2014 年 37 巻 1 号 p. 74-80
2014 年 37 巻 1 号 p. 74-80
Body and excrement extracts from Dermatophagoides farinae were used to study stimulation of Toll-like receptors (TLRs). The excrement extract stimulated nuclear factor (NF)-κB-dependent reporter activity to an extent similar to lipopolysaccharide (LPS) in a mouse macrophage cell line, J774A.1, but the activity of the body extract was negligible. The excrement extract also activated NF-κB in HEK293 cells expressing TLR1/TLR2, TLR2/TLR6 and CD14/TLR4/MD-2, whereas no activation was observed in cells expressing TLR3, TLR5, TLR7, TLR8 or TLR9. Although the excrement extract required co-expression of CD14, TLR4 and MD-2 in HEK293 cells to activate NF-κB, efficient activation was still observed in I-13.35 cells, a bone-marrow macrophage cell line established from LPS-hypo-responsive C3H/HeJ mice. The excrement extract activated NF-κB in HEK293 cells expressing TLR2 alone, but the activation was significantly increased by co-expression of CD14. Polymyxin B inhibited CD14/TLR4/MD-2- and CD14/TLR2-mediated activation of NF-κB but not the activation in I-13.35 cells. These results indicate that CD14/TLR4/MD-2-dependent and CD14/TLR2-dependent mechanisms are involved in the activation of NF-κB by the excrement extract of D. farinae and suggest that the extract also contains substances that activate NF-κB through non-TLR-mediated mechanisms.
Allergic asthma is often caused by exposure to aeroallergens, which include dust mites (bodies and excrement), mold and pet hairs. House dust mites, in particular, are a major source of indoor allergens that are associated with allergic asthma.1) Crucial in the development of allergy is the activation of antigen-presenting cells, which take up antigens and express them on the cell surface. However, antigen presentation alone does not lead to an allergic response, but rather leads to tolerance. Efficient induction of an allergic response requires activation of antigen-presenting cells through Toll-like receptors (TLRs).2)
Activation of the innate immune response is mainly mediated by TLR recognition of pathogen-associated molecular patterns (PAMPs). To date, 10 functional TLRs have been identified in humans and 12 in mice.3) TLR2 associates with TLR1 to recognize triacyl lipoproteins from Gram-negative bacteria and mycoplasma, whereas the combination of TLR2 and TLR6 recognizes diacyl lipoproteins from Gram-negative bacteria and mycoplasma. TLR3 binds the genomic RNA of reoviruses and double-stranded RNA (dsRNA) produced during viral replication. TLR4 and its accessory molecule MD-2 recognize lipopolysaccharide (LPS), a component of the outer membrane of gram-negative bacteria. TLR5 recognizes the flagellin protein component of bacterial flagella. TLR7 and TLR8 recognize single-stranded RNAs (ssRNAs) derived from RNA viruses. Finally, TLR9 recognizes unmethylated cytidine–phosphate–guanosine (CpG) sites primarily found in bacterial DNA.3) TLR stimulation, regardless of type, eventually leads to the activation of the nuclear factor (NF)-κB transcription factor and subsequent production of cytokines, chemokines and cell surface molecules involved in adaptive immune responses.
Dermatophagoides pteromyssinus and Dermatophagoides farinae have been reported to be the most common house dust mite species that produces allergens. Both species widely distributed in the United States and D. pteromyssinus has been regarded to be the predominant species in Europe although in certain European areas, D. farinae was common.4) In Japan, it has been reported that5) D. farinae was predominant in Nagoya and Tokyo, whereas D. pteromyssinus was predominant in Osaka, Sendai, Sapporo, Fukuoka, Tokushima and Hiroshima. The allergic inflammation induced by D. pteromyssinus was abolished in TLR4-deficient mice.6) In addition, Der p2, a major group II allergen from D. pteromyssinus, activates smooth muscle cells in a TLR2-dependent manner to induce an inflammatory response.7) Although D. farinae is also a major allergen, TLRs required for this species to stimulate innate immune responses have not been reported. Here we systematically studied TLRs required for extracts prepared from the body and excrement of D. farinae to stimulate NF-κB and found that the excrement extract activates NF-κB through CD14/TLR4/MD-2-dependent and CD14/TLR2-dependent mechanisms.
