingredients of Astragalus membranaceus (HuangQi). Chemically, it is a cycloartane triterpene saponin (Fig. 1). AS-IV has multiple pharmacological functions including anti-inflammation,1,2) antioxidation,3–5) antivirus,6,7) anti-apoptosis,8,9) anti-hyperglycemic,10,11) neuroprotection,13) gastroprotection,14) anti-chronic-asthma,15) and anti-lung-cancer.16) One of AS-IV’s targets is the cardiovascular system, and especially the heart. There are emerging evidences that AS-IV has cardio-protective effects, for example, AS-IV treatment inhibited compensatory hypertrophy of myocardial cells and lowered the number of apoptotic myocytes in long-term heart failure in rats.17) These beneficial effects of AS-IV may involve the prevention of the depression of the activity of Ca2+-ATPase on sarcoplasmic reticulum.18)
However, to our knowledge, there have been no evaluations of the effects of AS-IV upon the electrophysiological characteristics of cardiac ventricular myocytes. In the present study, we have investigated the effects of AS-IV on action potentials and ionic currents from freshly isolated ventricular myocytes of guinea-pig heart using the whole-cell patch clamp technique. We have found that AS-IV prolonged the action potential duration (APD) and inhibited the delayed rectifier K+ currents (IK) and voltage-gated Ca2+ currents (ICaL).
Healthy, 350±50 g, adult guinea-pigs were purchased from China Medical University Animal Center. Single ventricular myocytes of guinea-pig heart were obtained with an enzymatic dissociation protocol.19,20) Briefly, animals were anaesthetized intraperitoneally with 100 mg/kg sodium pentoparbital. The heart was dissected out quickly and was mounted on a Langendorff apparatus for digestion. After perfusion with Tyrode solution for 3 min, Ca2+-free Tyrode solution for 6 min, and then Ca2+-free Tyrode solution containing 0.08% collagenase (Yakult, Japan) for 15 min, the heart was washed thoroughly with 60 mL Kraftbrühe-modified (KB) solution. All solutions were maintained at 37°C. Then the digested left ventricle was cut away, shredded and filtered through a 105 µm stainless-steel mesh. The filtrate was collected and centrifuged with KB solution. The dispersed cells were maintained in the storage solution at 4°C until use.
Solutions and DrugsFor the isolation ventricular myocytes of guinea-pig heart, Tyrode solution contained (in mm): NaCl 135, KCl 5.4, NaH2PO4 0.33, MgCl2 1.0, glucose 5.5, CaCl2 1.8, and N-(2-hydroxyethyl)piperazine-N′-2-ethanesulfonic acid (HEPES) 10 (pH 7.4 adjusted by NaOH). KB solution contained (in mm): KOH 70, Glutamic acid 50, KCl 40, Taurine 20, KH2PO4 20, Glucose 10, ethylene glycol bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA) 0.5, MgCl2 3 and HEPES 10 (pH 7.4 by KOH). The whole-cell patch clamp technique was employed to record the action potential and membrane channel currents. The basic external perfusion solution was the Tyrode solution, except indicated especially. AS-IV (C41H68O14) was isolated from Astragalus membranaceus var. mongholicus by chromatographic techniques and identified through spectral analysis. The purity (>99%) of AS-IV was determined with the high performance liquid chromatography-evaporative light scattering detector (HPLC-ELSD) method. AS-IV was dissolved in dimethyl sulphoxide (DMSO), then diluted in the perfusion solution (final concentration of DMSO <0.1%). Three concentrations of AS-IV (1×10−7, 1×10−6, 1×10−5 m) were prepared in Tyrode solution.
Experimental GroupsFour groups of cells were used in electrophysiological experiments. The control group was bathed in normal Tyrode solution. The three experimental groups were bathed in Tyrode solution which contained 1×10−7 m, 1×10−6 m, and 1×10−5 m AS-IV, respectively. Experiments were conducted in cells immersed in these solutions for 30 min to 2 h.
