2014 年 37 巻 1 号 p. 174-177
2014 年 37 巻 1 号 p. 174-177
Ultrasound (US) is used in the clinical setting not only for diagnosis but also for therapy. As a therapeutic US technique, high-intensity focused ultrasound (HIFU) can be applied to treat cancer in a clinical setting. Microbubbles increased temperature and improved the low therapeutic efficiency under HIFU; however, microbubbles have room for improvement in size, stability, and targeting ability. To solve these issues, we reported that “Bubble liposomes” (BLs) containing the US imaging gas (perfluoropropane gas) liposomes were suitable for ultrasound imaging and gene delivery. In this study, we examined whether BLs and HIFU could enhance the ablation area of the tumor and the antitumor effect. First, we histologically analyzed the tumor after BLs and HIFU. The ablation area of the treatment of BLs and HIFU was broader than that of HIFU alone. Next, we monitored the temperature of the tumor, and examined the antitumor effect. The temperature increase with BLs and HIFU treatment was faster and higher than that with HIFU alone. Moreover, treatment with BLs and HIFU enhanced the antitumor effect, which was better than with HIFU alone. Thus, the combination of BLs and HIFU could be efficacious for cancer therapy.
Ultrasound (US) imaging is a widely used diagnostic technique that allows for real-time imaging and combines the advantages of noninvasiveness with easy access by the public and low cost. In addition, US is used in the clinical setting not only for diagnosis but also for therapy. Recently, as a therapeutic US technique, high-intensity focused ultrasound (HIFU) has been applied for the treatment of prostate cancer and liver cancer in a clinical setting.1) HIFU can induce thermal elevation and lead to ablation and coagulative necrosis for targeted tissue. Although the concept of HIFU can be ideal of cancer therapy, HIFU has some disadvantages. Because the ablation area of HIFU exposure is narrow, HIFU requires a long time for cancer therapy. Microbubbles increase heat generation mainly by increasing inertial cavitation and improve the therapeutic efficiency under HIFU exposure2); however, microbubbles have room for improvement in size, stability, and targeting ability. To solve these issues, we previously developed “Bubble liposomes” (BLs: approximately 500 nm). These are polyethylene glycol (PEG)-modified liposomes that contain echo-contrast gas, which can function as a gene and short interfering RNA (siRNA) delivery tool with US exposure in vitro and in vivo.3,4) However, it is not yet known whether BLs increase the temperature of tumor tissue and enhance the ablation area and antitumor effect under HIFU exposure as microbubbles. In this study, we examined whether BLs and HIFU could enhance the ablation area of tumors and the antitumor effect.
To prepare liposomes for BLs, 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) and 1,2-distearoyl-sn-glycero-3-phosphatidyl-ethanolamine-polyethyleneglycol (DSPE-PEG2000-OMe) were mixed at a molar ratio of 94 : 6. The liposomes were prepared by a reverse-phase evaporation method, as described previously.4) In brief, all the reagents were dissolved in 1 : 1 (v/v) chloroform–diisopropylether. Phosphate-buffered saline was added to the lipid solution, and the mixture was sonicated and then evaporated at 47°C. The organic solvent was completely removed, and the size of the liposomes was adjusted to less than 200 nm using extrusion equipment and a sizing filter (Nuclepore Track-Etch Membrane, 200 nm pore size, Whatman plc, U.K.). After being sized, the liposomes were passed through a sterile 0.45- µm syringe filter (Asahi Techno Glass Co., Chiba, Japan) for sterilization. The lipid concentration was measured using the Phospholipid C test (Wako Pure Chemical Industries, Ltd., Osaka, Japan). BLs were prepared from liposomes and perfluoropropane gas (Takachiho Chemical Inc., Co., Ltd., Tokyo, Japan). First, 5 mL sterilized vials containing 2 mL of liposome suspension (lipid concentration: 1 mg/mL) were filled with perfluoropropane gas, capped, and then pressurized with 7.5 mL of perfluoropropane gas. The vials were placed in a bath-type sonicator (42 kHz, 100 W, Bransonic 2510J-DTH, Branson Ultrasonics Co., Danbury, CT, U.S.A.) for 5 min to form BLs. The mean size of the BLs was determined using light-scattering with a zeta potential/particle sizer (Nicomp 380ZLS, Santa Barbara, CA, U.S.A.).
Animals and Tumor ModelsMale BALB/c mice (6 weeks old) were purchased from Tokyo Laboratory Animals Science Co., Ltd. (Tokyo, Japan). All animal use and relevant experimental procedures were approved by the Tokyo University School of Pharmacy and Life Science Committee on the Care and Use of Laboratory Animals. Colon26 cells (1×106 cells/mouse) and Matrigel 50 µL (BD Biosciences, San Diego, CA, U.S.A.) were inoculated subcutaneously in the flank of mice. In vivo histological and antitumor studies were performed when the tumors were 100–200 mm3. In vivo temperature rise test was performed when the tumors were approximately 300 mm3.
