2013 年 36 巻 5 号 p. 754-763
2013 年 36 巻 5 号 p. 754-763
Our previous study demonstrated that Erxian Decoction (EXD), a traditional Chinese herbal formula, inhibited angiogenesis in zebrafish embryos. To further investigate the anti-angiogenic activity and mechanism of EXD, we evaluated its inhibitory effect on angiogenesis in mammalian endothelial cells in vitro. Cell based assays included proliferation, apoptosis, migration, tube formation and cell cycle analysis. Real-time quantitative polymerase chain reaction (qPCR) and Western blotting were carried out to evaluate the molecular targets and signaling pathways of EXD in human umbilical vein endothelial cells (HUVECs). EXD inhibited proliferation, migration and tube formation in HUVECs. EXD also caused HUVEC apoptosis and cell increase in G0/G1 phase in cell cycle analysis. Furthermore, it decreased the mRNA expressions of vascular endothelial growth factor A (VEGF-A), VEGFR-1 and VEGFR-2 in HUVECs. It also inhibited extracellular signal-regulated kinase 1/2 (ERK1/2) and Akt activation, suggesting the involvement of these signaling pathways in the anti-angiogenic action of EXD in HUVECs. The anti-angiogenic activity of EXD provides new insights to its clinical application and may lead to potential drug development for treating various cancers, especially in menopausal period in the future.
Angiogenesis, which is the formation of new blood vessels from existing ones, plays an important role in tumor growth and metastasis.1) New blood vessels are essential for the delivery of oxygen and nutrients to the tumor microenvironment, especially when the tumor volume is 1–2 mm in diameter.2) Furthermore, the formation of new blood vessels seems critical by providing route for metastasis and thus it is considered as the major cause of cancer mortality.3) These have led to the design of therapeutic strategies including preventing the formation of new vessels called anti-angiogenesis and damaging existing vessels called vascular targeting, toward the tumor vasculature.4) Angiogenesis has been identified as a hallmark of tumor progression, and there is extensive evidence suggesting that anti-angiogenic therapy might be a promising anti-cancer therapeutic strategy.5)
Erxian Decoction (EXD), a traditional Chinese herbal formula, consists of six Chinese herbs: Epimedium sagittatum (Sieb. et Zucc) Maxim. (Berberidaceae, whole herb), Curculigo orchioides Gaertn. (Hypoxidaceae, rhizome), Morinda officinalis How (Rubiaceae, root), Angelica sinensis (Oliv.) Diels (Umbelliferae, root), Phellodendron chinense Schneid. (Rutaceae, bark) and Anemarrhena asphodeloides Bunge (Anthericaceae, rhizome). It has been used to treat osteoporotic disorders,6) menopausal symptoms7,8) or other aging diseases9) in elderly patients for several decades. Previous study from our group demonstrated that EXD inhibited angiogenesis in zebrafish embryos, and hypoxia-inducible factor 1 (HIF-1) mediated vascular endothelial growth factor A (VEGF-A) signaling pathway might be involved in the anti-angiogenic action of EXD.10) Our research team also found that EXD could reduce the metastasis of ovarian cancer cells significantly and inhibit the tumor growth in ovarian cancer nude mouse model when compared with the control.11) Therefore, we hypothesized that the anti-cancer activity of EXD could be partly attributed to its anti-angiogenic activity.
In order to further investigate the anti-angiogenic activity and mechanism of EXD, we evaluated its inhibitory effect on angiogenesis in mammalian endothelial cells in vitro. In this study, HUVECs was used as an in vitro model to detect the anti-angiogenic activities of EXD. The action mechanisms of EXD were further investigated.