Tripalmitoyl-Cys-Ser-Lys-Lys-Lys-Lys (Pam3CSK4) and MALP-2 were obtained from Bachem (Bubendorf, Switzerland) and suspended in 25 mM octyl glucoside. LPS prepared from Salmonella abortus equi was described previously.8–10) Poly(I : C) was purchased from GE Healthcare (Amersham Place, U.K.). Flagellin, CL-97, imiquimod and single-stranded RNA (ssRNA40) were obtained from InvivoGen (San Diego, CA, U.S.A.). CpG oligodeoxynucleotide (CpG ODN 2006) was synthesized at Operon Biotechnologies (Tokyo, Japan). Whole body (D. farinae body (DF-b); lot No.: FUF06032, SHE10015) and excrement (D. farinae excrement (DF-e); lot No.: SHE12031, FUF11021) extracts prepared from D. farinae were obtained from ITEA Inc. (Tokyo, Japan). The amounts of DF-b and DF-e were calibrated based on protein and Der f 1 content, respectively, with the extract amount containing 1 µg Der f 1 defined as 1 U. An endotoxin-specific limulus amebocyte lysate assay (Endospecy; SEIKAGAKU CORPORATION, Tokyo, Japan) showed that DF-b contained approximately 3 endotoxin units per µg protein while the DF-e had 7.8 endotoxin units per mU. Recombinant LPS-binding protein (LBP) has been described.11) The HEK293 and HeLa cell lines (ATCC, Manassas, VA, U.S.A.) were cultured in Dulbecco’s modified Eagle’s medium (Life Technologies, Grand Island, NY, U.S.A.) supplemented with 10% (v/v) heat-inactivated fetal bovine serum (Life Technologies), penicillin (100 U/mL) and streptomycin (100 µg/mL). The NF-κB-dependent luciferase reporter cell line J774-ELAM was previously described.11) The I-13.35 cell line, which was established from bone-marrow macrophages obtained from LPS-hypo-responsive C3H/HeJ mice was purchased from ATCC and cultured in the same manner as the HEK293 cells, with the exception that conditioned medium from RADMAC cells (ATCC) was included to 20% (v/v) as a source of M-CSF.
PlasmidsThe NF-κB-dependent luciferase reporter plasmid pELAM-L and expression plasmids for CD14, TLR4, MD-2 and TLR2 were described previously.10,12–14) The coding regions of TLR1 and TLR9 were amplified by reverse transcription polymerase chain reaction (RT-PCR) from total RNA prepared from differentiated THP-1 cells. The coding region of TLR3 was constructed by PCR from cDNA clones Hs.29499 (Stratagene, La Jolla, CA, U.S.A.), FLJ94938 and FLJ51380 (both from Toyobo, Osaka, Japan). I.M.A.G.E cDNA clones (obtained from Open Biosystems, Huntsville, AL) were used to amplify the coding regions of TLR5 (40035618), TLR7 (5582912) and TLR8 (40008788). The coding region of TLR6 was also amplified from I.M.A.G.E cDNA clones 40007924 and LIFESEQ5118201. These coding regions were inserted into mammalian expression vector pcDNA3 (Invitrogen, Carlsbad, CA, U.S.A.).
Reporter AssayThe luciferase reporter assay was performed as described previously.15) Briefly, HEK293 and HeLa cells were plated in 6-well plates and transfected the following day by calcium phosphate precipitation or with the FuGENE HD Transfection Reagent (Promega, Madison, WI, U.S.A.), according to the manufacturer’s instructions, with the indicated TLR plasmid and 0.2 µg pELAM-L and 5 ng phRL-TK (Promega) for normalization. At 24–32 h after transfection, the cells were stimulated in the culture medium described above for 6 h with DF-e or each TLR ligand. In Fig. 3, stimulation was performed in the culture medium supplemented with 100 ng/mL LBP in the absence of fetal bovine serum. Following stimulation, cellular extracts were prepared by adding a lysis buffer (10 mM N-(2-hydroxyethyl)piperazine-N′-2-ethanesulfonic acid (HEPES)-KOH, pH 7.9, 10 mM KCl, 5 mM ethylenediaminetetraacetic acid (EDTA), 40 mM β-glycerophosphate, 0.5% NP-40, 30 mM NaF, 1 mM Na3VO4, 1 mM dithiothreitol) containing a protease inhibitor cocktail (Nacalai Tesque, Kyoto, Japan). Reporter gene activity in the cellular extracts was measured using the Dual-Luciferase Reporter Assay System according to the manufacturer’s instructions (Promega). For some experiments, cell viability of the extracts was determined using the CellTiter-Glo Luminescent Cell Viability Assay Kit (Promega). Reporter activity was normalized to cell viability to compensate for differences in cell number and viability between wells.
