2013 年 36 巻 6 号 p. 1017-1023
2013 年 36 巻 6 号 p. 1017-1023
l-Pyroglutamic acid (PGA) is an endogenous molecule derived from l-glutamate. We demonstrate the effects of PGA on intraocular pressure (IOP) in experimentally induced ocular hypertension in rabbits. In the in vitro and in vivo transcorneal penetration studies, the PGA solution (PGA in saline) did not penetrate the rabbit cornea. On the other hand, the penetration of PGA was improved by the addition of zinc chloride and 2-hydroxypropyl-β-cyclodextrin (HPCD), and PGA penetration was enhanced with increasing HPCD concentration. Therefore, PGA solutions containing 0.5% zinc chloride and 5% or 10% HPCD (PGA/HPCD5% or 10% eye drops) were used to investigate the effects for IOP in this study. An elevation in IOP was induced by the rapid infusion of 5% glucose solution (15 mL/kg of body weight) through the marginal ear vein or maintaining under dark phase for 5 h. In the both models, the induced elevation in IOP was prevented by the instillation of PGA/HPCD eye drops, and the IOP-reducing effect enhanced with increasing HPCD concentration in the drops. Nitric oxide (NO) levels elevated in the aqueous humor following the infusion of 5% glucose solution, and this increase was also suppressed by the instillation of PGA/HPCD eye drops. In conclusion, the present study demonstrates that the instillation of PGA/HPCD eye drops has an IOP-reducing effect in rabbits with experimentally induced ocular hypertension, probably as a result of the suppression of NO production.
Glaucoma is characterized as nervous degeneration that caused the disappearance of retinal ganglion cells, visual field loss, excavation of the optic disk and ophthalmopathy.1,2) It is one of the most common causes of visual impairment and blindness in the world, and is more common in the elderly.3) The major risk factor for glaucoma is enhanced intraocular pressure (IOP). If the pressure is high or is maintained at an elevated level for a long period of time, apart from the damage to the ciliary artery, there is a mechanical damage to the optic nerve in the central visual areas.4) In treatments for glaucoma, the focus is reducing the IOP, and so damage to the retina and optic nerve. The principal pharmacological agents include β-blockers, prostaglandin agents, topical carbonic anhydrase inhibitors, α1-blockers, α,β-blockers and parasympathomimetic agents, and all of these focus on reducing elevated IOP. In addition, N-methyl-d-asparate inhibitors, which act to reduce retinal and optic nerve damage have also been investigated in the clinical setting.5) Drugs to protect the retina and optic nerve are generally administered via invasive methods, such as intravitreal or subtenon injections, and repeated injections are associated with potential risks of cataracts, vitreous hemorrhages and retinal detachment.6) Therefore, the search for effective agents and noninvasive delivery systems for the treatment of enhanced IOP, and damage to the retina and optic nerve is a high priority for glaucoma treatment. Based on these needs, Hironaka et al. reported a delivery system targeting the posterior segment of the eye using eye drops.7) However, an effective agent to both decrease IOP and prevent damage to the retina and optic nerve has not yet been introduced.
Pyroglutamic acid (PGA) is an endogenous molecule derived from l-glutamate, and a major intermediate in the γ-glutamyl cycle. This cycle is necessary to the synthesis and breakdown of glutathione and also to the intracellular transport of free amino acids.8) PGA is present in its free form in the mammalian brain, eye, plasma and cerebrospinal fluids.9,10) Recently, our group found that PGA promotes the survival of axotomized retinal ganglion cells in adult mammalians, possibly mediated by excitatory amino acid transfer.11) Therefore, it is possible that the PGA is an effective agent to both decrease IOP and prevent damage to the retina and optic nerve, if the PGA decrease the IOP. In this present study, we prepared eye drops containing PGA, which can penetrate the cornea, and demonstrate the effect of these eye drops on enhanced IOP in experimentally induced ocular hypertension in rabbits.
