2014 年 37 巻 1 号 p. 54-59
2014 年 37 巻 1 号 p. 54-59
Orally administrated diosgenin, a steroidal saponin found in the roots of Dioscorea villosa, improves reduced skin thickness in ovariectomized mice, and plays an important role in the treatment of hyperlipidemia. Diosgenin has been noticed as an active element in cosmeceutical and dietary supplements. We have already elucidated that the absolute oral bioavailability of diosgenin is very low; however, a high skin distribution of diosgenin was also observed. The aim of the present study was to examine and compare the effects of β-cyclodextrin (β-CD) and 3 kinds of its derivatives such as hydroxypropyl β-CD on the diosgenin permeability using a Caco-2 model and rat jejunal perfusion. These derivatives of β-CD greatly improved the low solubility of diosgenin. No significant increase was observed in the lactate dehydrogenase leakage from Caco-2 cell, while a slight decrease was found on the transepithelial electrical resistance by diosgenin and β-CD derivatives. However, β-CD derivatives, especially hydroxyethyl β-CD and hydroxypropyl β-CD, markedly enhanced diosgenin permeability across the Caco-2 monolayer and rat jejunum. The bioavailability of diosgenin in the presence of β-CD derivatives were about 4 to 11 fold higher than diosgenin suspension. The mechanisms of these enhancement effects may be due to improvements in solubility and tight junction opening.
Diosgenin is a steroid sapogenin that can be found in the roots of wild yam (Dioscorea villosa) and the seeds of fenugreek (Trigonella foenum greaecum).1,2) Several in vivo studies have shown that diosgenin is effective in treating various diseases such as hyperglycemia and hyperlipidemia and has been established as a starting material for the production of several steroidal hormones by the pharmaceutical industry.3–5) Diosgenin has also been used in hormone replacement therapy for menopausal women.6,7) Estrogen enhances the proliferation of estrogen-dependent cancer cells, whereas diosgenin has been shown to inhibit the proliferation of breast cancer cells.8,9) Orally administrated diosgenin has been shown to improve reduced skin thickness in ovariectomized mice and enhance DNA synthesis in a three dimensional human skin equivalent model.10) Thus, systemic action is expected with orally administered diosgenin. In B16 melanoma cells, diosgenin inhibited melanogenesis by activating the phosphatidylinositol-3-kinase pathway.11) Diosgenin has been noticed as an active element in cosmeceutical and dietary supplements.
In a preceding study, we demonstrated that diosgenin aqueous solubility was 0.7 ng/mL, absolute oral bioavailability was very low, and an inclusion complex with β-cyclodextrin (β-CD) increased oral absorption in rats.12) However, the absorption enhancement mechanisms of these complexes are still unknown. Although β-CD is a widely used natural CD for drug complexation, the aqueous solubility of β-CD (18.5 mg/mL) is lower than that of α- and γ-CD, which is problematic for clinical use.13,14) Such as hydrophilic CD derivatives include methyl (M) β-CD, hydroxyethyl (HE) β-CD, and hydroxypropyl (HP) β-CD. Chemical modifications to β-CD were broadly investigated in an attempt to improve its solubility.
Caco-2 cells, a human colon carcinoma cell line, retain many of the features of small intestinal cells.15) Furthermore, the use of Caco-2 cell monolayers has been widely adopted to the rapid screening of intestinal absorption. Previous rat in situ perfusion studies showed that the Caco-2 cell permeability was comparable to human intestinal permeability.16,17)
Few reports have compared the characteristics of β-CD and its derivatives.18) Therefore, in the present study, we evaluated the effect of β-CD and its derivatives on both the solubility and membrane permeability of diosgenin to elucidate the mechanism of the absorption enhancement by β-CD and its derivatives. Furthermore, the improvement effects of these complexes were investigated on the bioavailability of diosgenin.
Diosgenin was purchased from Sigma-Aldrich (MO, U.S.A.). Polyoxyethylene hydrogenated castor oil 60 (HCO-60) was supplied by Nikko Chemicals (Tokyo, Japan). Sodium pentobarbital was obtained from Kyoritsu Seiyaku (Tokyo, Japan). β-CD, M β-CD, HE β-CD, HP β-CD, 6-methyl diosgenin, Dulbecco’s modified Eagle’s medium (DMEM), Hanks’ balanced salt solution (HBSS), and other chemicals were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Fetal bovine serum (FBS) was obtained from Nichirei Biosciences (Tokyo, Japan). The mean molecular weight of M β-CD, HE β-CD, and HP β-CD were 1324.3, 1711.4, and 1668.3 which determined by LCQ DECA XPPlus mass spectrometer (Thermo Fisher Scientific, MA, U.S.A.) and the degree of substitution were 1.9, 1.9, and 1.3, respectively.