Statistical AnalysesFor comparison of two groups, the two-tailed Student’s t-test was used. For multiple comparisons, analysis was performed by two-way ANOVA, followed by the Tukey–Kramer method. Differences were considered significant for p<0.05.
Activation of NF-κB induced by DF-b and DF-e extracts were evaluated using J774-ELAM cells, a mouse macrophage cell line, stably carrying an NF-κB-dependent luciferase reporter plasmid because this cell line responds to all ligands of TLR1-TLR9 to activate NF-κB. Luciferase activity was measured after the cells were stimulated either with 0.1–100 mU/mL of DF-e, 0.1–10 µg/mL of DF-b or 0.1–100 ng/mL of LPS (Fig. 1). DF-e stimulated reporter activity to a level comparable to that produced by LPS. Only slight activity was also observed with the DF-b extract, but only at 10 µg/mL. Thus, we focused on the activation of NF-κB induced by DF-e.
The TLRs involved in DF-e-induced activation of NF-κB were identified by expressing each TLR in HEK293 cells and stimulating with DF-e and the corresponding TLR ligand (Fig. 2). HeLa cells were used with TLR7, TLR8 and TLR9 because HEK293 cells did not respond to the corresponding ligands, even when the TLRs were expressed. Neither cell line responded to the TLR ligands without concomitant expression of the appropriate TLR (data not shown). When TLR1/TLR2 (Fig. 2A) or TLR2/TLR6 were expressed in HEK 293 cells (Fig. 2B) with an NF-κB-dependent luciferase reporter gene, 100 mU/mL of DF-e increased reporter activity above baseline levels. Activities were approximately 20–30% of the corresponding TLR ligands, Pam3CSK4 (0.1 µg/mL) and MALP-2 (0.1 µg/mL), which at these concentrations led to maximal levels of activation. CD14, TLR4 and MD-2 are necessary for the response to TLR4 ligand. When all three were expressed in HEK293 cells (Fig. 2D), LPS (10 ng/mL) and DF-e (10 mU/mL) increased reporter activity. The activity at 100 mU/mL of DF-e reached the maximal level of activation, approximately 80% of the level observed with 10 ng/mL LPS stimulation. In HEK293 or HeLa cells expressing other TLRs, DF-e up to 100 mU/mL did not significantly increase reporter activity, although the corresponding TLR ligands did stimulate activity (Figs. 2C, E–H).

J774-ELAM cells were either left unstimulated or stimulated for 6 h with the indicated concentrations of DF-e, DF-b or LPS and NF-κB-dependent luciferase reporter activity was measured. Values are the means±S.E.M. from five independent experiments. ** p<0.01, compared to the activity without stimulation.

HEK293 cells were transiently transfected with TLR1 and TLR2 (A), TLR2 and TLR6 (B), TLR3 (C), CD14, MD-2 and TLR4 (D) or TLR5 (E) expression plasmids and an NF-κB-dependent luciferase reporter plasmid. HeLa cells were transiently transfected with TLR7 (F), TLR8 (G) or TLR9 (H) expression plasmids and an NF-κB-dependent luciferase reporter plasmid. After 24 h, cells were either left unstimulated or stimulated for 6 h with the indicated concentrations of DF-e or the corresponding TLR ligands and luciferase activity was measured. Values are the means±S.E.M. from at least four independent experiments. * p<0.05, ** p<0.01, compared to the activity without stimulation.