Male Japanese albino rabbits, 2.5–3.0 kg, were housed under standard conditions (12 h/d fluorescent light (07:00–19:00), 25°C room temperature) and allowed free access to a commercial diet (CR-3, Clea Japan Inc., Tokyo, Japan) and water. All procedures were performed in accordance with the regulations of the Kinki University Faculty of Pharmacy Committee for the Care and Use of Laboratory Animals and the Association for Research in Vision and Ophthalmology resolution on the use of animals in research. l-PGA and zinc chloride (ZnCl2) were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). d-PGA was not used in this study, since the prevention of IOP and nitric oxide (NO) don’t observed by the instillation of d-PGA. 2-Hydroxypropyl-β-cyclodextrin (HPCD, average molar substitution, 0.6; average molecular weight 1380) was a gift from Nihon Shokuhin Kako Co., Ltd. (Tokyo, Japan). Commercially available timolol maleate (0.5% Timoptol®, β-blockers) and 0.4% Benoxil were obtained from Santen Pharmaceutical Co., Ltd. (Osaka, Japan). All other chemicals used were of the highest purity commercially available.
Preparation of PGA Eye Drops Containing the ZnCl2 and HPCDThe eye drops used this study were solutions prepared in saline, adjusted to pH 5.8 with NaOH, and filtered through a Minisart CE (pore size of 0.20 µm, Costar Co., Massachusetts, U.S.A.). HPCD was added to saline solutions containing 0.5% ZnCl2 and PGA, and stirred at room temperature. The compositions of the PGA eye drops are shown in Table 1.
| Eye drops | Content (%) | ||
|---|---|---|---|
| Pyroglutamic acid | HPCD | ZnCl2 | |
| PGA solution | 0.5 | 0.0 | 0.0 |
| PGA eye drops | 0.5 | 0.0 | 0.5 |
| PGA/HPCD5% eye drops | 0.5 | 5.0 | 0.5 |
| PGA/HPCD10% eye drops | 0.5 | 10.0 | 0.5 |
The in vitro transcorneal penetration of eye drops containing PGA was examined using the method of Iwata et al.12) Adult Japanese albino rabbits, weighing 2.5 to 3.0 kg, were killed by injection of a lethal dose of pentobarbital into the marginal ear vein. The eyes were removed and the corneas were carefully separated from other ocular tissues. The individual corneas were placed on a methacrylate cell designed for transcorneal penetration studies. The side of the chamber (donor chamber) exposed to the exterior surface of the cornea was filled with eye drops as shown in Table 1. The other side of the chamber (reservoir chamber) was filled with 10 mm N-(2-hydroxyethyl)piperazine-N′-2-ethanesulfonic acid (HEPES) buffer (pH 7.4) containing 136.2 mm NaCl, 5.3 mm KCl, 1.0 mm K2HPO4, 1.7 mm CaCl2 and 5.5 mm glucose. The experiments were performed at 35°C over 5 h. Fifty microliters of sample solution was withdrawn from the reservoir chamber at the indicated time intervals and replaced with the same volume of buffer. PGA concentrations in the samples were determined by the following HPLC method. Fifty microliters of filtrate was added to 200 µL methanol containing 0.1 µg indomethacin (internal standard), and the mixtures were filtered through a Chromatodisk 4A (pore size of 0.45 µm, Kurabo Industries Ltd., Osaka, Japan). The solution (4 µL) was injected into a COSMOSIL® (5 µm, column size: 2.0 mm×150 mm) column (Nacalai Tesque Inc., Kyoto, Japan) using a Shimadzu LC-10AD system equipped with a column oven CTO-6A (Shimadzu Corp., Kyoto, Japan). The mobile phase consisted of a mixture of 0.01 m ammonium acetate and acetonitrile in a ratio of 50 : 50 (v/v). The flow rate was 0.5 mL/min, the column temperature was 35°C, and the wavelength for detection was 215 nm. Corneal viability was monitored by measuring thickness (average 0.0625 cm for 5 rabbits; no significant changes in thickness were observed over the 5 h period). The obtained data were analyzed by the following equations13):
 | (1) |
 | (2) |
 | (3) |
where Jc is the PGA penetration rate, Km is the cornea/preparation partition coefficient, D is the diffusion constant within the cornea, CPGA is the PGA content in the ophthalmic preparation, τ is the lag time, δ is the thickness of the cornea, Qt is the total amount of PGA appearing in the reservoir solution at time t, and A is the effective area of the cornea (0.78 cm2). Jc and τ were estimated by fitting each penetration profile to Eq. 3. The penetration coefficient through the cornea, Kp, is given by Jc/CPGA. A nonlinear least-squares computer program was employed for the calculations.14)
In Vivo Transcorneal Penetration of Various Eye Drop Preparations Containing PGAAdult Japanese albino rabbits weighing 2.5 to 3.0 kg were anesthetized by injecting pentobarbital (0.6 mg/kg) through the marginal ear vein, and a topical anesthetic (0.4% Benoxil) was instilled into each eye 3 min before sampling of the aqueous humor. Then, a 29 gauge injection needle connected to silicon tubing (inner diameter: 0.5 mm, Fuji Systems Co., Tokyo, Japan) joined to a 25 µL microsyringe (Ito Corp., Tokyo, Japan) was inserted into the eye to obtain aqueous humor samples, and 40 µL of various eye drop preparations containing PGA was instilled into the eyes. Samples of aqueous humor (5 µL each) were removed at the indicated times from the anterior chamber of the eye over a period from 0–90 min. The PGA concentrations were determined by HPLC as described above.