Primary human umbilical vein endothelial cells (HUVECs) were purchased from American Type Culture Collection (ATCC®, Manassas, VA, U.S.A.). HUVECs at early passages (passages 9–12) were used in all the experiments, and were cultured in gelatin-coated plates with Complete Growth Medium (Dulbecco’s modified Eagle’s medium: Nutrient Mixture F-12 (DMEM/F-12), 10% fetal bovine serum (FBS) (GIBCO®, Gaithersburg, MD, U.S.A.); 0.1 mg/mL heparin, 0.03–0.05% mg/mL endothelial cell growth supplement, and 1% P/S, Sigma-Aldrich®, St. Louis, MO, U.S.A.). Cells were incubated at 37°C in 5% CO2 in air.
SU5416 (1,3-Dihydro-3-[(3,5-dimethyl-1H-pyrrol-2-yl)methylene]-2H-indol-2-one, Sigma-Aldrich, St. Louis, MO, U.S.A.), a selective vascular endothelial growth factor (VEGF) receptor-2 inhibitor, was used as positive control.
EXD (powdered form) was kindly provided from Purapharm International (Hong Kong, China) Limited with GMP standard (Lot Number: A090612-01). The stock solution of EXD was prepared with the concentration of 10 mg/mL and filtered with a 0.2 µm filter before use. Aliquots of stock solution were stored at −20°C for subsequent use.
Liquid Chromatography-Mass Spectrometry AnalysisLC-MS analysis was performed as described in our previous study.10) Briefly, the sample was analyzed with an Agilent 6460 LC-MS Triple Quadrupole system (Santa Clara, CA, U.S.A.), coupled with an Ultra HPLC 1290, G4220A Infinity Binary Pump, G1316C Infinity TCC and G4226A Infinity Sampler. A Zorbax Eclipse XDB-C18 (4.6×250 mm, 5.0 µm, Agilent, U.S.A.) column was employed to separate the compounds in the LC system. The mobile phases were water (solvent A) and acetonitrile (solvent B). The flow rate was set at 1 mL/min, and the column temperature was held at 26°C. The gradient elution program was set as follows: 0–2 min, 5–15% solvent B; 2–2.1 min, 15–40% solvent B; 2.1–12 min, 40–80% solvent B; and 12–13 min, 80–99% solvent B. The LC column was then equilibrated for three more minutes. The column effluent was monitored at 254 nm.
Negative electrospray ionization (ESI) in multiple reaction monitoring (MRM) mode was used to record the signals of peaks and their isotope-labeled internal standards (ISs). The ion source and other MS/MS parameters were optimized by injecting approximately 100 µg/L of standard solution by an injector program or by using Automation Optimizer Software supplied by the vendor (Agilent, U.S.A.). The nitrogen gas temperature was held at 300°C at a flow rate of 10 L/min. The nebulizer was 45 psi, and the sheath gas temperature was kept at 300°C at a flow rate of 11 L/min. The nozzle voltages were set at 500 eV in negative ESI mode. The capillary was set at 2000 V. The chromatogram of HPLC was included in Supporting Information and the compounds identified were listed in Table S1.
In Vitro Proliferation or Cytotoxicity AssayProliferation assay was performed as described.12) HUVEC (8000 per well) were seeded in 96-well plate in the complete growth medium for 12 h for attachment. Then cells were treated for 24 h with growth medium containing 0.1% dimethyl sulfoxide (DMSO) (vehicle), SU5416 and various concentrations of EXD. Cell proliferation was measured by CellTiter 96® Aqueous One Solution (Promega, Madison, WI, U.S.A.). Each treatment was performed in triplicate.