Action Potential RecordingAction potentials were recorded from isolated myocytes using the patch clamp technique in current-clamp mode.21) Cells were bathed in solution in a recording chamber placed on an Olympus inverted microscope. Experiments were conducted at room temperature. A high-resistance seal (giga-seal) was established on a cell with Tyrode solution or drug solutions perfusion. The resistance of the pipette filled with the internal solution was 2–5 MΩ. Data were recorded with a patch-clamp amplifier (Axon 200B, Axon, America) filtered at 1 kHz, and fed to a computer at a sampling rate of 5 kHz. Action potentials were evoked with a 3 ms, 4 nA rectangular pulse. The external perfusion solution was the Tyrode solution. The patch pipettes were filled with (in mm): K-aspartate 110, KCl 30, MgCl2 5, K2ATP 5, Na2CrP 5, HEPES 5, EGTA 10, and CaCl2 1.43 (pH 7.2 by KOH).
Whole-Cell Membrane Current RecordingMembrane currents were recorded in voltage-clamp mode. After obtaining a high-resistance seal (giga-seal) to cell membrane, the whole-cell configuration was formed by rupturing the membrane with negative pressure. Na+, K+ and Ca2+ membrane currents were recorded with appropriate stimulus protocols.
The external perfusion solution for recording voltage-gated Na+ currents (INa) was (in mm): Choline chloride 100, NaCl 50, MgCl2 2, HEPES 10, Glucose 10, CsCl 20, CdCl2 0.3 (pH 7.3 by NaOH); and the pipette solution contained (in mm): CsCl 130, MgCl2 2, NaCl 10, HEPES 10 (pH 7.3 by CsOH). To record inward rectified K+ currents (IK1), BaCl2 (0.5 mm) were added to the external solution at the end of experiments, and the Ba2+-subtracted currents were considered as IK1 component. To record the delayed rectified K+ currents (IK), nifedipine (3 µm) and BaCl2 (0.5 mm) were added into the Tyrode solution as the external solution to block ICaL and IK1 currents, respectively; and the pipette solution was the same as applied in action potential recording. The tail current of IK (IK tail) which evoked on returning to a holding potential represented IK and has been analyzed. For recording voltage-gated L-type Ca2+ channel currents (ICaL), the internal and external solutions were the same as administrated in action potential recording.
Statistical AnalysisThe results were expressed as mean±standard error (S.E.). Statistical significance was determined by Student’s t-test or one-way ANOVA. p-values of less than 0.05 were considered significant.
All three concentrations of AS-IV significantly prolonged the APD (Fig. 2). The peak effect was achieved with AS-IV at 1×10−6 m. At that concentration, AS-IV increased APD25, APD50 and APD95 to 8.5, 3.0 and 2.5 times the control values (p=0.0055, p=0.0005, p=0.0001). AS-IV at 1×10−7 m and 1×10−5 m showed similar but lesser effects on APD. We also found that neither the resting membrane potential (RMP) nor the action potential amplitude (APA) was significantly changed by AS-IV at all three concentrations tested (Table 1).
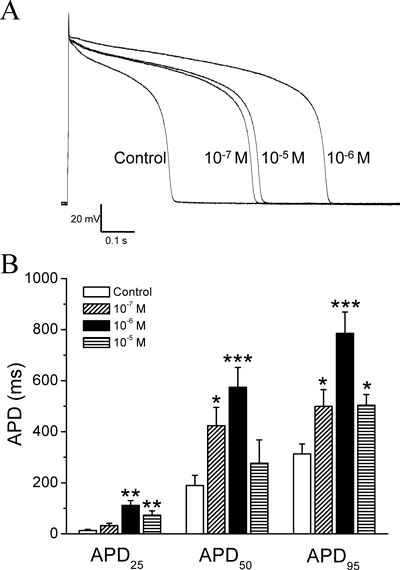
Action potentials were recorded in current-clamp mode. The supra-threshold current pulses were applied every 3 s. (A) Representative action potential recordings obtained from different groups of ventricular myocytes in normal Tyrode solution (Control) and in the presence of the indicated concentrations of AS-IV. (B) The mean values of action potential duration at 25%, 50% and 95% repolarization (APD25, APD50, APD95) in the presence of AS-IV at 1×10−7 m, 1×10−6 m, and 1×10−5 m. n=4–8. * p<0.05, ** p<0.01, and *** p<0.001 versus control.