While LPS requires the combination of CD14, TLR4 and MD-2 to activate NF-κB, it was not known if DF-e also requires these molecules. Therefore, HEK293 cells were transfected with each molecule or combinations of CD14, TLR4 and MD-2 and an NF-κB-dependent luciferase reporter gene. The cells were then stimulated with 100 mU/mL of DF-e or 10 ng/mL of LPS (Fig. 3A). To avoid the influence of soluble forms of CD14 and MD-2, which may be in fetal bovine serum, the stimulation was performed in the absence of serum and LBP was included when LPS was used. Although a slight increase in the reporter activity was observed in HEK293 cells expressing MD-2 plus TLR4 without stimulation and in cells expressing CD14 plus TLR4 and MD-2 plus TLR4 in response to LPS and DF-e, respectively, both DF-e and LPS significantly increased the reporter activity when all three of the molecules (CD14, TLR4 and MD-2) were expressed, indicating that DF-e also requires all three to activate NF-κB. To confirm this, a similar experiment was performed in HeLa cells (Fig. 3B). DF-e slightly increased the reporter activity in HeLa cells with vector alone (i.e., without CD14, TLR4 and MD-2) and the activity was enhanced when CD14 was expressed. Expression of TLR4 or MD-2 in addition to CD14 did not affect activity; however, the expression of all of these molecules further enhanced reporter activity. In contrast, LPS-induced activation was observed when CD14 and MD-2 were simultaneously expressed, and activity was significantly increased when CD14, TLR4 and MD-2 were all expressed together.

HEK293 cells (A) or HeLa cells (B) were transiently transfected with the indicated combination of CD14, MD-2 and TLR4 expression plasmids or an empty vector together with an NF-κB-dependent luciferase reporter plasmid. After 24 h, cells were either left unstimulated or stimulated with 100 mU/mL DF-e or 10 ng/mL LPS in a serum-free condition. When stimulated with LPS, 100 ng/mL LBP was included. Luciferase activity was measured after 6 h. Values are the means±S.E.M. from four independent experiments. * p<0.05, ** p<0.01, compared to the activity in vector (vec)-transfected cells.
Since DF-e activated NF-κB in HeLa cells expressing CD14 (Fig. 3B) and in HEK293 cells expressing TLR1/TLR2 (Fig. 2A) or TLR2/TLR6 (Fig. 2B), the effect of DF-e was examined in the I-13.35 cell line, which was established from bone-marrow macrophages obtained from LPS-hypo-responsive C3H/HeJ mice. I-13.35 cells were transfected with an NF-κB-dependent luciferase reporter gene with or without an expression plasmid for TLR4 and stimulated with DF-e, LPS or Pam3CSK4 (Fig. 4). LPS stimulation only slightly increased reporter activity whereas a significant increase in activity was observed upon TLR4 expression, confirming the LPS-hypo-responsiveness of this cell line. DF-e increased reporter activity, which was slightly enhanced by TLR4 expression, in a concentration-dependent manner. This result indicated that TLR4-indepent signaling also contributes to DF-e-induced activation of NF-κB.
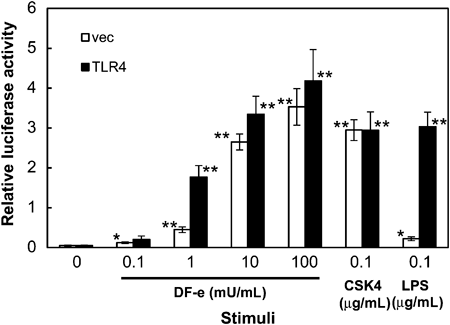
LPS-hypo-responsive I-13.35 cells were transiently transfected with a TLR4 expression plasmid or an empty vector and an NF-κB-dependent luciferase reporter plasmid. After 24 h, cells were either left unstimulated or stimulated for 6 h with the indicated concentration of DF-e, 0.1 µg/mL Pam3CSK4 (CSK4) or 0.1 µg/mL LPS and luciferase activity was measured. Values are the means±S.E.M. from four independent experiments. * p<0.05, ** p<0.01, compared to the activity without stimulation.