The area under the PGA concentration–time curve (AUCPGA) was calculated according to the following equation (Eq. 4):
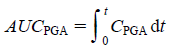 | (4) |
Briefly, AUC was determined according to the trapezoidal rule up to the last PGA concentration measurement point.
Measurement of Intraocular Pressure in RabbitsThe experiment was carried out according to Bonomi et al.15) The IOP in rabbits was measured with an electronic tonometer (Medtronic SOLAN, Jacksonville, FL, U.S.A.) under surface anesthesia (0.4% Benoxil).
An increase in IOP was induced by the rapid infusion of a 5% glucose solution through the marginal ear vein (this experiment was carried out between 16:00–18:00). The amounts injected were 15 mL/kg of body weight and the infusion was accomplished in all animals within 20 s. The various eye drop preaparations containing PGA were instilled 30 min prior to the infusion of the 5% glucose solution.
On the other hand, the enhancement of IOP was also obtained by maintaining under dark phase for 5 h (12:00–17:00). The various PGA eye drops (40 µL) were instilled into the eyes of rabbits, and the eyes were kept open for about 1 min after instillation to prevent the eye drops from overflowing.
The area under the curve (AUCΔIOP) of the ΔIOP (mmHg) versus time (min) (the area under ΔIOP–time curve) was calculated according to the following equations (Eqs. 5, 6):
 | (5) |
 | (6) |
Briefly, AUC was determined according to the trapezoidal rule up to the last IOP measurement point.
Measurement of NO Levels in the Aqueous HumorAdult Japanese albino rabbits weighing 2.5 to 3.0 kg were anesthetized by injecting pentobarbital (0.6 mg/kg) through the marginal ear vein, and a topical anesthetic (0.4% Benoxil) was instilled into each eye 3 min before sampling of the aqueous humor. Then, a concentric microdialysis probe (A-1-20-05, 5 mm length; Eicom, Kyoto, Japan) was inserted into the eye to measure NO levels, and perfused with Ringer’s solution (140 mm NaCl, 4 mm KCl, 1.26 mm CaCl2, and 1.15 mm MgCl2, pH 7.4) at a constant flow rate of 2 µL/min using a micro syringe pump (ESP-64, Eicom, Kyoto, Japan). NO2− and NO3− in the aqueous humor were separated on a reverse-phase separation column packed with polystyrene polymer (NO-PAK, 4.6×50 mm, Eicom, Kyoto, Japan); NO3− was reduced to NO2− in a reduction column packed with copper-plated cadmium filings (NO-RED, Eicom, Kyoto, Japan). NO2− was mixed with Griess regent to form a purple azo dye in a reaction coil, and placed in a column oven set at 35°C. The absorbance of the colored product dye at 540 nm was determined by a flow-through spectrophotometer (NOD-10, Eicom, Kyoto, Japan) with a mobile phase consisting of 10% methanol containing 0.15 m NaCl–NH4Cl and 0.5 g/L Na4–EDTA delivered by a pump at a flow rate of 0.33 mL/min. Griess reagent, 1.25% HCl containing 5 g/L sulfanilamide with 0.25 g/L N-naphthylethylenediamine, was delivered at a rate of 0.1 mL/min. In this paper, NO amounts reflect the level of the NO2− metabolite, which is produced from NO. The area under the curve (AUCΔNO) of ΔNO (nmol/mL) versus time (min) (the area under ΔNO–time curve) was calculated according to the following equations (Eqs. 7, 8):
 | (7) |
 | (8) |
Briefly, AUC was determined according to the trapezoidal rule up to the last NO measurement point.