Histological AnalysisTumor-bearing mice were treated with intratumoral injection of BLs (50 µg/50 µL) and HIFU exposure (Frequency: 3 MHz, Duty: 100%, Intensity: 1.5 kW/cm2, Time: 60 s). Next day, each tumor dissected, and fixed with 4% paraformaldehyde substituted with 20% sucrose and then embedded in optimal cutting temperature compound (Sakura Finetech, Co., Ltd., Tokyo, Japan) and frozen at −80°C. Each tumor section was prepared with a width of 6 µm and mounted on a poly-L-lysine coated slide. The tumor section were processed by both hematoxylin and eosin (H&E) and nicotinamide adenine dinucleotide reduced (NADH) tetrazolium reductase (NADH-TR) staining.5) The tissue sections were incubated at 37°C for 1 h in 150 mL of Tris–HCl buffer (pH 7.4) containing 150 mg of nitro blue tetrazolium (WAKO, Japan) and 120 mg of β-NADH (WAKO, Japan). The tissue sections were washed with distilled water, various concentrations of acetone (30, 60, 90, 60, and 30%), and finally mounted with glycerol gelatin (Sigma-Aldrich). H&E staining was also performed on the tumor sections using the standard technique. The ablated area seen by NADH-TR staining was measured using ImageJ software.
In Vivo Antitumor EfficacyTumor-bearing mice were treated with intratumoral injection of BLs (50 µg/50 µL) and HIFU exposure (Frequency: 3 MHz, Duty: 100%, Intensity: 1.5 kW/cm2, Time: 60 s), next day tumor volume was monitored. Then, this cycle was repeated three times.
First, we performed histological analysis of tumor tissue after BLs and HIFU exposure. BLs (lipid volume: 50 µg/50 µL) were administered using an intratumoral injection (n=5). Then, tumor-bearing mice were exposed to HIFU (frequency: 3 MHz, rate: 100%, intensity: 1.5 kW/cm2, time: 60 s). Tumor sections were prepared and processed by both H&E staining and NADH-TR staining. To identify tumor ablation area, NADH-TR staining was used to differentiate the viable (blue regions) and nonviable (white/clear regions) tumor tissue. The ablation area of the treatment of BLs and HIFU was ten times more than treatment with HIFU alone (Fig. 1). When tumor-bearing mice were also exposed to HIFU, outward damage (e.g., ablation, necrosis) to normal tissue (e.g., liver, kidney, and spleen) was not observed (data not shown). From these results, in order to verify that the ablation of tumor was due to thermal elevation by the treatment of BLs and HIFU exposure, we monitored temperature of tumor tissue (n=7) in time-course by thermometer (TM-300, IK-300, AS ONE, Osaka, Japan) (Fig. 2). As shown in Fig. 2, the temperature of the tumor tissue rose to 45°C with continuous exposure to HIFU for 3 min, while the temperature of tumor tissue by treatment with BLs and HIFU reached 45°C in a mere 20 s, rose to 50°C in 1 min, and finally reached 55°C in 3 min. Generally, tissue heated to over 55°C by an ablation technique such as HIFU has been reported as “ablative hyperthermia,” which has been used as a definitive treatment of accessible solid tumor, whereas tissue heated to 40–45°C has been reported as “mild hyperthermia,” which has been shown to be effective as an adjuvant for both radiotherapy and chemotherapy.6,7) These results suggested that the ablation area of the tumor in this study was due to high temperature (50–55°C).
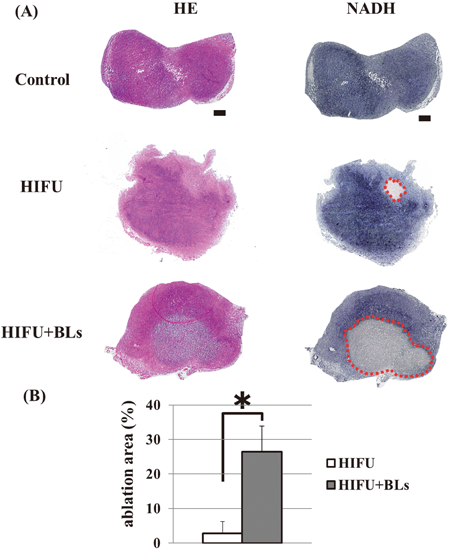
Mice were implanted s.c. with 1×106 of colon26 cells into the flank. After 5–6 d of implantation, they were treated with intratumoral injection of BLs and HIFU exposure (frequency: 3 MHz, rate: 100%, intensity: 1.5 kW/cm2, time: 60 s). Each tumor was dissected, and the tumor section were sliced at 6 µm (n=5). (A) Each section underwent H&E staining (left panel) and NADH-TR staining (right panel). Scale bars represent 500 µm. NADH-TR staining show viable tissue (blue) and nonviable tissue (clear/white). (B) The ablation area (red circle) of the tumor was measured by Image J. * p<0.005.