Since HeLa cells respond to TLR2 ligands to activate NF-κB, we examined the involvement of CD14 and TLR2 in DF-e-induced activation of NF-κB in HEK293 cells, which express neither of these proteins. HEK293 cells were expressed with each or combination of CD14 and TLR2 (Fig. 5 inset) and an NF-κB-dependent reporter gene. Reporter activity in response to DF-e was observed (Fig. 5). DF-e did not increase reporter activity when transfected with CD14 alone. Transfection with TLR2 resulted in an increase in activity only at 10 and 100 mU/mL of DF-e, but when combined with CD14, activity was significantly enhanced (p<0.01, by Tukey–Kramer method) and the increase was observed as low as 1 mU/mL. These results indicate that CD14/TLR4/MD-2-dependent and CD14/TLR2-dependent mechanisms are involved in DF-e-induced activation of NF-κB.

HEK293 cells were transiently transfected with either, or a combination of, CD14 and TLR2 expression plasmids together with an NF-κB-dependent luciferase reporter plasmid. After 24 h cells were either left unstimulated or stimulated for 6 h with the indicated concentration of DF-e and luciferase activity was measured. Supernatants from cellular extracts were subjected to SDS-PAGE followed by Western blot analysis to detect expression of CD14 and TLR2 (inset). Values are the means±S.E.M. from five independent experiments. * p<0.05, ** p<0.01, compared to the activity without stimulation in corresponding transfection.
We next addressed the involvement of endotoxin in the CD14/TLR4/MD-2-mediated activation of NF-κB by DF-e because DF-e used in this study contained a considerable amount of endotoxin as determined by an endotoxin-specific limulus amebocyte lysate assay. HEK293 cells were expressed with CD14, TLR4 and MD-2 together with an NF-κB-dependent reporter gene. Reporter activity in response to DF-e and LPS was observed in the presence of polymyxin B, which is known to neutralize biological activity of LPS (Fig. 6A). Polymyxin B inhibited LPS-induced activation of NF-κB and DF-e-induced activation was also inhibited by polymyxin B in a concentration-dependent manner. Since DF-e activated NF-κB through a CD14/TLR2-dependent mechanism, the effect of polymyxin B was also studied (Fig. 6B). DF-e-induced activation of NF-κB in HEK293 cells expressing CD14/TLR2 was inhibited by polymyxin B with a similar concentration range that inhibited the CD14/TLR4/MD-2-mediated activation whereas polymyxin B did not affect Pam3CSK4-induced activation. We also addressed whether polymyxin B inhibits DF-e-induced activation in I-13.35 cells (Fig. 6C). Polymyxin B did not significantly affect the activation of NF-κB in response to DF-e and Pam3CSK4. These results indicate that DF-e contains polymyxin B-sensitive substance(s) that activate(s) NF-κB through CD14/TLR4/MD-2-dependent and CD14/TLR2-dependent mechanisms and that polymyxin B-insensitive substance(s) was also involved in the activation of NF-κB.

HEK293 cells were transiently transfected with CD14, TLR4 and MD-2 (A) or CD14 and TLR2 (B) expression plasmids together with an NF-κB-dependent luciferase reporter plasmid. After 24 h cells were treated with the indicated concentration of polymyxin B followed by DF-e (10 mU/mL), LPS (10 ng/mL) or Pam3CSK4 (CSK4; 100 ng/mL). I-13.35 cells (C) were transfected with an NF-κB-dependent luciferase reporter plasmid and treated as above. After 6 h luciferase activity was measured. Values are the means±S.E.M. from 3–8 independent experiments. * p<0.05, ** p<0.01, compared to the activity in response to corresponding stimulation in the absence of polymyxin B.