Statistical AnalysisAll data are expressed as the mean±standard error (S.E.) of the mean (n=3–5). Unpaired Student’s or Aspin–Welch’s t-tests were used to evaluate statistical difference, and multiple groups were evaluated by one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison. p Values less than 0.05 were considered significant.
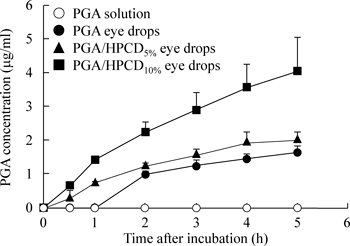
Figure 1 shows in vitro transcorneal penetration of PGA from various eye drop preparations containing PGA through rabbit corneas, and Table 2 summarizes the pharmacokinetic parameters calculated from the in vitro transcorneal penetration data. PGA in the PGA solutions was not detected in the reservoir chamber. On the other hand, transcorneal PGA penetration began after a lag time of 0.7 h lag time, and its penetration rate was very low. Penetration from the PGA eye drop preparations containing 5% or 10% HPCD was observed immediately, and the PGA penetration rate from the drops containing 10% HPCD was significantly increased in comparison with preparations containing no HPCD. Figure 2 shows the PGA concentrations in the aqueous humor after the instillation of eye drops containing PGA into rabbit eyes, and Table 3 summarizes the AUCPGA calculated from the in vivo transcorneal penetration data. No PGA penetration from PGA solutions was detected in the aqueous humor. In contrast to the results for the PGA solutions, the penetration of PGA from PGA eye drops with or without HPCD was detected, with a peak concentration observed at approximately 20 min after instillation. PGA penetration increased by the addition of HPCD, and the AUCPGA value for the PGA/HPCD10% eye drops was 2.5-fold higher than that of the PGA/HPCD5% eye drops.
The donor chamber exposed to the exterior surface of the cornea was filled with various eye drop preparations. PGA solution (○), PGA eye drops (●), PGA eye drops containing 5% HPCD (▲), PGA eye drops containing 10% HPCD (■). The data are presented as the means±S.E. of 3–5 rabbit corneas.

Rabbits were instilled with 40 µL of various eye drop preparations containing HPMC. PGA solution (○), PGA eye drops (●), PGA eye drops containing 5% HPCD (▲), PGA eye drops containing 10% HPCD (■). The data are presented as the means±S.E. of 3–5 rabbit corneas.
| Jc (nmol/cm2/h) | Kp (×10−4/h) | Km | τ (/h) | D (×10−2 cm2/h) | |
|---|---|---|---|---|---|
| PGA eye drops | 10.52±1.25 | 0.09±0.01 | 0.69±0.02 | 0.80±0.02 | 0.81±0.02 |
| PGA/HPCD5% eye drops | 14.18±2.22 | 0.12±0.02 | 0.09±0.02* | 0.08±0.01* | 8.55±1.09* |
| PGA/HPCD10% eye drops | 27.83±4.14*,** | 0.24±0.05*,** | 0.23±0.03*,** | 0.10±0.01*,** | 6.61±0.88 |
| AUCPGA (mg·min/mL) | |
|---|---|
| PGA solution | N.D. |
| PGA eye drops | 10.81±1.84 |
| PGA/HPCD5% eye drops | 36.16±5.43* |
| PGA/HPCD10% eye drops | 89.25±14.17*,** |
AUCPGA, the area under the PGA concentration–time curve. AUCPGA was calculated using Eq. 4. N.D., not detectable. Each value represents the mean±S.E. of 3–5 experiments. * p<0.05, vs. PGA eye drops. ** p<0.05, vs. PGA/HPCD5% eye drops.