MethodsSolubility StudyIn the solubility study, diosgenin was dissolved in methanol and dispensed into each test tube. Methanol was removed by evaporation in a 40°C heating block. The solubility of diosgenin to water which calculated by ACD/Labs Software V11.02 (Advanced Chemistry Development, Ontario, Canada) is not influenced by solvent pH. Each β-CD was dissolved in water at 20 mM and the solutions (2 mL) were added to diosgenin (0.2 mg). Sample suspensions were shaken at 400 rpm in a thermo-controlled incubator at 37°C for 24 h. Suspensions were filtered using 0.2 µm membrane filters (Advantec, Tokyo, Japan). Diosgenin content was determined using a liquid chromatography mass spectrometry (LCMS) system. Separation was achieved by an MXY01-01 (Michrom Bioresources, CA, U.S.A.) with a TSK gel ODS-100 V column, 2.0×50 mm, 3 µm, (TOSOH, Tokyo, Japan) at room temperature. The mobile phase consisted of methanol (90%) and distilled water (10%) containing 10 mM ammonium acetate. The flow rate was set to 150 µL/min and detection was carried out using a mass spectrometer.
Phase solubility study was performed as described in a previous paper.19) Different concentrations (0 to 20 mM) of each β-CD solutions were added to diosgenin. After attained equilibrium, suspensions were filtrated and diosgenin contents were determined. Stability constants were calculated by diosgenin solubility-each β-CD concentration plot.
Caco-2 Cell CultureThe Caco-2 cell line was obtained from the European Collection of Cell Cultures (Wiltshire, U.K.). Cells were maintained in DMEM containing 10% FBS in 5% CO2 at 37°C. The passage number of the cells was between 45 and 50. Lactate dehydrogenase (LDH) leakage from Caco-2 cells were measured to estimate membrane integrity.20) Caco-2 cells were seeded at 1.0×104 cells/well on 96 well plates. Twenty-four hours after seeding, the remaining medium was removed, and cells were washed with HBSS (pH 7.4). Then, 200 µL of HBSS pH 6.0 containing 10 µM of diosgenin and 20 mM of β-CD, M β-CD, HE β-CD, or HP β-CD solution was added to each well and incubated in humidified atmosphere of 5% CO2 at 37°C for 2 h. Negative control cultures in HBSS without any test compounds and positive control cultures in HBSS with 1% Triton X-100. Plates were centrifuged at 400×g for 5 min and supernatant was transferred from another plates. LDH leakage was determined using LDH Cytotoxicity Detection Kit (Takara Bio, Shiga, Japan). Absorbance was measured at 490 nm by Spectra Max® M2e (Molecular Devices, CA, U.S.A.).
Caco-2 Monolayer Transport StudiesCaco-2 cells were seeded at a density of 2.5×105 cells/well on a Transwell® of 6 wells (Corning Costar, MA, U.S.A.) and polycarbonate filters (pore size 0.4 µm, surface area 4.67 cm2). Culture medium was replaced with fresh medium every 2–3 d in the inserts. Monolayers were used for the experiments between 21 and 28 d after seeding. Before the experiments, cells were washed with HBSS (pH 6.0). When measuring the apical to basal permeability, the donor solution containing 10 µM diosgenin and 20 mM of each β-CD in HBSS (pH 6.0) was added to the apical side, and the receiver solution containing 20 mM of each β-CD in HBSS (pH 7.4) was added to the basal side. The transport of diosgenin with or without CDs across Caco-2 cell monolayers was studied for 2 h at 37°C. Samples (100 µL) were taken from the basal side and immediately replaced by the fresh receiver solution. Diosgenin content was determined by an LCMS system. The apparent permeability (Papp) of diosgenin was calculated according to the following equation:
 | (1) |
where dQ/dt (µg/s) is the rate of appearance of the drug on the basal side, A (4.67 cm2) is the surface area of the monolayers, and C0 (µg/mL) is the initial drug concentration on the apical side. Transepithelial electrical resistance (TEER) was measured with a Millicell® ERS-2 (Millipore, MA, U.S.A.). Inserts were used for experiments when TEER values were over 1000 Ω·cm2. Before and after the experiments, TEER was measured to evaluate possible damage to the monolayers.