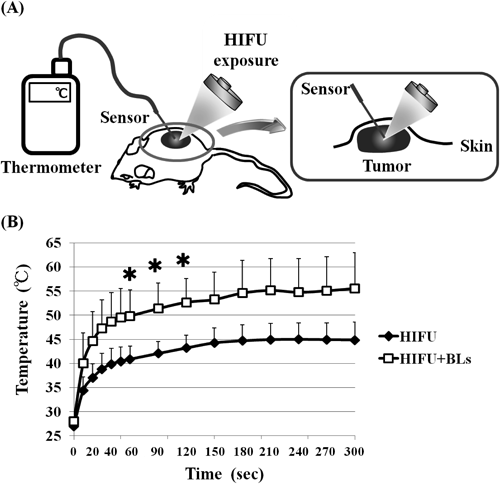
Tumor-bearing mice were treated with BLs and HIFU exposure (frequency: 3 MHz, rate: 100%, intensity: 1.5 kW/cm2, time: 300 s, BLs: 50 µg/50 µL), and the tumor temperature monitored. Each value is the average and S.D. (n=7). * p<0.005 compared with HIFU. (A) Scheme of tumor temperature measurement; (B) Temperature increase in tumor treated with HIFU alone or BLs and HIFU.
Next, as treatment with BLs and HIFU showed that the ablation area of the tumor was broader than that with HIFU alone by increasing temperature, the antitumor effect of BLs and HIFU treatment was evaluated in tumor-bearing mice. Tumor-bearing mice were treated with intratumoral injection of BLs and HIFU exposure, and tumor volume was monitored the following day. This cycle was repeated three times. As shown in Fig. 3, the BLs and HIFU group had significantly suppressed tumor growth compared with the control group (p<0.01). In addition, the tumor growth inhibition efficacy of the BLs and HIFU group was better than that of the HIFU alone group (p<0.05). It has been reported that local high-temperature hyperthermia (50–55°C) enhanced the indices of the T helper 1 (Th1) immune response, such as interleukin-2 (IL-2), interferon-gamma (IFN-γ), and tumor necrosis factor-alpha (TNF-α)8); therefore, it could be suggested that the antitumor effect of BLs and HIFU exposure was due to the ablation effect and immune response.
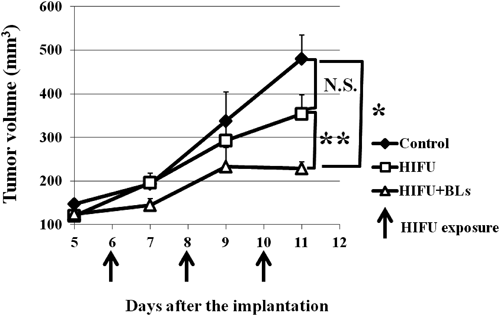
Tumor-bearing mice were treated with intratumoral injection of BLs and HIFU exposure (frequency: 3 MHz, rate: 100%, intensity: 1.5 kW/cm2, time: 60 s, BLs: 50 µg/50 µL). Tumor volume of tumor-bearing mice was monitored. Each value is the average and S.D. (n=4). * p<0.01, ** p<0.05.
In this study, we observed that treatment with BLs and HIFU could enhance the ablation area of the tumor and the antitumor effect. The ablation area with BLs and HIFU treatment was broader than with HIFU alone. We next monitored the temperature of tumor tissue with BLs and HIFU treatment. In this experiment, the temperature increase with BLs and HIFU treatment was faster and higher than with HIFU alone. Moreover, BLs and HIFU treatment enhanced the antitumor effect, which was better than with HIFU alone. Thus, the combination of BLs and HIFU could be efficacious treatment for cancer therapy.
We are grateful to Prof. Katsuro Tachibana (Department of Anatomy, School of Medicine, Fukuoka University) for technical advice regarding the induction of cavitation with US and to Mr. Yasuhiko Hayakawa and Mr. Kosho Suzuki (NEPA GENE Co., Ltd.) for technical advice regarding US exposure. This study was supported in part by the Research Foundation for Pharmaceutical Sciences and the Industrial Technology Research Grant Program (04A05010) from New Energy, the Industrial Technology Development Organization (NEDO) of Japan.