Apoptosis AssayThe HUVECs were seeded in 25 cm2 flasks with complete medium. After the cells had reached confluence, they were starved with low-serum (0.5% FBS) medium for 24 h to render them quiescent. The samples were then treated with complete medium containing different concentrations of EXD for another 24 h. The cells treated with tert-butyl hydroperoxide (t-BHP), which is an organic peroxide that induces apoptosis in a wide-variety of cells by induction of oxidative stress, served as a positive control. After treatment, the cells were harvested and fixed in 1% (w/v) para-formaldehyde for 15 min. Following fixation, the cells were further fixed overnight in pre-chilled 70% ethanol at −20°C. The terminal deoxynucleotidyl transferase mediated deoxyuridine (dUTP) nick-end labeling (TUNEL) assays were performed with the APO-BrdU™ TUNEL Assay Kit (Invitrogen). The cells were incubated for 1 h at 37°C with DNA-labeling solution containing terminal deoxynucleotidyl transferase enzyme and bromodeoxyuridine triphosphate (BrdUTP), and then for 30 min with anti-BrdU antibody. The labeled cells were analyzed with a FACSCalibur flow cytometer (BD, Franklin Lakes, NJ, U.S.A.). The fluorescent signal was detected through the fluorescein isothiocyanate (FITC) channel.
In Vitro Migration AssayHUVEC migration assay was performed in a modified Boyden chamber (Neuroprobe, Cabin John, MD, U.S.A.).12) Twenty-seven microliter of M199 (Gibco, Gaithersburg, MD, U.S.A.) supplemented with 0.1% bovine serum albumin (BSA, Sigma-Aldrich) plus 1% P/S (Life Technologies, Grand Island, NY, U.S.A.) containing 0.1% DMSO (vehicle) or various concentrations of EXD was added to the lower compartment of each chamber. Gelatin-precoated membrane was used to separate each chamber into upper and lower compartments. HUVEC suspension (4×104 cells per well) in M199 medium with 0.1% BSA and 1% P/S was added to the upper compartments. After 8-h incubation in an incubator, the non-migrated cells from the upper face of the membrane were removed by phosphate buffer saline (PBS)-soaked cotton swab. The migrated cells on the bottom face of the membrane were fixed with methanol and stained with hematoxylin and eosin (HE). Each treatment was done in triplicate and five randomly selected fields of each well were scored for migrated cells using a 40× objective.
In Vitro Network Formation AssayHUVEC (2×104 cells/well) in complete growth medium were seeded in 48-well plate precoated with Matrigel (150 µL/well, 8 mg/mL, BD Biosciences, Bedford, MA, U.S.A.) and exposed to 0.1% DMSO and EXD with indicated concentrations for 18 h.12) The network formation was visualized and imaged under inverted microscope (Nikon, Tokyo, Japan) at 40× magnification. The anti-angiogenic activities were assayed by counting the branch points of the tubes formed, and the average numbers of branch points were calculated from three random fields.
Cell Cycle Analysis of HUVECsThe HUVEC cells were seeded in cell culture flasks with complete medium. After the cells had reached around 90% confluence, they were starved with low-serum (0.5% FBS) medium for 24 h to render them quiescent. The samples were then treated with complete medium containing different concentrations of EXD for 24 h, followed by another 24 h treatment with 10 ng/mL VEGF. After treatment, the cells were trypsinized, washed with PBS, and fixed overnight in pre-chilled 70% ethanol at −20°C. The fixed samples were washed with PBS, and then incubated with propidium iodide (5 µg/mL) and RNase (10 µg/mL) for 30 min. The stained cells were analyzed with a FACSCalibur flow cytometer (BD, Franklin Lakes, NJ, U.S.A.). The fluorescent signal was detected through the FL2-H channel. The DNA content in the G0/G1, S, and G2/M phases was analyzed using ModFit LT version 3.0 software (Verity Software House, Topsham, U.S.A.).