We demonstrate here that an excrement extract (DF-e) prepared from D. farinae, a major indoor allergen, activates NF-κB through TLR4-dependent and CD14/TLR2-dependent mechanisms. It has been reported6) that a body extract prepared from another major indoor allergen, D. pteromyssinus, induces asthma via TLR4 triggering of airway structural cells. In this study, a body extract (DF-b) from D. farinae only slightly activated NF-κB whereas a significant activation was observed with its excrement extract in a mouse macrophage cell line (Fig. 1). An analysis of allergen contents in the extracts revealed that group I allergens were found primarily in excrement extracts whereas group II allergens were detected in both body and excrement extracts.16) It has been reported17) that Der p2, a group II allergen found in D. pteromyssinus, in conjunction with LPS activates TLR4 signaling even in the absence of MD-2, an accessary molecule of TLR4. They found that Der p2 functioned as MD-2-like chaperon, resulting in efficient induction of cytokine production in HEK293 cells expressing CD14 and TLR4 or in peritoneal macrophages obtained from MD-2−/− mice. However, in the present study, DF-e required the transfection of MD-2 in addition to CD14 and TLR4 to activate NF-κB in HEK293 cells (Fig. 3A). D. farinae possesses a Der p2 homolog Der f2, which also reportedly exhibits MD-2-like activity.18) However, Der f2 expression did not confer LPS responsiveness in HEK293 cells expressing CD14 and TLR4 (data not shown). Recombinant Der f2 also failed to activate NF-κB in HeLa cells expressing CD14 and TLR4 (data not shown). We also examined the ability of Der f1, group I allergen found in D. farinae, to confer LPS responsiveness although Der f1 is structurally unrelated to MD-2. However, expression of Der f1, alone or in conjunction with LPS, also failed to activate NF-κB in HeLa cells expressing CD14 and TLR4 (data not shown). Therefore, it is unlikely that Der f2 and Der f1 are involved in the DF-e-induced activation of NF-κB observed in this study.
The development and severity of asthma are thought to be determined by an endotoxin.19,20) According to Braun-Fahrlander et al.,19) endotoxin levels in house dust mites have been correlated with a modifying effect on allergic sensitization in children. Trompette et al.17) have reported that recombinant Der p2 complexed with a sub-effective amount of LPS stimulated TLR4-dependent innate cytokine production by mouse peritoneal macrophages in the presence and absence of MD-2. House dust mite extracts contain endotoxin originating from colonizing bacteria and environmental pollution.17) The DF-e used in this study also contains a considerable amount of endotoxin. Since DF-e used in this study was prepared from a laboratory-cultured D. farinae, it is unlikely that the endotoxin was derived from environmental pollution. An endotoxin-specific limulus amebocyte lysate assay revealed that 1 mU/mL of DF-e contained 7.8 endotoxin unit per ml, which corresponds to 2.3 ng/mL of S. abortus equi LPS used in this study. The concentration of LPS was enough to stimulate mouse macrophage cells (Fig. 1). Furthermore, the activation of NF-κB in response to DF-e was inhibited by polymyxin B with a concentration range that also inhibited LPS-induced activation in HEK293 cells expressing CD14, TLR4 and MD-2 (Fig. 6A) suggesting that an endotoxin is responsible for the majority of the CD14/TLR4/MD-2-dependent activity of DF-e. However, the activation of NF-κB in response to DF-e in HEK293 cells expressing CD14 and TLR2 was also inhibited by these concentrations of polymyxin B (Fig. 6B). Thus, it is possible that DF-e contains polymyxin B-sensitive substances, other than LPS, that activate NF-κB through both CD14/TLR4/MD-2-dependent and CD14/TLR2-dependent mechanisms.
DF-e also activated NF-κB in I-13.35 cells, which was derived from bone-marrow macrophages obtained from LPS-hypo-responsive C3H/HeJ mice (Fig. 4). Since this cell line responds to LPS poorly due to a mutation in the tlr4 gene, it is unlikely that the activation was mediated through TLR4. Involvement of TLR2 could also be ruled out because the activation was not affected by polymyxin B (Fig. 6C) despite being effectively inhibited in CD14/TLR2-transfected HEK293 cells. Since HEK293 cells without transfection did not respond to DF-e, the activity observed in I-13.35 cells may be macrophage-specific. We are currently trying to identify these active substances that exert their effects through TLR2/4-mediated and non-TLR-mediated mechanisms.
This research was supported in part by Health and Labour Sciences Research Grants from the Ministry of Health, Labour and Welfare of Japan. We thank Ryosuke Sohma and Nozomi Ishiguro for technical assistance.