| RMP (mV) | APA (mV) | APD25 (ms) | APD50 (ms) | APD95 (ms) | |
|---|---|---|---|---|---|
| Control | −75.5±1.7 | 158±4.6 | 13.1±4.1 | 189.2±40.3 | 313.1±38.9 |
| 10−7 m AS-IV | −74.5±2.2 | 150.3±5.1 | 32.4±8.9 | 423.6±72.0* | 499.6±65.5* |
| 10−6 m AS-IV | −77.6±4.5 | 153.3±7.4 | 111.5±18.9** | 574.3±77.4*** | 785.3±83.7*** |
| 10−5 m AS-IV | −75.9±6.1 | 161.2±23.4 | 73.4±16.6** | 276.9±91 | 503.7±42.1* |
RMP: resting membrane potential; APA: action potential amplitude; APD25, APD50 and APD95: action potential duration at 25%, 50% and 95% of repolarization. n=4–8. * p<0.05, ** p<0.01, and *** p<0.001, compared with control group.
The voltage-dependent Na+ currents (INa) are responsible for the rising phase of the action potential in cardiac myocytes, we thus examined the effects of AS-IV on INa. Neither the I–V curves (Fig. 3A) nor the inward peak amplitude of INa (Fig. 3B) was affected by AS-IV. These suggest that AS-IV does not affect INa, which is consistent with the result that AS-IV is failed to affect APA. Representative traces of INa are shown in Fig. 3C.
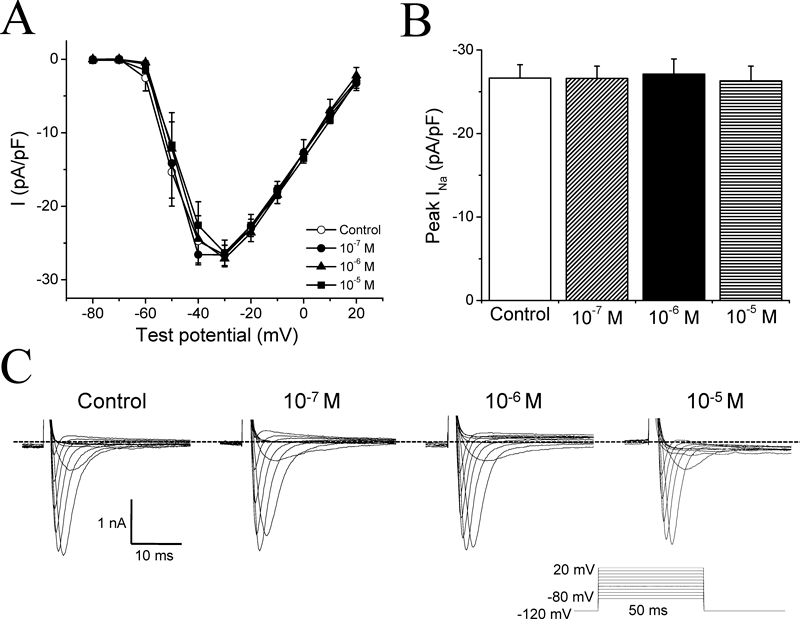
(A) Current–voltage (I–V) curves for INa. Open circles (○) represent control data, filled circles (●), filled triangles (▲) and filled squares (■) represent the effects of 1×10−7 m, 1×10−6 m, and 1×10−5 m AS-IV, respectively. The amplitudes of INa were normalized by cell capacitance. n=9. (B) The peak amplitude of INa in control and in the presence of different concentrations of AS-IV (1×10−7, 1×10−6, 1×10−5 m). There was no significant difference among the four groups. (C) Representative INa recorded from different myocytes with the indicated concentrations of AS-IV. The voltage-clamp protocol is shown in the inset. INa was elicited by 50 ms voltage steps from the holding potential of −120 mV to between −80 and +20 mV in 10 mV increments.