Total RNA Isolation, Reverse Transcription and Real-Time Quantitative Polymerase Chain Reaction (qPCR) Quantification of mRNA ExpressionTotal RNA samples were isolated from HUVEC cells using Tri-Reagent (Molecular Research Center, Cincinnati, OH, U.S.A.) according to the manufacturer’s protocol. The amount and purity of the RNA were determined by spectrophotometry. Reverse transcription (RT) was performed at 37°C for 2 h in a total volume of 10 µL reaction solution containing 1× moloney murine leukemia virus (MMLV) reverse transcriptase buffer, 0.5 mm of each deoxyribonucleotide triphosphate (dNTP), 0.5 µg oligo(dT), and 100 U MMLV reverse transcriptase (Invitrogen, Carlsbad, CA). The primers of all tested genes were synthesized by Tech Dragon Limited (Hong Kong, China), and their sequences were listed in Table 1.
| Gene | Accession No. | Primer sequence | Expected size (bp) |
|---|---|---|---|
| ef1a | NM_001402.5 | 5′-AAAATGACCCACCAATGGAA-3′5′-GCAGCATCACCAGACTTCAA-3′ | 211 |
| vegfa | NM_001171623.1 | 5′-CCCACTGAGGAGTCCAACAT-3′5′-TTTCTTGCGCTTTCGTTTTT-3′ | 186 |
| kdr/vegfr-2 | NM_002253.2 | 5′-GTGACCAACATGGAGTCGTG-3′5′-TGCTTCACAGAAGACCATGC-3′ | 218 |
| vegfr-1 | NM_002019.4 | 5′-GGCTCTGTGGAAAGTTCAGC-3′5′-GCTCACACTGCTCATCCAAA-3′ | 223 |
| angpt1 | NM_001146.3 | 5′-GAAGGGAACCGAGCCTATTC-3′5′-GGGCACATTTGCACATACAG-3′ | 181 |
| angpt2 | NM_001147.2 | 5′-TTATCACAGCACCAGCAAGC-3′5′-CGCGAGAACAAATGTGAGAA-3′ | 221 |
| tie1 | NM_005424.4 | 5′-GACTGACCCAGCTTTTGCTC-3′5′-CTGCAATCTTGGAGGCTAGG-3′ | 182 |
| tie2 | NM_000459.3 | 5′-TACACCTGCCTCATGCTCAG-3′5′-TTCACAAGCCTTCTCACACG-3′ | 242 |
Real-time qPCR was performed for quantitative analysis. The standards for the tested genes and elongation factor 1 alpha (ef1a, housekeeping gene) were prepared by RT-PCR amplification of cDNA fragments with specific primers. The amplicons were resolved by agarose gel electrophoresis, purified, and quantitated by electrophoresis along with the Mass Ruler DNA marker (MBI Fermentas, Hanover, MD, U.S.A.). These amplified amplicons were used to construct standard curves in all real-time qPCR assays.
Real-time qPCR was performed on the iCycler iQ Real-time PCR Detection System (Bio-Rad, Hercules, CA, U.S.A.) in a volume of 30 µL containing 10 µL 1 : 15 diluted RT reaction mix, 1× PCR buffer, 0.2 mm each dNTP, 2.5 mm MgCl2, 0.2 µm each primer, 0.75 U Taq polymerase, 0.5× Eva Green (20× concentrated; Biotium, Hayward, CA), and 20 nm fluorescein (Bio-Rad). The reaction profile consisted of 40 cycles of 94°C for 30 s, 60°C for 30 s, 72°C for 1 min, and 80°C for 7 s for signal detection. A melt-curve analysis was performed at the end of the reaction consisting of 180 cycles of 7 s with temperature increased at 0.2°C/cycle to demonstrate the specificity of the amplification.