Figure 3 shows the effects of the instillation of eye drops containing PGA on IOP in rabbits with experimentally induced ocular hypertension. The elevation in IOP in rabbits was induced by the rapid infusion of 5% glucose solution (15 mL/kg) through the marginal ear vein. IOP reached a maximum 5 min after the infusion, and returned to the pre-infusion level by 50 min after infusion. The instillation of PGA/HPCD eye drops prior to glucose infusion resulted in a significantly lower elevation in IOP as compared with the instillation of saline. Figure 4 shows the changes in the NO levels in the aqueous humor of rabbits instilled with saline or various eye drop preparations containing PGA. NO levels in the aqueous humor of rabbits rose following the rapid infusion of 5% glucose solution into the marginal ear vein, and reached a maximum at 20 min. The instillation of PGA/HPCD eye drops also reduced the degree of NO elevation in the aqueous humor. Table 4 shows the AUC values for IOP and NO in the aqueous humor of rabbits after the instillation of eye drops. The IOP- and NO-reducing effect enhanced with increasing HPCD concentration in the eye drops. In addition, the AUCΔIOP of PGA/HPCD10% eye drops was similar to that of commercially available timolol eye drops (73.9±36.9 mmHg·min, means±S.E. of 3 independent rabbits). Figure 5 shows the effects of the instillation of eye drops containing PGA on IOP in rabbits induced ocular hypertension by maintaining under dark phase. The IOP in rabbit under dark phase was increased by maintaining under dark phase for 5 h, and the IOP in rabbit under dark phase was approximate 6 mmHg higher than that under light phase (under light phase; 12.35±0.11, under dark phase; 18.07±0.13, mmHg, means±S.E. of 5 independent rabbits). The instillation of eye drops containing PGA decreased the IOP under dark phase, and the IOP in eye of rabbit instilled the PGA/HPCD10% eye drops was clear lower than that of PGA eye drops. The lowest IOP trough in eye of rabbit instilled the PGA/HPCD10% eye drops was observed at 50 min, and the IOP-reducing was continued until 120 min after instillation of the eye drops. Although, the lowest IOP trough in eye of rabbit instilled the PGA/HPCD10% eye drops was similar to that of commercially available timolol eye drops, and the reaction time of PGA/HPCD10% eye drops was obviously longer than that of commercially available timolol eye drops.

The eyes of rabbits receiving a rapid infusion of a 5% glucose solution were first instilled with 40 µL of saline or various eye drop preparations containing PGA. IOP was measured with an electronic tonometer. Saline instilled rabbits (○), PGA eye drops instilled rabbits (●), PGA eye drops containing 5% HPCD instilled rabbits (▲), PGA eye drops containing 10% HPCD instilled rabbits (■). The data represent the means±S.E. of 3–5 independent rabbits. * p<0.05, vs. Saline instilled rabbits.

The eyes of rabbits receiving a rapid infusion of a 5% glucose solution were first instilled with 40 µL of saline or eye drops containing PGA. NO levels were measured by the microdialysis method. Saline instilled rabbits (○), PGA eye drops instilled rabbits (●), PGA eye drops containing 5% HPCD instilled rabbits (▲), PGA eye drops containing 10% HPCD instilled rabbits (■). The data represent the means±S.E. of 3–5 independent rabbits. * p<0.05, vs. Saline instilled rabbits.

The enhancement of IOP was obtained by maintaining under dark phase for 5 h (12:00–17:00). The rabbits under dark phase were instilled with 40 µL of saline or various eye drop preparations containing PGA. IOP was measured with an electronic tonometer. Saline instilled rabbits (○), PGA eye drops instilled rabbits (●), PGA eye drops containing 5% HPCD instilled rabbits (▲), PGA eye drops containing 10% HPCD instilled rabbits (■), commercially available timolol maleate (0.5% Timoptol®) instilled rabbits (◆). The data represent the means±S.E. of 3–5 independent rabbits. * p<0.05, vs. Saline instilled rabbits.
| AUCΔIOP (mmHg·min) | AUCΔNO (nmol·min/mL) | |
|---|---|---|
| Saline | 204.7±25.1 | 1358±181 |
| PGA eye drops | 178.2±18.6 | 1215±159 |
| PGA/HPCD5% eye drops | 143.4±14.9* | 967±122* |
| PGA/HPCD10% eye drops | 83.2±14.1*,**,*** | 655±101*,**,*** |
AUCΔIOP, the area under the ΔIOP–time curve; AUCΔNO, the area under the ΔNO concentration–time curve. AUCΔIOP and AUCΔNO were calculated using Eqs. 6 and 8, respectively. Each value represents the mean±S.E. of 3–5 experiments. * p<0.05, vs. saline for each category. ** p<0.05, vs. PGA eye drops for each category. *** p<0.05, vs. PGA/HPCD5% eye drops for each category.