We then proceeded to investigate the effects of AS-IV on K+ currents which are known to contribute to the repolarization of the action potential. Voltage-clamp protocols used to elicit both inward rectifier K+ currents (IK1) and delayed rectifier K+ currents (IK) were shown in the inlets of Figs. 4 and 5. Ba2+-subtracted current was taken as IK1 (see Materials and Methods), AS-IV was likely to increase IK1 (Fig. 4), but decrease IK at all three concentrations (Fig. 5). However, there were no significant differences among three different concentrations of AS-IV. Especially, the middle dose (1×10−6 m) of AS-IV induced the smallest increase in IK1 (Fig. 4B); and this was also the only concentration of AS-IV which showed significant suppression of IK (Fig. 5B).
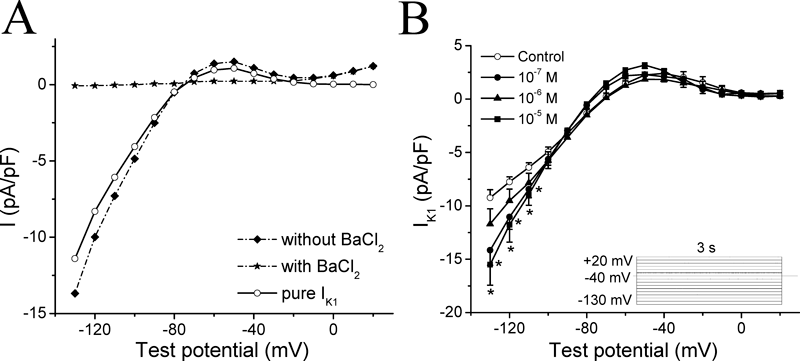
(A) Analysis of IK1 in a ventricular muocyte of guinea-pig heart. The dash dot line with diamonds (◆) shows the current due to a voltage-clamp step stimulation applied to a ventricular cell in nifedipine (3 µm) containing Tyrode solution. Following the superfusion of 0.5 mm BaCl2 in the Tyrode solution, a second stimulation was applied. Membrane current under this condition is shown as the dash dot line with stars (★). The value of pure IK1 is the difference current between without and with BaCl2 shown as the solid line with open circles (○). (B) Current–voltage (I–V) curves for IK1 currents recorded with the inset voltage-clamp protocol. From a holding potential of −40 mV, 3 s steps were applied from −130 to +20 mV with 10 mV steps. Open circles (○) in each graph represent control data, filled circles (●), filled triangles (▲) and filled squares (■) represent results obtained in the presence of 1×10−7 m, 1×10−6 m and 1×10−5 m AS-IV, respectively. n=6. * p<0.05 versus control.
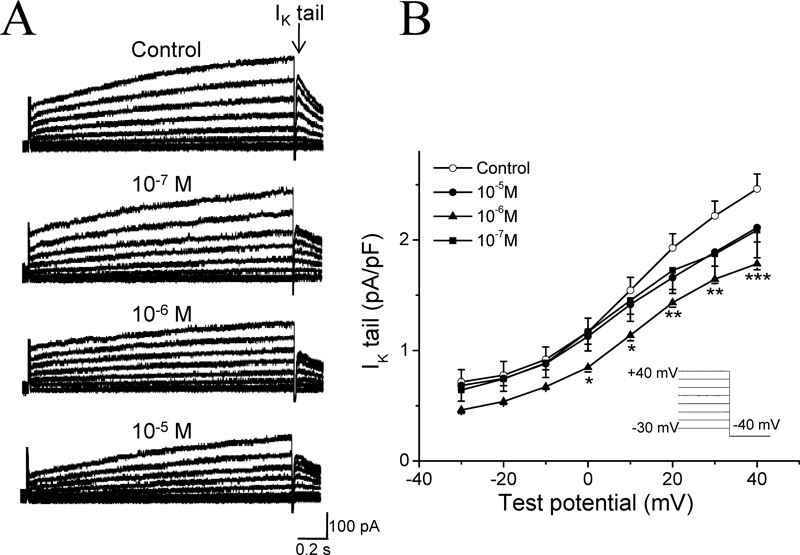
(A) Representative tail current traces of IK in control, 1×10−7 m, 1×10−6 m, and 1×10−5 m AS-IV. (B) I–V curves for IK tail currents were recorded during 3-s depolarizing test pulses from −30 to +40 mV, followed by 0.2 s repolarization to −40 mV. Open circles (○) in each graph represent control data, filled circles (●), filled triangles (▲) and filled squares (■) represent results obtained in the presence of 1×10−7 m, 1×10−6 m and 1×10−5 m AS-IV, respectively. n=6. * p<0.05, ** p<0.01, *** p<0.001 versus control. The IK tail currents were recorded in Tyrode solution with 3 µm nifedipine and 0.5 mm BaCl2 to avoid ICaL and IK1 contamination.