Western BlottingThe cells were treated with different concentrations of EXD for 12 h and lysed with 80 µL of sodium dodecyl sulfate (SDS) sample buffer (62.5 mm Tris–HCl [pH 6.8], 1% SDS, 10% glycerol, and 5% 2-mercaptoethanol). The samples collected above were boiled at 95°C for 10 min before loading onto the acrylamide gel (12%) for SDS-polyaclylamide gel electrophoresis (PAGE). A biotinylated ladder (Cell Signaling Technology, Danvers, MA, U.S.A) was used as a size marker. After the proteins were separated by SDS-PAGE, they were transferred onto a nitrocellulose membrane at 90 V for 90 min. The membrane was blocked with 5% skim milk for 1 h after the blotting procedure, followed by washing twice with 1× Tris-buffered saline (TBS; 20 mm Tris–HCl, 136 mm NaCl, pH 7.6) with 1% Tween-20 (called TTBS below; USB) for 10 min. The primary antibody against phosphor-Akt, total Akt, phosphor-extracellular signal-regulated kinase 1/2 (ERK1/2) and total ERK1/2 (Cell Signaling Technology) or β-actin (Santa Cruz, CA, U.S.A.) was diluted at 1 : 1000 in 5% skim milk and incubated with the membrane overnight at 4°C. On the next day, the membrane was washed twice with TTBS for 10 min, followed by incubation with the secondary antibody (bovine anti-rabbit-horseradish peroxidase; Santa Cruz Biotechnology) at 1 : 2000 dilution for 1 h at room temperature. The membrane was washed twice with TTBS for 10 min before detection using the SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Fisher Scientific, Rockford, IL, U.S.A.). The chemiluminescent signal was visualized and analyzed with the Lumi-imager and the software LumiAnalyst 3.1 (Roche Applied Science, Indianapolis, IN, U.S.A.).
StatisticsAll experiments were repeated at least three times. Values are given as means±S.E.M. Data were analyzed using GraphPad Prism 5.0 software. Statistical significance was assessed by one-way ANOVA. p values less than 0.05 were considered significant.
Since angiogenic factor-stimulated endothelial cell proliferation is a key component of the angiogenic response, we investigated the effect of EXD on the mitogenesis of HUVECs. EXD significantly inhibited the basal level of HUVECs proliferation in a clear dose-dependent manner (Fig. 1A).

Treatment with t-BHP served as a positive control. Each point represents mean±S.E.M. (n=4) from a representative experiment. * p<0.05, ** p<0.01, *** p<0.001 in one-way ANOVA followed by the Dunnett’s test.
In the cytotoxicity assay, HUVEC cells were treated with 1 mg/mL EXD for up to 48 h. EXD showed limited toxicity to HUVECs at this concentration and over 80% of cells survived 48 h after treatment (Fig. 1B).
In order to investigate the mechanism of the anti-proliferative effect of EXD in HUVECs, an apoptosis assay with TUNEL staining was conducted. EXD induced HUVEC apoptosis in a dose-dependent manner, with 1 mg/mL causing a significant number of cells to undergo apoptosis (Fig. 1C).
Inhibition of HUVEC MigrationTo determine whether EXD was capable of influencing HUVEC migration, cells were treated with different concentrations of EXD in a 2-D migration assay. We observed that the HUVEC migration was significantly inhibited by the treatment in a dose-dependent manner. The ability of the cells to migrate was reduced to 82.08%, 70.50% and 51.4% following treatments at doses of 0.1, 0.5 and 1.0 mg/mL, respectively; whereas the migration of cells treated with 4 µm SU5416 was decreased to 54.63% (Fig. 2).

Each point represents mean±S.E.M. (n=3) from a representative experiment. ** p<0.01, *** p<0.001 in one-way ANOVA followed by the Dunnett’s test.
Tube formation is another key step for angiogenesis. EXD and SU5416 treatment suppressed the formation of tubular structures. Exposure to the angiogenesis inhibitor SU5416 resulted in a reduction of the capillary-like structures on the Matrigel™, as was proposed. There were much fewer branch points observed in the treated group with 1 mg/mL EXD (Fig. 3D) than that in the control (Fig. 3A). Quantitative measurements confirmed that exposure to EXD resulted in a significant decrease in the mean number of branch points (Fig. 3F). This result suggests that EXD may have an inhibitory effect on angiogenesis.

HUVECs were treated with 0.1 mg/mL (B), 0.5 mg/mL (C) and 1 mg/mL (D) EXD and 4 µm SU5416 (E), respectively. Each point represents mean±S.E.M. (n=3) from a representative experiment. *** p<0.001 in one-way ANOVA followed by the Dunnett’s test.