Glaucoma is a major cause of blindness, with an estimated 70 million people affected worldwide. In treatments for glaucoma, reducing IOP, and preventing retina and optic nerve damage is of the utmost importance. However, effective agents that both reduce IOP and protect against optic nerve damage have not yet been introduced. Recently, our group reported that PGA can repair damaged optic nerves.11) In this study, we investigated the effects of eye drops containing PGA on preventing enhanced IOP in experimentally induced ocular hypertension in rabbits, and found the instillation of eye drops containing PGA reduces the levels of IOP.
The cornea is considered to be the major route of ocular penetration for topically applied drugs.16) The cornea comprises three main layers including the epithelium, stroma, and endothelium. The outermost layer, the corneal epithelium, is the major rate limiting barrier for drug absorption because it is a protective barrier that limits the access of foreign substances into the eye.16,17) In the in vitro and in vivo transcorneal penetration experiments using rabbit corneas, the PGA solution did not penetrate the rabbit cornea. Therefore, we used cyclodextrins and a chelating complex with zinc to improve transcorneal penetration. Cyclodextrins are cyclic oligosaccharides comprising R-d-glucose molecules linked by R-(1–4) glucosidic bonds. The potential use of natural cyclodextrins and their synthetic derivatives in improving certain properties of drugs, such as solubility, stability, and/or bioavailability has been studied extensively.18) HPCD is a cyclic oligosaccharide with a hydrophilic outer surface and a lipophilic cavity at its centre, and is capable of forming inclusion complexes with many drugs by taking up the drug molecule, or part of it, into its cavity.19,20) Moreover, it has been reported that HPCD ranks second in safety only to γ-CD among a variety of CD derivatives for use in the application of eye drops.21) Jansen et al.22) have reported no observable irritation of the eye membrane by solutions containing HPCD levels of less than 12.5%. Therefore, we used solutions containing 5 and 10% HPCD, less than the concentration used in that report (Table 1). In this study, it is no observable irritation of the eye by instillation of eye drops containing PGA using Draize test for 7 d (instillation (50 µL) is 4 times per day). The PGA in eye drop solutions was observed to penetrate the cornea, and the addition of HPCD significantly increased its penetration rate further (Tables 2, 3). The HPCD may affect the cornea, resulting in elevating the penetration rate, since, Kp and D was enhanced by the addition of HPCD and the amount of HPCD (5% or 10%) was obviously higher in comparison with the amount of PGA (0.5%).
The intravenous administration of a 5% glucose solution into rabbits is a simple and reproducible technique for screening anti-glaucoma agents.15) In this study, the IOP in rabbits initially rose following the rapid infusion of the 5% glucose solution, and returned to baseline levels by 50 min after infusion. This indicates that the increase in IOP in this experiment may provide a suitable model for acute glaucoma. We also used rabbits to investigate the efficacy of eye drops containing PGA. The level to which the IOP rose following the infusion of the 5% glucose solution was reduced by the instillation of PGA eye drops containing Zn and HPCD, with the instillation of PGA/HPCD10% eye drops providing an IOP-reducing effect sufficient to protect against glaucoma. In addition, the IOP-reducing effect was enhanced by higher transcorneal penetration of PGA (Tables 2, 3), and the AUCΔIOP of PGA/HPCD10% eye drops was similar to that of commercially available timolol eye drops. These results demonstrate that eye drops containing PGA may be useful agents for the treatment of glaucoma.