The effect of AS-IV in prolonging the APD suggested that membrane voltage-gated L-type Ca2+ currents (ICaL) might be affected by AS-IV. Thus we examined the effects of AS-IV on ICaL. Free Ca2+ concentration in the patch pipette solution was 80 nm, which is the physiological intracellular Ca2+ concentration at rest condition. Following 60-ms voltage steps from the holding potential of −80 to −40 mV to inactivate the INa, ICaL was then evoked by subsequent 200-ms depolarizations from −40 to +40 mV in 5 mV increments. AS-IV decreased the amplitude of ICaL at all tested potentials (Fig. 6A). All three doses of AS-IV significantly reduced the inward peak amplitude of ICaL to 61.2±7.2% (p<0.05), 52.8±6.3% (p<0.01), and 60.9±5.8% (p<0.05) of the control group value, respectively (Fig. 6B). Interestingly, as we may see from the reduction percentage, the middle dose (1×10−6 m) of AS-IV reduced the ICaL the most effectively. It also induced a significant negative shift of the ICaL inactivation curve [V1/2 (10−6 m)=−24.4±1.4 mV versus V1/2 (control)=−19.4±0.7 mV, p=0.005], while 1×10−7 m and 1×10−5 m AS-IV had no effect [V1/2 (10−7 m)=−19.9±1.0 mV, P=0.673; V1/2 (10−5 m)=−20.1±1.4 mV, p=0.675] (Fig. 6C). In no case did the slope factor of inactivation change, k (10−7 m)=6.2±0.6, k (10−6 m)=7.4±0.7, k (10−5 m)=6.1±0.3, versus k (control)=6.7±0.4.
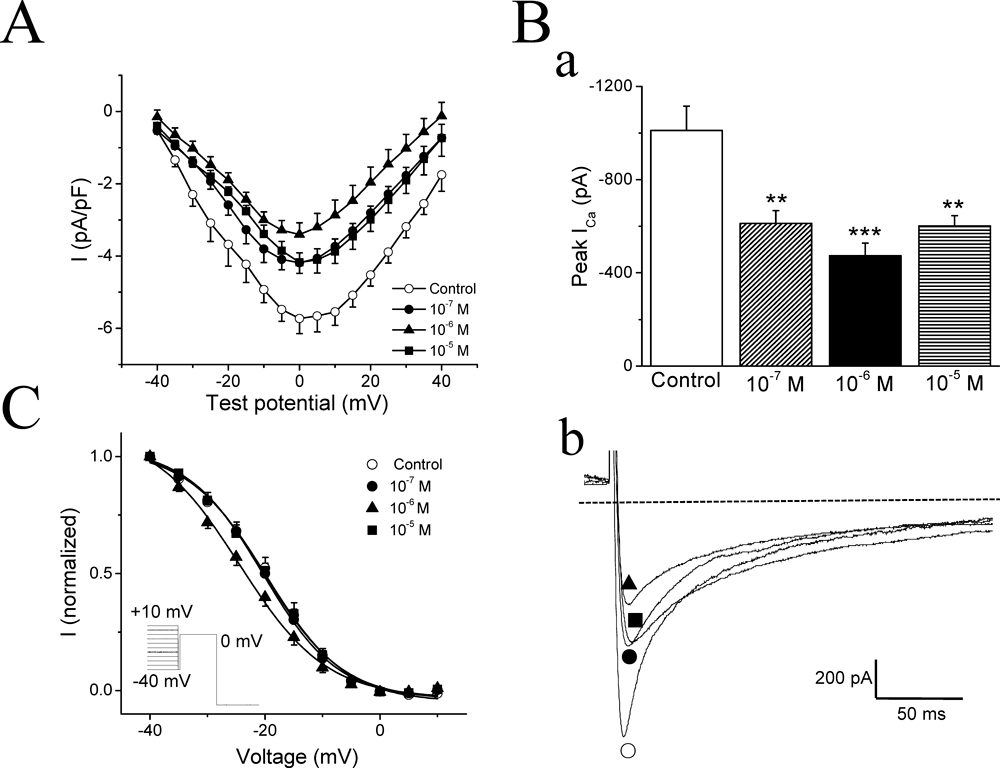
(A) Current–voltage (I–V) curves for ICaL. The voltage-clamp protocol was a 200 ms voltage step from −40 to +40 mV with a 5 mV increment from a holding potential of −80 mV. Open circles (○) represent control data, filled circles (●), filled triangles (▲) and filled squares (■) represent 1×10−7 m, 1×10−6 m and 1×10−5 m AS-IV, respectively. n=7. (B) a) Mean maximum values of ICaL in the different groups of myocytes. b) Representative current traces in control (○), 1×10−7 m (●), 1×10−6 m (▲), and 1×10−5 m (■) AS-IV. ** p<0.01, *** p<0.001 versus control. The broken lines indicated the zero current level. (C) Inactivation curves for voltage-gated Ca2+ channels. I: normalized channel availability. Data points were fitted with the Boltzmann equation: y=A2+(A1−A2)/(1+exp((V−V1/2)/k)), where A1, A2, V1/2, and k are the initial value, final value, mid-point voltage, and slope factor, respectively. Insert: voltage clamp protocol with the pre-steps ranging from −40 to +10 mV, followed by the 100 ms test step to 0 mV.
HuangQi, as a tradition Chinese medicine, has been widely used for heart disease treatment in clinical conditions. AS-IV, as one of its active elements, has been revealed its pharmacological mechanisms to some degree. Recent researches have suggested that AS-IV might protect from cardiomyopathy via diverse effects upon a variety of intracellular signaling pathways, including the activation of Src/mitogen-activated protein kinase (MEK)/extracellular signal-regulated kinase (ERK) pathway,22) inhibiting tumor growth factor (TGF)-β123) and ERK1/2 signaling pathways,24) upregulating superoxide dismutase-1 levels,3,25) antioxidative and nitric oxide-inducing pathway,26) and improving intracellular calcium handling.27,28) The aim of our study is to clarify the basic electrophysiological effects of AS-IV on cardiac myocytes, and we have found that AS-IV prolonged APD, and decreased IK and ICaL in guinea-pig cardiac myocytes.