The cell cycle analysis on the HUVECs treated with EXD for 24 h also showed a dose-dependent increase in cells in G0/G1 phase, with 0.5 and 1 mg/mL EXD causing a significant increase in cells in this phase (Fig. 4).

All experiments were repeated 3 times, and data were expressed as mean±S.E.M. * p<0.05, ** p<0.01 versus control in G0/G1 phase.
Due to the potent anti-angiogenic activities of EXD, its action mechanism deserved further study. VEGF-VEGFR and ANGIOPOIETING (ANGPT)-TIE are two most widely studied signaling pathways involved in angiogenesis. The major molecules participating in these two signaling pathways including vegfa, vegfr-2, vegfr-1, angpt1, angpt2, tie1 and tie2 were thus investigated. In the time course experiment (Fig. 5), EXD significantly decreased the mRNA level of vegfr-2 and vegfr-1 as early as 24 h treatment (48 hpf), and also decreased the vegfa level after 48 h treatment. It seemed to have no effect on the mRNA levels of other genes.

gapdh was used as internal control. The expression levels were first normalized to gapdh and then expressed as the fold change of control. The black column indicates control groups while the white column indicates EXD-treated groups. Each value represents the mean±S.E.M. (n=3). *** p<0.001 in one-way ANOVA followed by the Dunnett’s test.
Further examination was carried out. In the dose response study, the inhibitory effect of EXD on vegfa, vegfr-2 and vegfr-1 levels exhibited a clear dose-dependent manner at 72 hpf (Fig. 6).

gapdh was used as internal control. The expression levels were first normalized to gapdh and then expressed as the fold change of control. Each value represents the mean±S.E.M. (n=3). * p<0.05, ** p<0.01, *** p<0.001 in one-way ANOVA followed by the Dunnett’s test.
ERK1/2 and Akt pathways are the two major ones among the endothelial cell signaling pathways that regulates endothelial cell proliferation and migration, and they are necessary for essential cellular procedures of endothelial cells and angiogenesis after activation.13,14) Since EXD demonstrated to have inhibitory effects on angiogenesis, its effect on the activation of ERK1/2 and Akt were investigated by Western blotting to further clarify the molecular mechanisms. As shown in Fig. 7, Western blotting analysis revealed that EXD down-regulated the expression and related phosphorylation of ERK1/2 pathway, and the phosphorylation of Akt pathway, suggesting that EXD might exert anti-angiogenesis capacity via inhibition of the ERK1/2 and Akt signaling pathways.
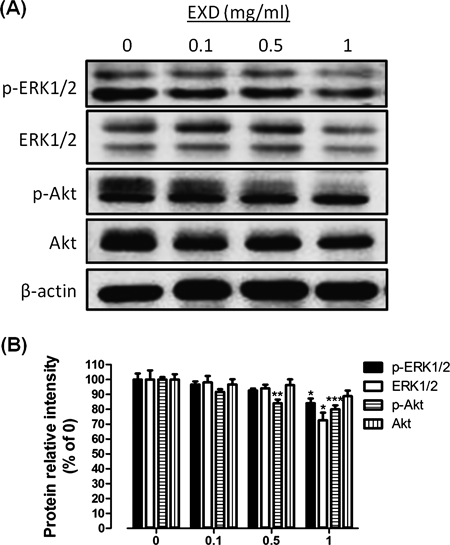
HUVEC cells were cultured with EXD (0, 0.1, 0.5, 1 mg/mL) for 12 h, and whole cell lysates were subjected to SDS-PAGE followed by Western blotting with antibodies against phosphor-ERK1/2, ERK1/2, phsophor-Akt and Akt. β-Actin was used as internal control to ensure equal protein loading. The protein levels were first normalized to β-actin and then expressed as the percentage of control in each group. Each value represents the mean±S.E.M. (n=3). * p<0.05, ** p<0.01, *** p<0.001 in one-way ANOVA followed by the Dunnett’s test.