AnimalsTen-week-old male Wistar rats (200 to 250 g) were purchased from Japan SLC (Shizuoka, Japan) and housed in a room maintained at 23±2°C with a 12 h light-dark cycle. Prior to the experiments, rats were fasted overnight with free access to water. The animal care protocol was approved by the Animal Care and Use Committee of Josai University (Saitama, Japan).
Rat Jejunal PerfusionThe procedure for the in situ single-pass jejunal perfusion followed previous reports.21,22) Briefly, rats were anesthetized with an intraperitoneal injection of 88 mM sodium pentobarbital solution at a dose of 0.18 mmol/kg. The abdomen was opened by a midline incision of 1 cm. A jejunal segment of 10 cm was carefully exposed and cannulated on two ends with PVC tubing. The jejunal segment was then flushed with phosphate buffered saline (PBS) (pH 6.5) to remove residual intestinal contents. Suspensions of diosgenin (10 mM) were prepared with β-CD, HE β-CD, or HP β-CD at a concentration of 20 mM in PBS (pH 6.5). Perfusion solutions were delivered with a peristaltic pump at a flow rate of 0.2 mL/min through an inlet tubing water bath at 37°C before its entry into the jejunal segment. The perfusion buffer was first perfused for 30 min in order to ensure steady state conditions. Blood samples (300 µL) were taken from the tail vein at 10 min intervals for 40 min. Plasma was immediately separated by centrifugation and stored at −30°C until analysis. Diosgenin content was determined by an LCMS system. Following the termination of the experiment, the length of each perfused jejunal segment was measured. The area under the plasma concentration–time curve (AUC) was calculated by the liner trapezoidal rule. Methyl β-CD was excluded in this study because of its hemolytic effect on human erythrocytes.23)
Pharmacokinetic StudiesIntravenous and oral administration studies were performed to compare the pharmacokinetic parameters of diosgenin and its β-CD derivative complexes. Diosgenin and its complexes were dissolved or suspended in physiological saline containing 1% HCO-60, which was added as a suspending agent. In the intravenous administration, 61 µg/mL of diosgenin solution was prepared, and 122 µg/kg of diosgenin was injected into the tail vein. In the oral administration, 100 mg/kg of diosgenin suspension or an equivalent diosgenin amount of each β-CD complex, which were prepared by shaking diosgenin and each β-CD in water, were administered using a gastric tube. At a time range from 0 to 120 h, blood was collected in heparinized tubes by the tail vein and immediately separated by centrifugation.
Analytical ProcedureTo analyze diosgenin in plasma samples, 6-methyl diosgenin was used as an internal standard. Plasma diosgenin were extracted with methanol by sonication at 37°C. Samples were centrifuged at 15000×g for 5 min at 25°C and the supernatants were collected. Diosgenin levels were determined using an LCMS system.
Pharmacokinetics and Statistical AnalysisAfter the intravenous administration, the elimination of diosgenin was best fitted to the linear 1-compartment model.24) Pharmacokinetics analysis was performed with non-liner least squares fitting. The AUC was calculated by the liner trapezoidal rule. Absolute bioavailability was determined as AUCpo/AUCiv, using the mean AUC values for oral and intravenous doses. Dunnett’s multiple comparison tests were used to assess the significance of differences between groups. A p value of less than 0.05 was considered significant.
Table 1 summarized increasing effect of diosgenin aqueous solubility by β-CD and its derivatives (M β-CD, HE β-CD, and HP β-CD). Although β-CD had an increasing effect on the diosgenin solubility in water, M β-CD, HE β-CD, and HP β-CD had a greater effect on the solubility: the solubilities of diosgenin by β-CD and its derivatives were about 1000 times higher than that without β-CDs and 20 times higher than that by original β-CD. These results indicate that the inclusion complex may take place between diosgenin and each β-CD to increase diosgenin solubility.