The action potential is formed by multiple ion currents gated by different ion channels. Na+ channels are responsible for the initial membrane depolarization (phase 0) during the action potential. AS-IV had no effect on INa, and correspondingly, APA (Fig. 3). Current clamp results (Table 1) also showed that AS-IV had no effect on RMP. IK1 is the major determinant of RMP, but AS-IV, which was shown to increase IK1, had no effect on this parameter. We noticed that although AS-IV increased IK1 at −130 mV, −120 mV and −110 mV (Fig. 4), indeed, it had no measurable effect on IK1 at the membrane potentials around RMP, for example at −70 mV. Perhaps this could explain why it has no influence upon RMP.
The action potential plateau and repolarization result from complex affections between Ca2+ and K+ channel currents. Delayed repolarization and thus APD elongation may result from decreased outward currents or enhanced inward currents during the action potential plateau and the repolarization phase. IK is the major outward current involved in respolarization of ventricular myocytes. It consists of two major components with different biophysical properties and different drug sensitivities: one is rapid activation component (IKr), which rectifies inwardly at depolarized membrane potential due to C-type inactivation, the other is slow activation component (IKs), which has almost linear current voltage-relationship.29,30) Reductions in IKr prolong the APD, which consequently activate more IKs to prevent excess repolarization delay. In most mammalian species, IKr including the human ether-à-go-go-related gene (hERG) currents, play a pivotal role in the repolarization of cardiac action potentail. Actually IKr/hERG represents an important target for pharmacological management of arrhythmias.31) Blockers of IKr prolong the cardiac APD, exhibiting actions of Class III antiarrhythmic drugs. For example, teisamil, a novel antiarrythmic compound, inhibited the K+ currents, thus contributed to the potent antiarrythmic action, underlying predominant Class III antiarrhythmic characteristics.32) In the present study, although IKr was not observed alone, we found that the total IK currents were inhibited by AS-IV (Fig. 5). It has been reported that AS-IV remarkably decreased the incidence of ventricular arrhythmia on the arrhythmia mice model caused by toad venom.33) We speculate that the inhibition of IK by AS-IV may cause APD prolongation, which could be a mechanism for its terminating effects on tachyarrhythmia. On the other hand, excessive prolongation of APD with drugs which inhibit IK may lead to torsades de pointes (TdP) arrhythmias associated with acquired long QT syndrome,34) especially the drugs which prolong the APD through the inhibition of hERG channels. To predict the potential cardiac toxicity of compounds, the effects of compounds on hERG channels should be observed in the early stage of drug development. Interestingly, our data showed that AS-IV at the higher concentration (1×10−5 m) didn’t exhibit potent inhibition on IK and prolongation of APD, whereas the middle dose (1×10−6 m) of AS-IV reduced the IK current the most. This kind of dose-dependency was different from most western medicines, which may benefit for the clinical application of AS-IV safely.