In this study, we are the first group to demonstrate that EXD inhibits angiogenesis in HUVECs, probably via VEGF–VEGFR pathway, thus demonstrating its anti-angiogenic activity. Our experimental results demonstrate that EXD inhibited the proliferation, migration and tube formation abilities of HUVECs in vitro (Figs. 1–3). In response to EXD treatment, HUVECs tended to undergo anti-angiogenesis. EXD exposure attenuated HUVEC proliferation in a concentration-dependent manner (Fig. 1). Endothelial cell migration is a key step during the process of angiogenesis. EXD exposure also resulted in a reduction in the number of migrated cells, a characteristic feature of different angiogenic phenotypes of ECs (Fig. 2). Furthermore, EXD treatment blocked HUVEC tube formation in vitro (Fig. 3). EXD treatment also caused HUVECs to undergo apoptosis (Fig. 1C) and a concentration-dependent increase at G0/G1 phase in HUVECs (Fig. 4), indicating that anti-proliferative activity of EXD might due to its ability to induce apoptosis and cell cycle arrest. The eukaryotic cell cycle is regulated by signal transduction pathways mediated by a series of cell-cycle regulators, including cyclins, cyclin dependent kinases (CDKs) and CDK inhibitors (CDKIs). Progression through the G1 phase requires both cyclin D and cyclin E to activate CDK4/6 and CDK2, respectively.15) The cyclin D1-CDK4/6 complexes formed during G1 phase phosphorylate retinoblastoma (Rb) protein and activate the transcriptional factor E2F-1 which initiates the transcription of key cell cycle regulators such as cyclins E and A and in the process, driving cells into the S phase.16,17) Therefore, we hypothesize that EXD binds to, and subsequently inhibits CDKs and their downstream molecules to suppress HUVECs growth through cell cycle arrest. Work has already been carried out in our group to elucidate how EXD induces G0/G1 cell cycle arrest.
Our experiments revealed that EXD significantly inhibited the expression of vegfa, vegfr-1 and vegfr-2 (Figs. 5, 6). The complex process of angiogenesis is managed by angiogenic signaling networks.18,19) Numerous pro-angiogenic and anti-angiogenic factors have been identified and studied, among which VEGF is a key mediator that stimulates angiogenesis.20) VEGF-induced activation of specific receptors is critically involved in differentiation of endothelial cell progenitors, endothelial cell sprouting, increased endothelial cell permeability, migration, proliferation, and cell survival.21) Two tyrosine kinase receptors that specifically bind VEGF have been identified on endothelial cells, VEGF receptor-1 (VEGFR-1, Flt-1), and VEGFR-2 (KDR).22) VEGF and VEGF receptors have been implicated in the angiogenesis that occurs in many solid tumors, such as breast cancer,23) colon cancer,24) hepatoma,25) ovarian cancer,26) gastric cancer27) and prostate cancer.28) Thus, anti-angiogenesis, especially neutralizing VEGFA and blocking its signaling, has been considered to be a highly promising avenue for anticancer therapy.3,29) Here, we showed a novel candidate for the anti-VEGF-signaling inhibitor, EXD. Among all the genes tested in this study, EXD only specifically suppressed those involved in VEGF–VEGFR pathway. Results of real-time PCR illustrated that the inhibitory effect of EXD was in a clear time- and dose-dependent manner (Figs. 5, 6). To our surprise, EXD significantly down-regulated not only the expression level of vegfr-2 but also that of vegfr-1.