To evaluate the interaction of diosgenin and β-CD derivatives, phase solubility study was performed. Figure 1 shows the phase solubility diagrams obtained for diosgenin against β-CD and its derivatives concentrations in distilled water. Typical incremental curve for the diosgenin concentration was observed against β-CD or its derivatives added in the solution. Then, the stability constants (K11 and K12) were determined by a method as described earlier.25) Table 2 lists the calculated stability constants K11 and K12. K11 increased in the order of M β-CD (30900 M−1)>HP β-CD (7800 M−1)>β-CD (6420 M−1)>HE β-CD (1020 M−1).
| Diosgenin alone | β-CD | M β-CD | HE β-CD | HP β-CD | |
|---|---|---|---|---|---|
| Solubilitya) (mM) | 2.36×10−5 | 3.06×10−3 | 0.480 | 3.59×10−2 | 0.144 |
a) The solubility of diosgenin was determined in distilled water containing 20 mM of each β-CD at 37°C for 24 h.

○, diosgenin/β-CD; ▲, diosgenin/M β-CD; □, diosgenin/HE β-CD and ●, diosgenin/HP β-CD.
| β-CD | M β-CD | HE β-CD | HP β-CD | |
|---|---|---|---|---|
| K11 (M−1) | 6420 | 30900 | 1020 | 7800 |
| K12 (M−1) | 610 | 1600 | 3700 | 1900 |
Excess amount of diosgenin was suspended in 0–20 mM of each β-CD solution. After equilibration at 37°C, diosgenin concentrations were determined. The stability constants were calculated by diosgenin solubility–each β-CD concentration plot.
The LDH leakage of 10 µM of diosgenin and 20 mM of β-CD and its derivative against the Caco-2 cells were determined (data not shown). It was not significantly increased by diosgenin and β-CD derivatives for 2 h, which is the average residence time of nutrients in the stomach. These results indicate that diosgenin and each β-CD did not exhibit a cytotoxic ability towards Caco-2 cells. In the following Caco-2 transport study, we applied 10 µM diosgenin with 20 mM of each β-CD.
Caco-2 Transport StudyTEER measurements through Caco-2 monolayer were performed in order to verify the effect of β-CD and its derivatives on the tightness of junctions between cells. Barrier function of tight junction must be closely related to the drug transport through Caco-2 layer. TEER values obtained before and after permeation of diosgenin with β-CD or its derivatives are listed in Fig. 2. The TEER decreases by β-CD (45.8%), M β-CD (54.2%), HE β-CD (63.3%), and HP β-CD (97.5%) suggest tight junction opening by β-CD, M β-CD, and HE β-CD.
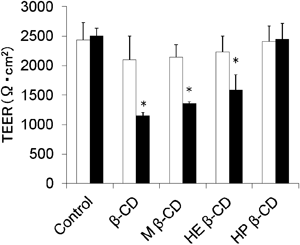
Caco-2 monolayers were incubated for 120 min at 37°C in the presence of 10 µM diosgenin and 20 mM of each β-CD. □, before permeability; ■, after permeability. Values are mean±S.E., n=4. *Significantly different from the control (p<0.05, Dunnett’s test).
Next, the effect of each β-CD on the diosgenin permeability was evaluated using Caco-2 monolayers. Figure 3 shows the amount of diosgenin permeating across the monolayer by β-CD and its derivatives. Table 3 also shows permeability coefficient, Papp, of diosgenin. The membrane permeations of diosgenin were greatly improved by HE β-CD and HP β-CD. The Papp values of diosgenin in the presence of β-CD, M β-CD, HE β-CD, or HP β-CD were 30.8×109, 127×109, 351×109, and 340×109 cm/s, respectively. These are higher than diosgenin alone (8.48×109 cm/s). The effect of M β-CD on the diosgenin permeability was lower than HE β-CD and HP β-CD. β-CD slightly increased diosgenin permeability across the Caco-2 monolayer.

△, diosgenin; ○, diosgenin/β-CD; ▲, diosgenin/M β-CD; □, diosgenin/HE β-CD and ●, diosgenin/HP β-CD. Data are presented as the mean±S.E., n=6–8. *Significantly different from diosgenin (p<0.05, Dunnett’s test).
| Diosgenin alone | β-CD | M β-CD | HE β-CD | HP β-CD | |
|---|---|---|---|---|---|
| Solubilitya) (ng/mL) | 0.712±0.218 | 43.9±17.3* | 1030±420* | 774±240* | 1120±380* |
| Pappb) (×109 cm/s) | 8.48±1.56 | 30.8±15.5* | 127±25* | 351±76* | 340±64* |
a) The solubility of diosgenin was determined in HBSS (pH 6.0) containing 20 mM of each β-CD at 37°C for 24 h. b) Diosgenin (20 nmol) was suspended in 2 mL HBSS (pH 6.0). Apical to basal Papp values were measured. Data are presented as the mean±S.E., n=6–8. *Significantly different from the diosgenin alone (p<0.05, Dunnett’s test).