It is very important to elucidate the mechanism by which PGA eye drops reduce the increase in IOP in rabbits following the rapid infusion of 5% glucose solution. Shah et al.23) have reported that the rapid infusion of a 5% glucose solution into rabbits leads to a reduction in blood osmolarity, which leads to the transfer of water into the eye thus causing the elevation in IOP. Kiel et al.24,25) reported that the inhibition of nitric oxide synthase (NOS) by NG-nitro-l-arginine methyl ester (l-NAME) causes a decrease in water production in rabbits due to ciliary vasoconstriction, resulting in a decrease in IOP. In addition, it is known that the increase in IOP in rabbits following the rapid infusion of 5% glucose is caused by a rapid production of aqueous humor,15) and our previously reports showed that enhanced NO in the aqueous humor causes this increase in IOP using NOS inhibitor, disulfiram (DSF) eye drops.26) In this study, the NO levels in the aqueous humor increased following the rapid infusion of 5% glucose solution, and the instillation of PGA/HPCD eye drops reduced this increase (Fig. 4, Table 4), and the behavior of IOP and NO levels in rabbit instilled PGA/HPCD eye drops was similar to that instilled DSF eye drops.26) The increased IOP and NO levels in rabbits who did receive the 5% glucose infusion were not reduced by instilling the eye drop vehicle (saline containing 0.5% ZnCl2 and 10% HPCD; data not shown). Thus, we hypothesize that the PGA in the eye drop preparations penetrates the cornea, and may cause a reduction in water production by inhibiting NO production, resulting in a reduction in IOP. On the other hand, the elevation in IOP occurs prior to the induction of NO in the aqueous humor. In this study, the amounts of NO reflect the levels of NO2− metabolites, which are the products of NO. Therefore, the high NO levels reached 20 min after the infusion may reflect NO metabolites. As incipient NO produced in the eye may be consumed during ciliary vasoconstriction, the apparent enhancement of NO in the aqueous humor may not change.
Rabbits have circadaian rhythms of IOP and aqueous flow; both IOP and flow are lowest during the light phase and highest during the dark phase.27–33) The enhancement of IOP in dark phase was caused an increase of norepinephrine, the adrenergic neurotransmitter and cyclic adenosine monophosphate, the second messenger for beta-adrenergic signal transduction in aqueous humor.34) The rabbit enhanced IOP by dark phase was used for development of anti-glaucoma eye drops.35) We also used the rabbit for investigation of PGA eye drops in this study. Although, the instillation of PGA/HPCD eye drops did not affect the IOP of normal rabbits (under light phase, 17:00, not receiving the 5% glucose solution), the enhancement of IOP in rabbit under dark phase was decreased by instillation of PGA eye drops containing Zn and HPCD. The lowest IOP trough in eye of rabbit instilled the PGA/HPCD10% eye drops was similar to that of commercially available timolol eye drops, however, the reaction time of PGA/HPCD10% eye drops was obviously longer than that of commercially available timolol eye drops (the IOP-reducing was continued until 120 min after instillation of the PGA/HPCD10% eye drops, Fig. 5). In addition, the IOP was decreased with the enhancement in PGA transcorneal penetration (Fig. 5), and the NO levels in rabbit under dark phase was significantly higher than that in rabbit light phase (under light phase; 7.88±0.92, under dark phase; 11.05±0.81 nmol/mL, means±S.E. of 5 independent rabbits). This result suggested that the instillation of PGA decreased the enhancement of IOP in rabbit by circadaian rhythms, and the eye drops may be useful agents in the clinical utility.
Further studies are needed for the development of an eye drop delivery system targeting both the anterior and posterior segments of the eye. In addition, it is important to clarify the precise mechanisms for the IOP-reducing effect of eye drops containing PGA. Therefore, we are now investigating the effect of eye drops containing PGA on water production, and attempting to develop a delivery system for PGA that targets both the anterior and posterior segments of the eye.
In conclusion, the present study demonstrates that the instillation of eye drops containing PGA has a potent IOP-reducing effect in rabbits with experimentally induced high IOP, probably caused by inhibiting the elevation in NO levels. The development of a delivery system targeting both the anterior and posterior segments of the eye by eye drops containing PGA may provide an effective anti-glaucoma drug that can both decrease IOP and prevent damage to the retina and optic nerve. These findings provide significant information that can be used to design further studies to develop anti-glaucoma drugs.