VEGFR-2 is regarded as the major angiogenic receptor to mediate the growth and permeability actions of VEGF.30) EXD is a traditional Chinese medicine formula and consists of six medicinal herbs. Its components and the working mechanisms are very complicated. Herba epimedii is one of the six herbs in EXD. The ethyl acetate fraction of its ethanol extract showed anti-angiogenic effect in both in vivo and in vitro models, and it inhibited the mRNA level of VEGFA.31) Colchicine, identified in its ethyl acetate fraction, has been reported to inhibit angiogenesis by down-regulating VEGF–VEGFR pathway.31,32) In addition, the existence of other bioactive components in EXD is possible. Characterization of these components will benefit future drug development and clinical therapy. Further studies are being carried out in our group.
Unlike VEGFR-2, the functional role of VEGFR-1 in angiogenesis is controversial.33) Some reports demonstrated that soluble VEGFR-1 plays a negative role by acting as a decoy receptor or suppressing signaling through VEGFR-2,34,35) while others indicated the stimulatory effect of VEGFR-1 in tumor growth and metastasis.36–38) Activation of VEGFR-1 induces tumor angiogenesis via stimulation of monocytes and macrophages. These cells migrate into tumor and inflammatory lesions to produce VEGF-A, VEGF-C and cytokines, resulting in tumor angiogenesis and lymphoangiogensis via VEGFR-2 and VEGFR-3.36,37) Matrix metalloproteinase-9 is induced via VEGFR-1 in endothelial cells and macrophages, leading to lung metastasis.38) Mice expressing a mutant allele of vegfr-1 displayed defects in tumor vessel formation and metastasis38,39) and inhibition of VEGFR-1 led to defects in neovascularization of the eye.40) Furthermore, other studies41,42) with pro- or anti-angiogenic drugs demonstrated that VEGFR-1 positively regulates angiogenesis in HUVECs.
Activation of VEGFR-2 by VEGF leads to phosphorylation of specific downstream signaling transduction mediators, mainly phospholipase C γ (PLCG) and PI3K. Phosphoinositide 4,5 bisphosphate (PI4,5P2, PIP2) plays a key role in intracellular downstream transmission of VEGF-dependent signal, serving as the proximal substrate for both major signaling branches. PI3K utilizes PIP2 to generate PI(3,4,5)P3 which stimulates Akt signaling, whereas PLCγ hydrolyzes PIP2 to produce inositol 1,4,5-triphosphate (IP3) and diacylglycerol (DAG), which further activate Ca2+ metabolism, protein kinase C, NFAT and ERK1/2.43,44) Therefore, disruption of either of these two signaling branches causes defects in angiogenesis. In the present study, we found that EXD decreased the expression and related phosphorylation ERK1/2 pathway, and the phosphorylation of Akt (Fig. 7). Both ERK1/2 and Akt pathways are cellular survival pathways that are devoted to cell survival signaling.45) Recent reports have shown that elevation of ERK1/2 or Akt levels and activities could result in apoptosis inhibition.46–49) Therefore, EXD treatment may inhibit the total levels or activities of ERK1/2 and Akt, and then induce HUVEC apoptosis. In addition, changes of ERK1/2 and Akt expression are also observed in many cancers, including hepatocellular cancer,50,51) colon cancer,52) breast cancer,53) human acute leukemias,54) and follicular and papillary thyroid cancer.55–57) When Sprague-Dawley rats were administered diethylnitrosamine for up to 3 months, they developed hepatomas, which showed that changes in ERK1/2 and Akt levels and activities were associated with cyclin D1 up-regulation.51) Therefore, EXD inhibited cyclin D1, and subsequently induced G0/G1 cell cycle arrest and down-regulation of ERK1/2 and Akt levels or activities.
In conclusion, we provide evidence in the present study that EXD suppresses HUVEC viability, migration and tube formation in vitro. Our studies also reveal that EXD may target VEGF-mediated signaling cascades. Thus, EXD could serve as a potential candidate for anti-angiogenic therapy.
This study was supported by Grants from Small Project Funding (No. 201007176075), the University of Hong Kong, and Nong’s Company Limited (Member of PuraPharm Group).