The jejunal absorption of diosgenin with an addition of each β-CD is determined and obtained AUC is summarized in Table 4. In the in situ jejunal perfusion experiments, mean AUC of diosgenin alone, β-CD, HE β-CD, and HP β-CD were 450, 817, 560, and 1750 µg·min/cm2, respectively. The concentration of diosgenin absorbed into the general circulation was most increased by HP β-CD. This enhancement effect is not confirmed when perused with HE β-CD, although the reason is unclear.
| Diosgenin alone | β-CD | HE β-CD | HP β-CD | |
|---|---|---|---|---|
| AUC (µg·min/cm2) | 450±102 | 817±167 | 560±110 | 1750±907* |
Rat jejunal loops were perfused with PBS buffer (pH 6.5) containing 10 mM diosgenin or 20 mM of each β-CD. The perfusion flow rate for the jejunal loop was 0.2 mL/min. Data are presented as the mean±S.E., n=3–5. *Significantly different from the diosgenin alone (p<0.05, Dunnett’s test).
Figure 4 shows the plasma diosgenin concentration-time profile after the oral administration of diosgenin and each β-CD complex suspension at a dose of 100 mg/kg in rats. The mean AUCiv value after the intravenous administration of 61 µg/mL diosgenin solution was 113±18 ng·h/mL. The pharmacokinetic parameters obtained are summarized in Table 5. Diosgenin bioavailability was higher with all β-CD derivatives than with the diosgenin suspension. Cmax, AUCpo, and bioavailability were significantly higher in the HP β-CD complex than in diosgenin alone. The bioavailability of diosgenin when administrated after diosgenin suspension, β-CD, HE β-CD, and HP β-CD complexes were 4.45, 33.5, 17.3, and 49.5%, respectively.
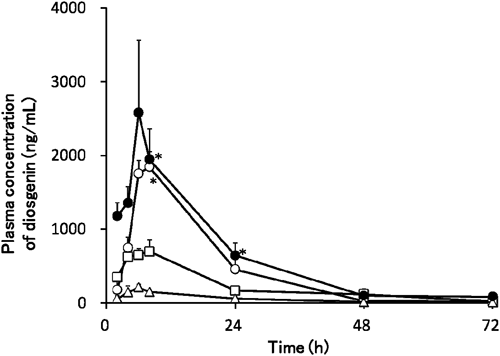
△, diosgenin; ○, diosgenin/β-CD; □, diosgenin/HE β-CD and ●, diosgenin/HP β-CD. Means±S.E. of 3 to 8 experiments are shown. *Significantly different from diosgenin (p<0.05, Dunnett’s test).
| AUCpo (ng·h/mL) | Cmax (ng/mL) | Tmax (h) | MRT (h) | Bioavailability (%) | |
|---|---|---|---|---|---|
| Diosgenin alone | 4121.9±1354.7 | 132.5±48.2 | 5.01±0.55 | 28.9±2.4 | 4.45±1.46 |
| β-CD | 31373±2967 | 1292±91* | 6.21±1.14 | 19.8±1.2 | 33.5±3.1 |
| HE β-CD | 16271±2020 | 561.8±38.5 | 4.95±0.59 | 25.2±2.3 | 17.3±2.2 |
| HP β-CD | 46374±5289* | 1776±159* | 3.35±0.88 | 22.8±1.1 | 49.5±5.7* |
Data are presented as the mean±S.E., n=3–8. *Significantly different from the diosgenin alone (p<0.05, Dunnett’s test).