An important inward current that contributes to the plateau phase is ICaL. We found that AS-IV reduced ICaL (Fig. 6). Inhibition of ICaL would normally shorten APD, but given the observation that AS-IV prolonged APD, its inhibitory effect on ICaL must have been compensated by some other effects, for example the reduction of IK. Therefore, AS-IV could be regard as a multi-channel blocker. Rather than pure channel blockers, compounds with multiple channel targets have come to be preferred as antiarrhythmics.35,36) Dronedarone, a new Class III antiarrhythmic, with fewer adverse effects, had multi-channel blocking effect, inhibiting INa, IK and ICaL, and was highly effective in treating many arrhythmias.37) Verapamil, a Ca2+ channel blocker, classified as a Class IV antiarrhythmic drug, has been further reported not only to block the Ca2+ channel, but also to exert prolonging APD by blocking K+ channels. It is believed that this multi-electrophysiology actions probably explained why verapamil was free from QT prolongation in human.38) It seems that AS-IV not only inhibited IK to prolong APD, but also suppressed ICaL to prevent APD over-prolongation which can further cause arrhythmias. This multi-target effect could be taken into account when considering the possible clinical application of AS-IV as an antiarrhythmic drug.
We found that the dose-dependent effect of AS-IV upon the parameters measured in the present study was not typical “S” shaped, especially the effects on ICaL. Actually, this kind of phenomenon has also been reported in other systems. For example, as Han et al. observed, the effects of calmodulin (CaM) on Ca2+ channel activity in inside-out patch was bell-shaped, and they believed that the dual effects of CaM were due to two CaM-binding sites, which had opposite effects in the regulation of Ca2+ channel activity.19) Waston et al. suggested another complex response pattern with multiple peaks, in which the actions of estrogens and xenoestrogens via non-genomic signaling mechanisms, including their oscillating time courses and non-monotonic concentration-responses, were observed. They suggested that the multi-peak patterns could be probably dictated by the influences from the complement of estrogen receptors engaged and the availability of signaling partners in given tissues and cell types.39) A possible explanation for the none-‘S’-shaped dose-dependency of AS-IV’s effects on the electrophysiological properties of cardiac myocytes is that it is also a complex involving multiple factors, not only including the membrane ionic channels but also other intracellular signaling pathways. In our study, we also found that AS-IV at 1×10−5 m and 1×10−7 m significantly prolonged APD, but only slightly inhibited IK, which is thought the major current affecting APD. This phenomenon also suggests that AS-IV may have effects on other targets, such as intracellular Ca2+ handling. Therefore, further studies are required for searching the other targets of AS-IV, especially the intracellular pathways that may affect channel activities and APD.
In summary, the present study provided some explanations for potential mechanism of AS-IV’s effects on the heart by observing the alterations of electrophysiological parameters of cardiac ventricular myocytes. These effects might be expected to protect from cardiac disorders, for instance tachyarrhythmia, mainly via prolonging the APD and/or blocking the Ca2+ and the K+ channels.
The study was supported by Research Grants form Nation Nature Science Foundation of China Grants to L. Y. Hao (30870907, 31071004) and X. S. Huang (30600783).