| Diosgenin | β-CD | M β-CD | HE β-CD | HP β-CD | |
|---|---|---|---|---|---|
| Solubilitya) | 1 | 1690 | 20300 | 1520 | 6090 |
| 1/TEERb) | 1 | 2.19 | 1.85 | 1.58 | 1.03 |
| Caco-2c) | 1 | 10.2 | 54.0 | 214 | 165 |
| Jejunumd) | 1 | 1.82 | - | 1.24 | 3.89 |
| Bioavailabilitye) | 1 | 7.61 | - | 3.94 | 11.3 |
a) Solubility of diosgenin with the existence of 20 mM each β-CD. b) TEER decreasing ratio after incubation with 10 µM diosgenin and 20 mM of each β-CD for 2 h. c) Caco-2 permeability ratio of diosgenin after treatment by 10 µM diosgenin and 20 mM of each β-CD for 2 h. d) Diosgenin AUC ratio after jejunal perfusion of diosgenin suspension containing 20 mM each β-CD. e) Diosgenin bioavailability ratio after oral administration of diosgenin and each β-CD complex.
The enhancement ratios by each β-CD compared with diosgenin alone on the drug solubility, 1/TEER, Caco-2 permeability, jujunal permeability, and bioavailability were summarized in Table 6. The solubility of diosgenin improved all β-CD derivatives, and concave curvatures were obtained between diosgenin solubility and all of the CD concentrations, suggesting high order complexation taking place between diosgenin and each β-CD. Our previous study suggests that diosgenin and β-CD formed 1 : 2 molar ratio complexes.24) M β-CD showed the highest effect on the diosgenin solubility among the β-CD derivatives tested. The results may be due to the role of methyl substituents in hydrophobic interactions.
An LDH leakage form cell is well known as a useful index for membrane integrity. The LDH results demonstrate that expose of diosgenin and each β-CD for 2 h did not break down Caco-2 cell membrane. The 1/TEER ratios, which related with tight junction opening, of β-CD, M β-CD, HE β-CD, and HP β-CD compared with diosgenin alone were 2.19, 1.85, 1.58, and 1.03, respectively. It has been reported that TEER decreases the paracellular permeability of drugs by an increase in connection with tight junction opening.26) Since TEER was never below 1000 Ω·cm2, which indicates a slight cellular damage taking place during the permeation experiments.27,28) Therefore, each β-CD open tight junction and the effect of tight junction opening was higher by HE β-CD than HP β-CD.
The Caco-2 permeation ratios of β-CD, M β-CD, HE β-CD, and HP β-CD compared with diosgenin alone were 10.3, 54.6, 221, and 169, respectively. The lower effect of M β-CD may depend on its higher K11 which decreases the uncombined drug content. β-CD slightly increased diosgenin permeability across the Caco-2 monolayer, which is probably due to its lower solubility. Although HE β-CD has a lower K11, it markedly enhanced the diosgenins permeability through Caco-2 monolayer, which seems to indicate that HE β-CD improved solubility and opened tight junctions. It is suggested that HP β-CD does not affect tight junctions; however, it has a suitable stability constants and solubility enhancement effect.
The AUC ratios of diosgenin plasma concentration after the jejunal perfusion experiment were 1.82, 2.02, and 3.89 by β-CD, HE β-CD, and HP β-CD, respectively, compared with diosgenin alone. The enhancement ratios of diosgenin bioavailability by β-CD, HE β-CD, and HP β-CD complexes were 7.61-, 3.94-, and 11.3-fold higher than that of diosgenin alone, respectively. The result of jejunal permeability and bioavailability correlates with solubility study rather than Caco-2 cell studies. It is suggested that the oral absorption of diosgenin could infer by water solubility and it was mostly improved by HP β-CD.
In the solubility study, all β-CD derivatives improved the solubility of diosgenin. The calculated K11 value of HP β-CD was higher than that of HE β-CD. However, the stimulated effect of diosgenin permeability across the Caco-2 monolayer was highest by HE β-CD. It is suggest that the effect of tight junction opening was higher by HE β-CD. This phenomenon can be confirmed by the TEER decrease. In the rat jejunum perfusion study, improvements in diosgenin permeability were higher by HP β-CD than by HE β-CD. Therefore, the contribution of these improved effects was estimated to be higher in solubility than in permeability with diosgenin bioavailability. In conclusion, these studies suggest the possibility that HE β-CD enhances diosgenin permeability across the Caco-2 monolayer by tight junction opening and HP β-CD improves rat intestinal absorption by a solubilization effect. Although further study is needed, these results may support the elucidation of the absorption enhancement mechanism for drugs with poor solubility by β-CD derivative.